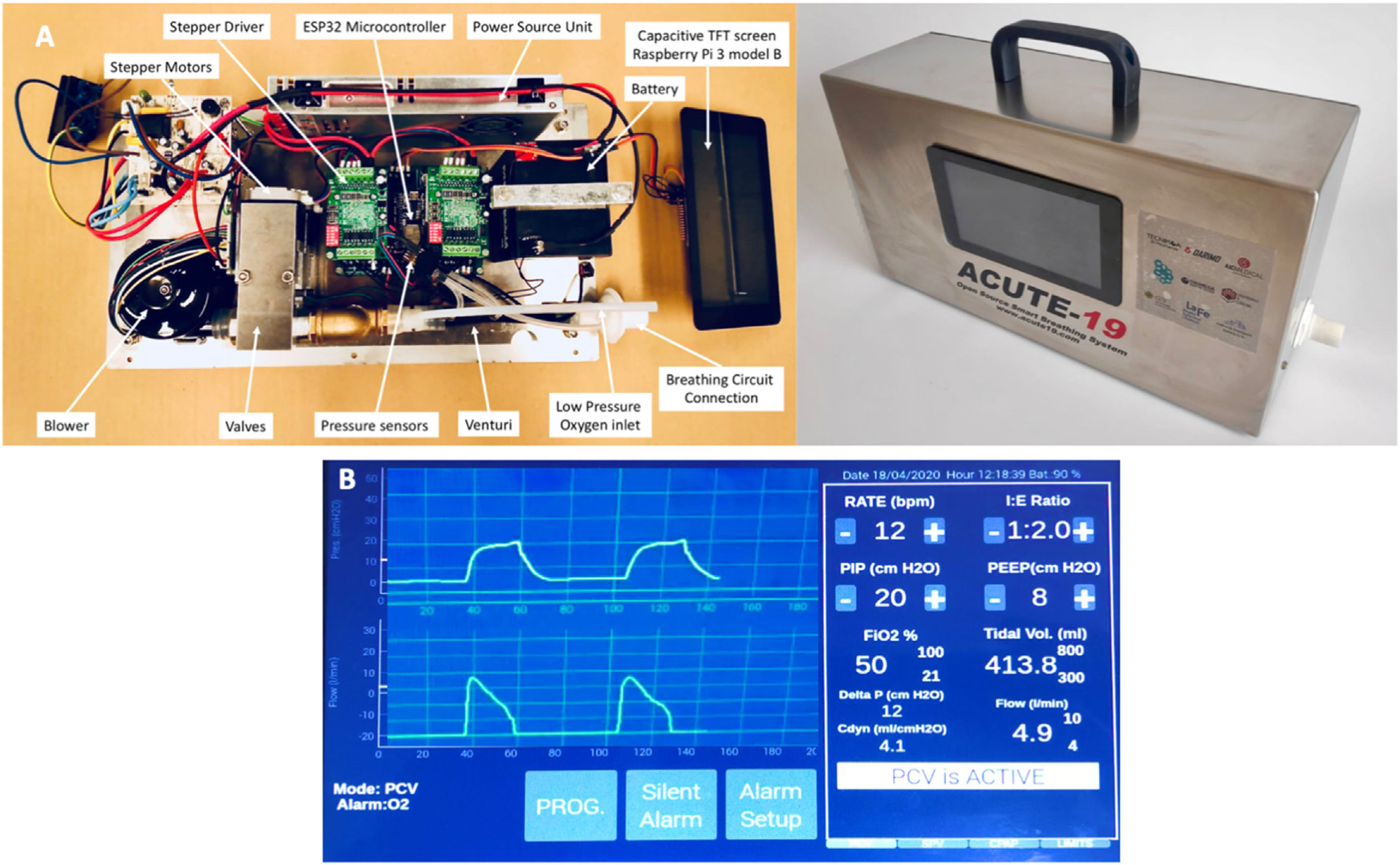
The Severe Acute Respiratory Syndrome (SARS)–Coronavirus 2 (CoV–2) pandemic pressure on healthcare systems can exhaust ventilator resources, especially where resources are restricted. Our objective was a rapid preclinical evaluation of a newly developed turbine–based ventilator, named the ACUTE–19, for invasive ventilation.
