Near-infrared spectroscopy combined with a vascular occlusion test (VOT) could indicate an impairment of microvascular reactivity (MVR) in septic patients by detecting changes in dynamic variables of muscle O2 saturation (StO2). However, in the perioperative context the consequences of surgical trauma on dynamic variables of muscle StO2 as indicators of MVR are still unknown.
MethodsThis study is a sub-analysis of a randomized controlled trial in patients with metastatic primary ovarian cancer undergoing debulking surgery, during which a goal-directed haemodynamic algorithm was applied using oesophageal Doppler. During a 3min VOT, near-infrared spectroscopy was used to assess dynamic variables arising from changes in muscle StO2.
ResultsAt the beginning of surgery, values of desaturation and recovery slope were comparable to values obtained in healthy volunteers. During the course of surgery, both desaturation and recovery slope showed a gradual decrease. Concomitantly, the study population underwent a transition to a surgically induced systemic inflammatory response state shown by a gradual increase in norepinephrine administration, heart rate, and interleukin-6, with a peak immediately after the end of surgery. Higher rates of norepinephrine and a higher heart rate were related to a faster decline in StO2 during vascular occlusion.
ConclusionsUsing near-infrared spectroscopy combined with a VOT during surgery showed a gradual deterioration of MVR in patients treated with optimal haemodynamic care. The deterioration of MVR was accompanied by the transition to a surgically induced systemic inflammatory response state.
En pacientes sépticos, la espectroscopia cercana a infrarrojos combinada con un test de oclusión vascular (VOT) puede indicar alteraciones de la reactividad microvascular (RMV) detectando cambios dinámicos de la saturación de oxígeno muscular (StO2). Sin embargo, se desconocen las consecuencias del trauma quirúrgico sobre la StO2 como indicador de RMV perioperatoria.
MétodosSubanálisis de un ensayo clínico aleatorizado en pacientes con metástasis de cáncer primario de ovario sometidos a cirugía citorreductora donde se aplicó un algoritmo de terapia hemodinámica dirigida a objetivo mediante doppler esofágico. Tras un VOT de 3min, se valoraron cambios dinámicos de la StO2 muscular mediante espectroscopia cercana a infrarrojo.
ResultadosAl inicio de la cirugía, los valores de desaturación y las pendientes de recuperación de valores basales fueron comparables a los valores obtenidos en voluntarios sanos pero ambas mostraron disminuciones progresivas durante el transcurso de la misma. Simultáneamente, la población a estudio sufrió una transición a un estado de respuesta inflamatoria sistémica por estrés quirúrgico, mostrándose por un incremento progresivo de los requerimientos de norepinefrina, de la frecuencia cardiaca y de interleucina 6, y produciéndose un pico inmediatamente tras la cirugía. Las dosis altas de norepinefrina y la frecuencia cardiaca se correlacionaron con una disminución más rápida de StO2 durante el VOT.
ConclusionesEl uso combinado de espectroscopia cercana a infrarrojo y VOT durante la cirugía mostró un deterioro progresivo de la RMV en pacientes hemodinámicamente tratados de forma óptima. El deterioro de la RMV se acompañó de una transición a un estado de respuesta inflamatoria sistémica inducida por cirugía.
In recent years, several studies shown that advanced haemodynamic monitoring can reduce postoperative morbidity and hospital length of stay.1 Despite this, however, mortality following non-cardiac surgery remains high2 and not even goal-directed haemodynamic algorithms to optimize stroke volume, maintain mean arterial pressure and avoid low cardiac output states by advanced haemodynamic monitoring will prevent a high rate of postoperative complications in major non-cardiac surgery.3 One reason for this high complication rate could be impaired microvascular reactivity,4 while altered tissue oxygen consumption may be a contributor to organ dysfunction.4,5
Near-infrared spectroscopy (NIRS) is an indirect method of assessing microcirculation that uses muscle O2 saturation as a surrogate variable of microvascular perfusion.6 NIRS combined with a vascular occlusion test (VOT) could detect changes in microvascular reactivity by indicating an impairment of dynamic muscle O2 saturation (StO2) response in septic7,8 and trauma patients,9 associating this value with organ dysfunction and mortality.7,8 In contrast to direct visualization of the microvascular bed (e.g., with microscopy), NIRS in combination with a VOT is non-invasive and simple to use, and could be a more feasible evaluation technique, especially during limited access surgery.
However, intraoperative changes in the dynamic StO2 response and their association with symptoms remain unclear. Two recent studies10,11 have evaluated the association between intraoperative absolute StO2 measurements and perioperative characteristics. In these studies, however, no goal-directed haemodynamic algorithm was used, and VOT, which gives a clearer picture of StO2 response to ischaemia and reperfusion, was not performed.
The aims of this study were twofold: to investigate changes over time in dynamic muscle O2 saturation (StO2) response shown by NIRS measurement of changes in muscle StO2 over different VOT periods; and to evaluate pre-, intra- and postoperative clinical characteristics associated with dynamic StO2 variables in ovarian cancer patients undergoing major cytoreductive surgery.
MethodsThis is a sub-analysis of a previously published randomized controlled trial comparing balanced crystalloid with balanced colloid infusion within a goal-directed haemodynamic algorithm (BalaCriCo, ISRCTN 53154834).3 This sub-analysis investigates the significance of dynamic muscle StO2 response, including data obtained by NIRS that was not reported in the main study. All data was obtained from the per-protocol group of the BalaCriCo trial, resulting in a subset of 48 patients with metastatic ovarian carcinoma undergoing cytoreductive surgery. The study was duly registered (EudraCT 2008-006135-12; ISRCTN 53154834), and authorization to proceed was obtained from the competent German authorities (Bundesinstitut für Arzneimittel und Medizinprodukte; BfArM-Nr. 4034705) and from the corresponding independent ethics committee (Ethikkommission des Landes Berlin; Nr. EK 12 581/08). The trial was conducted at Charité – University Medicine Berlin, Campus Virchow Klinikum, Berlin, Germany, and written informed consent was obtained from all patients. Subjects were treated according to the previously published interdisciplinary clinical pathway defined by standard operating procedures accessible on the intranet of the University Hospital of the Charité–University Medicine, Berlin, Germany.3