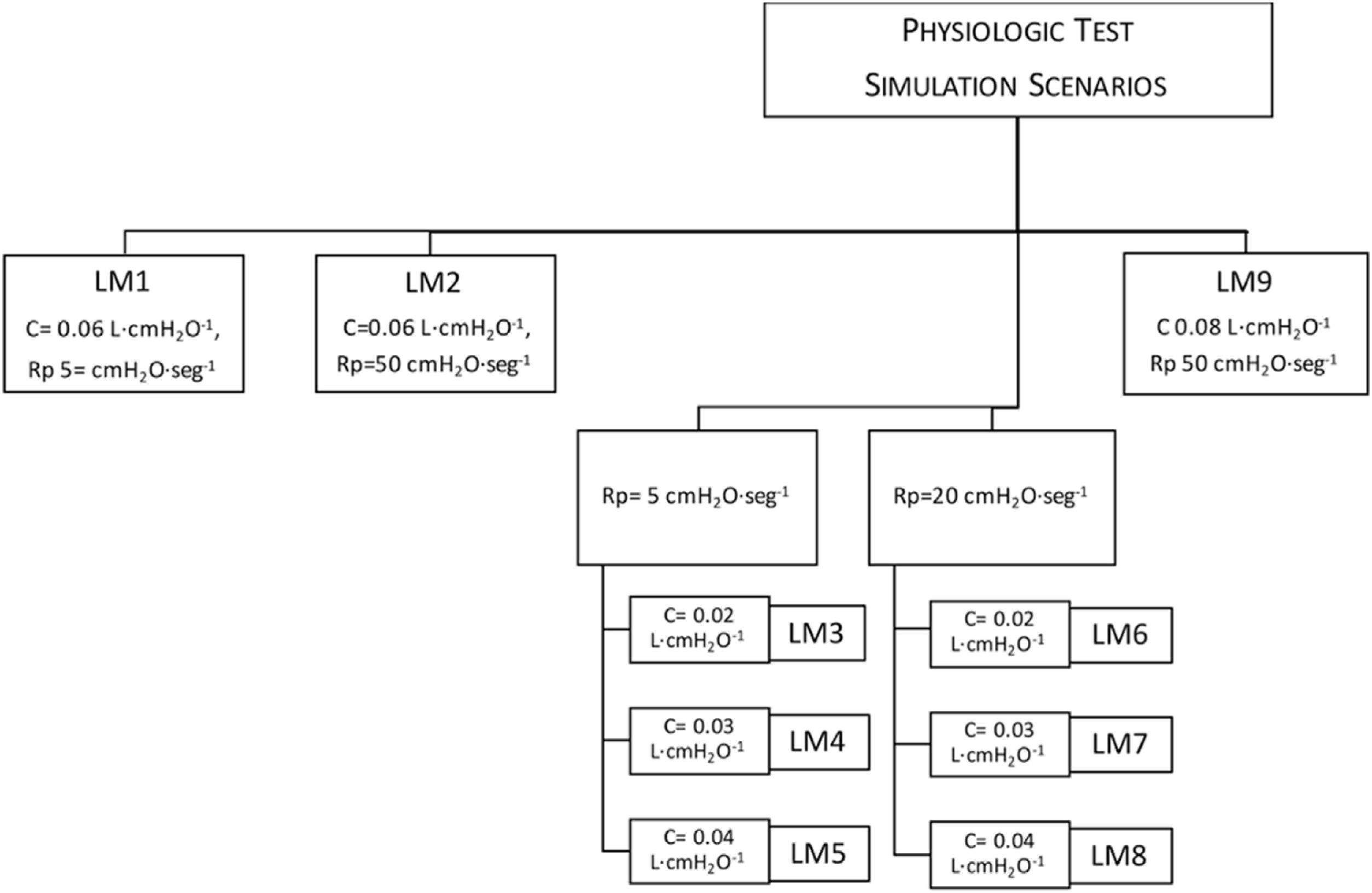
MethodsValidation consisted of (a) testing tidal volume (VT) delivery in 11 simulated models, with various resistances and compliances; (b) comparison with a commercial ventilator (VIVO–50) adapting the United Kingdom Medicines and Healthcare products Regulatory Agency–recommendations for rapidly manufactured ventilators; and (c) in vivo testing in a sheep before and after inducing acute respiratory distress syndrome (ARDS) by saline lavage.