Intraoperative haemodynamic managementHaemodynamic management followed the previously published, goal-directed, oesophageal Doppler-guided12 (ODM, CardioQ-ODM™, Deltex Medical, Chichester, UK) haemodynamic algorithm.3 A brief description is given in Appendix.
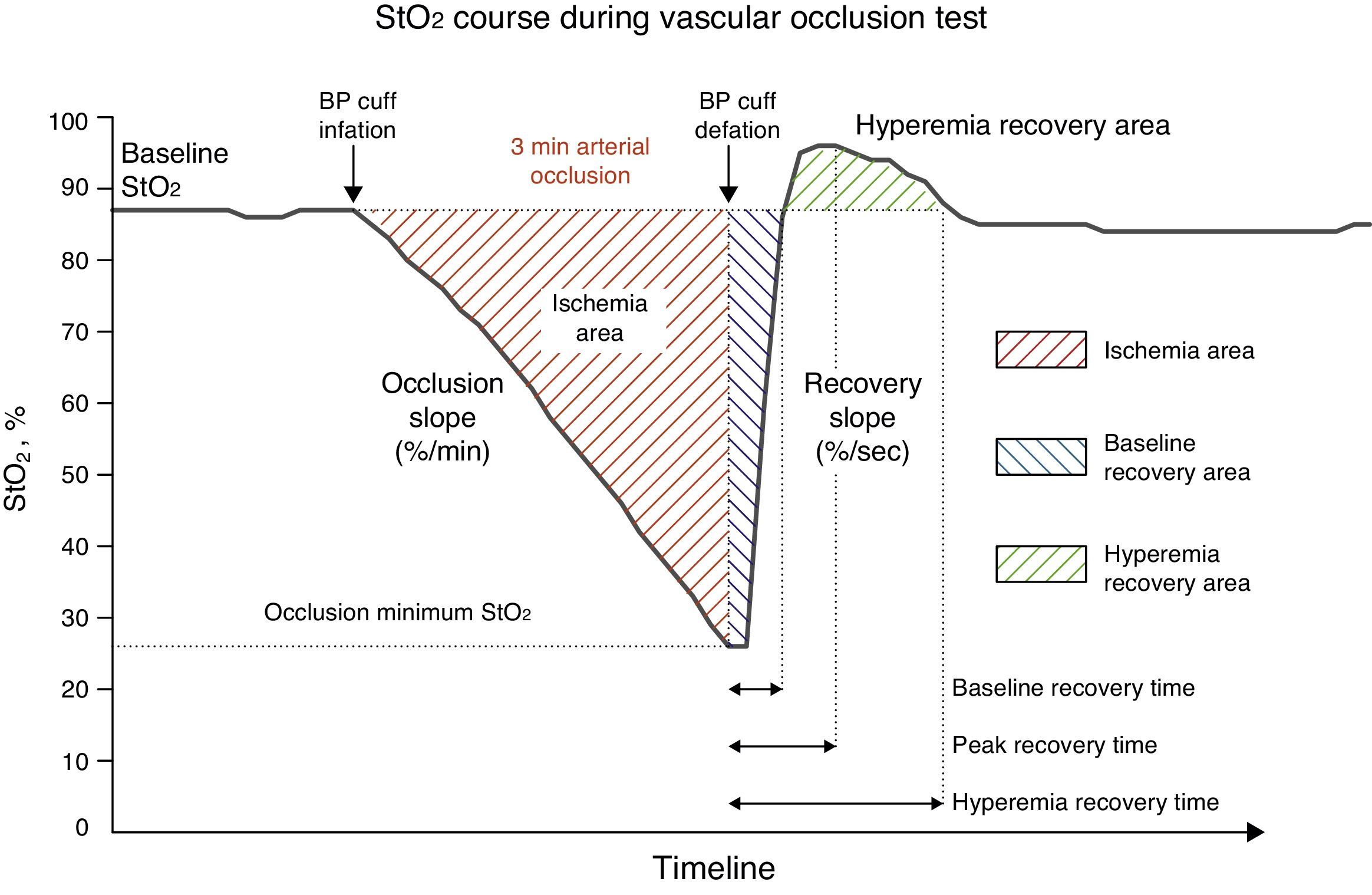
Near infrared spectroscopy and vascular occlusion testAfter induction of anaesthesia and start of haemodynamic monitoring, a 15mm NIRS sensor (Sensor 1615, Hutchinson Technology, Inc., Hutchinson, MN, USA) was placed on the patient's thenar eminence and connected to the InSpectra™ near-infrared spectroscopy StO2 monitor (Model 650, Hutchinson Technology, Inc., Hutchinson, MN, USA). The sensor emits light at a wavelength of 680–800nm, and can detect 95% of StO2 to a depth of 14mm. Continuous intraoperative StO2 values were obtained and digitally recorded every 2s. The intraoperative vascular occlusion test (VOT) was performed every 30min. A blood pressure cuff was placed around the upper arm and inflated to 50mmHg above the pressure measured by the arterial line. Pressure was maintained for 3min (arterial occlusion), and was then rapidly and completely deflated. VOT allowed us to define four different periods: pre-VOT, ischaemia, reperfusion and hyperaemia. Using InSpectra™ software (Version 4.03) we obtained the following variables for each of the foregoing VOT periods: (1) Baseline StO, pre-VOT (baseStO2), (2) desaturation slope (desStO2) during arterial occlusion, (3) recovery slope (recStO2) during reperfusion, and (4) hyperaemia recovery area during hyperaemia (Fig. 1).