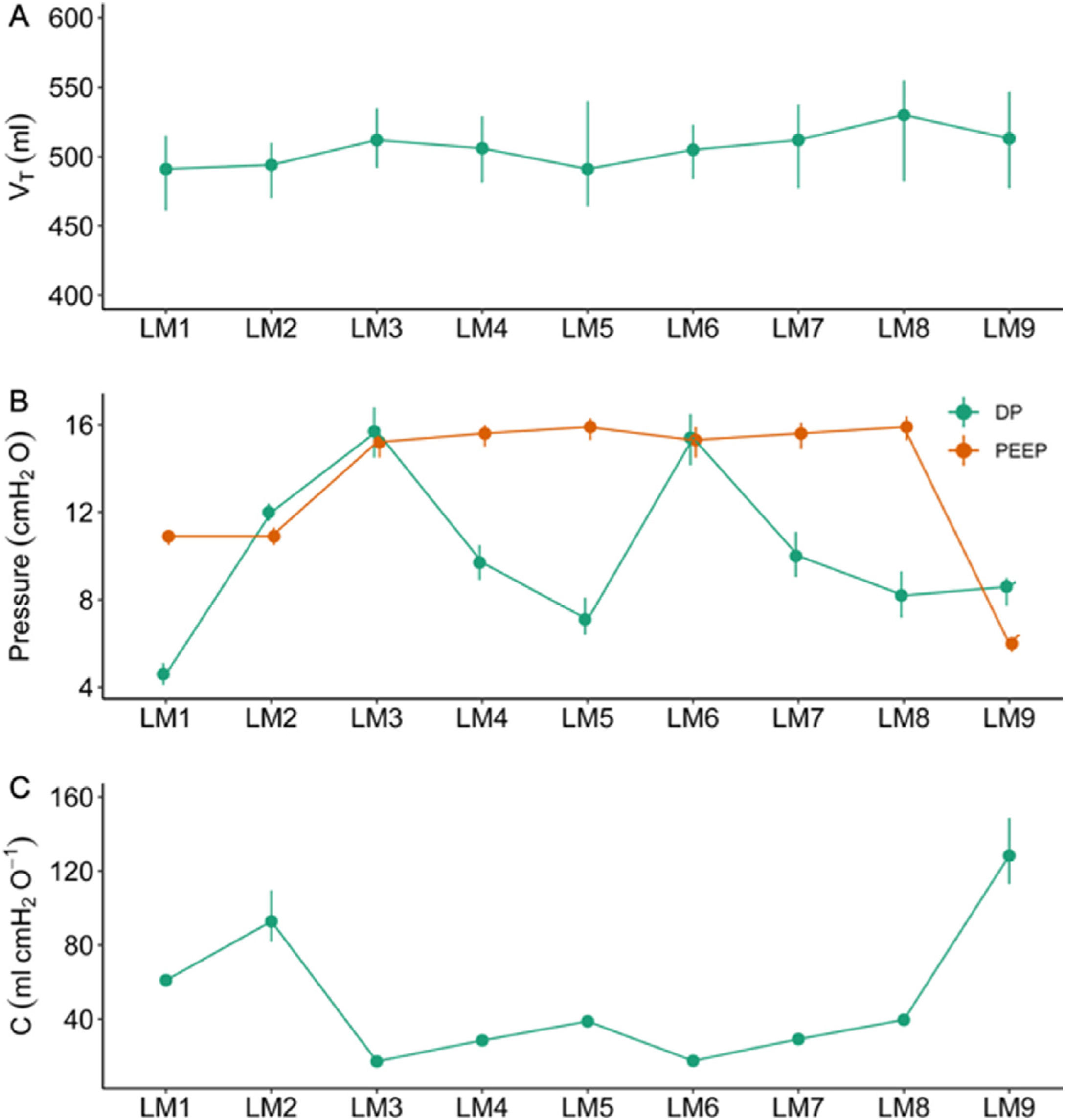
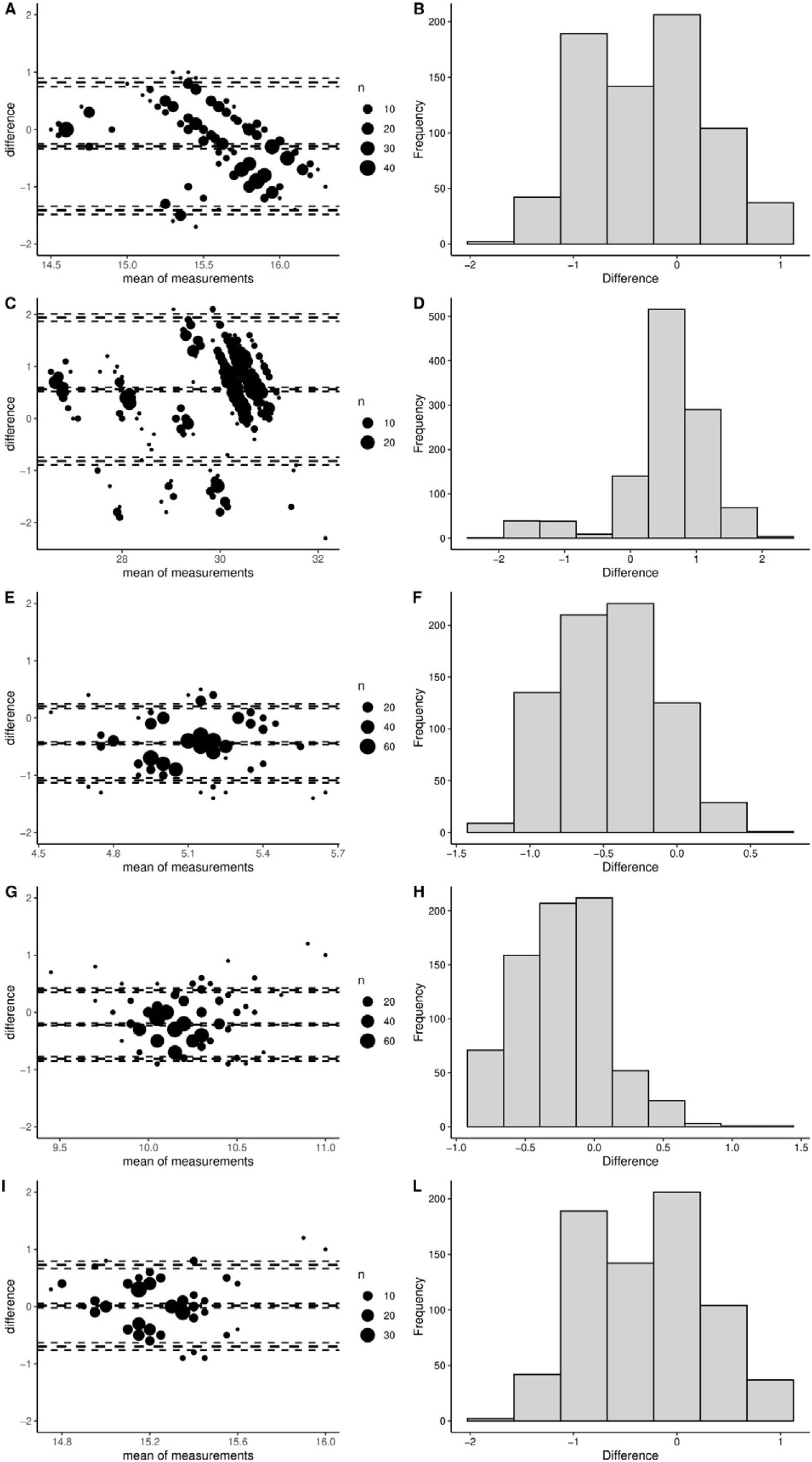
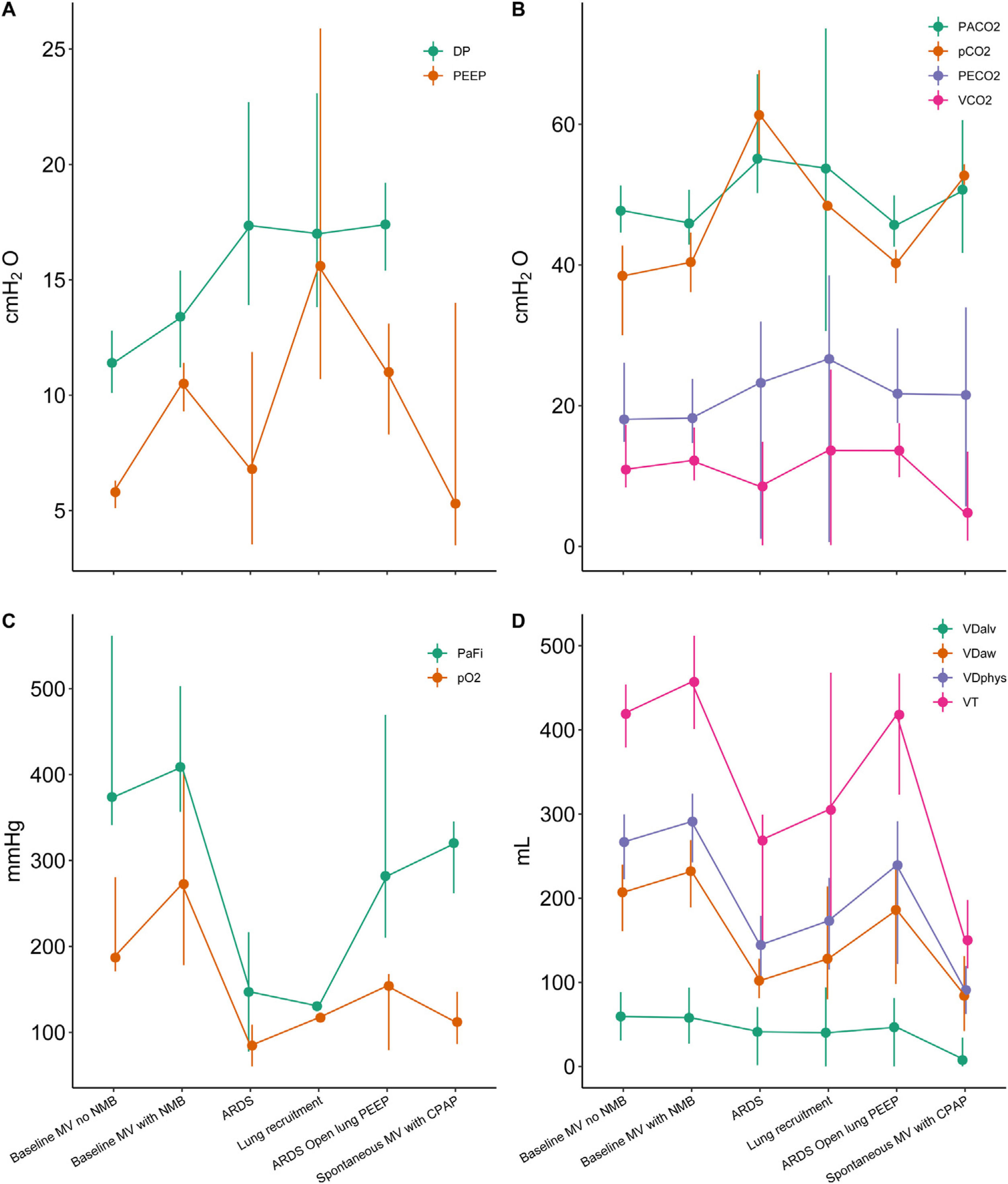
ResultsDifferences in VT in the simulated models were marginally different (largest difference 33ml [95%–confidence interval (CI) 31–36]; P<.001ml). Plateau pressure (Pplat) was not different (–0.3cmH2O [95%–CI –0.9 to 0.3]; P=.409), and positive end-expiratory pressure (PEEP) was marginally different (0.3 cmH2O [95%–CI 0.2 to 0.3]; P<.001) between the ACUTE–19 and the commercial ventilator. Bland–Altman analyses showed good agreement (mean bias, –0.29, [limits of agreement, 0.82 to –1.42], and mean bias 0.56 [limits of agreement, 1.94 to –0.81], at a Pplat of 15 and 30cmH2O, respectively). The ACUTE–19 achieved optimal oxygenation and ventilation before and after ARDS induction.
ConclusionsThe ACUTE–19 performed accurately in simulated and animal models yielding a comparable performance with a VIVO-50 commercial device. The acute 19 can provide the basis for the development of a future affordable commercial ventilator.
La pandemia producida por el Síndrome Respiratorio Agudo Severo (SARS) por Coronavirus 2 (CoV-2) puede agotar los recursos sanitarios, especialmente de respiradores, en situaciones de escasez de recursos sanitarios. Nuestro objetivo fue realizar una evaluación preclínica rápida de un prototipo de respirador de turbina para la ventilación invasiva denominado ACUTE-19.
MétodosLa validación consistió en (a) evaluación de la administración de un volumen corriente (VT) en 11 modelos pulmonares simulados, con diversas resistencias y compliancias; (b) comparación con un ventilador comercial (VIVO-50) adaptando las recomendaciones de la Agencia Reguladora de Medicamentos y Productos Sanitarios del Reino Unido para ventiladores de fabricación rápida; y (c) realización pruebas in vivo en una oveja antes y después de inducir el síndrome de distrés respiratorio agudo (SDRA) mediante lavado salino.
ResultadosLas diferencias de VT en los modelos simulados fueron mínimamente diferentes (la mayor diferencia fue de 33ml [intervalo de confianza (IC) del 95%: 31 a 36]; P<,001ml). La presión de meseta (Pplat) no fue diferente (−0,3 cmH2O [IC del 95%: −0,9 a 0,3]; P=,409), y la presión positiva al final de la espiración (PEEP) fue levemente diferente (0,3cmH2O [IC del 95%: 0,2 a 0,3]; P<,001) comparando el ACUTE-19 y el ventilador comercial. El análisis de Bland-Altman mostró una buena concordancia (bias medio, −0,29, [límites de concordancia, 0,82 a −1,42], y bias medio 0,56 [límites de concordancia, 1,94 a −0,81], a una Pplat de 15 y 30cmH2O, respectivamente). El ACUTE-19 consiguió una oxigenación y ventilación óptimas antes y después de la inducción del SDRA en el modelo animal.
ConclusionesEl ACUTE-19 se comportó con precisión en los modelos simulados y animales, con un rendimiento comparable al del dispositivo comercial VIVO-50. El ACUTE-19 puede servir de base para el desarrollo de un futuro ventilador comercial asequible.