Changes in StO2 during the 3-min vascular occlusion test (VOT). Baseline StO2 levels show absolute StO2 values before and after VOT. Dynamic StO2 response is shown by the occlusion slope during ischaemia, the recovery slope during reperfusion, and the hyperaemic recovery area during hyperaemia.
Data were excluded from the statistical analysis, if: (a) the vascular occlusion time varied by more than 10% of 3min; (b) the period between two VOTs was less than 15min; and (c) the measured StO2 response curve during VOTs could not be analyzed correctly by the analysis software.
The entire dataset of a particular patient was excluded because InSpectra™ near-infrared spectroscopy StO2 monitoring was not performed before the start of surgery.
Sampling and measurement of interleukin-6 and intercellular adhesion molecule 1 levelsTo measure plasma interleukin 6 (IL-6) and intracellular adhesion molecule-1 (ICAM-1) levels, blood samples were drawn 1 day before surgery, at 2h after the start of surgery, at 1 and 6h after surgery, and on postoperative days 1 and 3.
Plasma IL-6 and ICAM-1 levels were measured by flow cytometry (FACSCalibur™, BD Biosciences, San Jose, CA, USA) after processing in a bead immunoassay kit (BD Biosciences).
Data management and statistical analysisSince the main study was a randomized controlled trial, patients were randomized to receive either a balanced crystalloid or balanced colloid solution within a goal-directed algorithm. In view of the randomization process and the knowledge that colloid solutions have been seen to improve microperfusion in experimental animal models and critical patients13–16 we looked for significant correlation between any of the dynamic StO2 response variables and the randomized study groups by means of multivariate generalized estimating equation (GEE) model adjusted for multiple measurements per patient. The type of study solution given was also included in all regression analysis to adjust all other covariables.
Due to the limited sample sizes and non-normal distributions of measurements, data were expressed as median [25th–75th percentile], or frequencies [%], respectively. After global testing, post hoc analyses were carried out to detect specific inter-group differences at fixed time points (Mann–Whitney tests), or intra-group difference at two time points (Wilcoxon test). A two-tailed p-value <0.05 was considered statistically significant. All tests have to be interpreted in the context of exploratory data analysis; therefore, no adjustments for multiple testing were made.
Changes over time in study outcomes were analyzed using multivariate nonparametric analysis of longitudinal data in a bifactorial model (1st (independent) factor: groups, 2nd (dependent) factor: repetitions over time). Therefore, all time points were simultaneously compared on the corresponding response curves.
The following comparisons, with their respective analyses, were performed: differences between groups (over time) (Group), systematic changes over time (over groups) (total time), group/time interactions (Group×Time), as well as systematic changes over time for each group (changes over time).
To study clinical factors associated with the changes over time in VOT variable, desStO2 and recStO2, regression analysis was performed using a multivariate generalized estimating equation (GEE) adjusted for multiple measurements per patient. Preoperative clinical factors, intraoperative variables and postoperative outcomes were tested in a univariate analysis before creating the multivariate GEE model for the VOT variable. Adjusted odds ratios (OR) with 95% confidence interval were calculated. To facilitate clinical interpretation of the data, the odds ratios of norepinephrine, heart rate and temperature were stepped (s.c.) at intervals of 0.1μgkg−1min−1, 5beatsmin−1 and 0.1°C, respectively. Due to their close correlation, postoperative clinical outcomes were analyzed with univariate GEE for each VOT variable.
All numerical calculations were performed with IBM© SPSS© Statistics (version 20, Copyright© 1989, 2010 SPSS, Inc.), SAS, version 9.1, (Copyright© SAS Institute, Inc., Cary, NC, USA), and R project for Statistical Computing, version 3.0.2.
ResultsOf the 48 patients included in the main study, 17 were excluded because near-infrared spectroscopy was not performed during surgery. Another patient was excluded because the first measurement was obtained 3h after start of surgery. In total, therefore, the data from 30 patients were analyzed. Neither the changes over time in absolute mean baseline StO2 levels nor the changes over time in any dynamic StO2 variables were associated with any of the randomized groups in the main study.
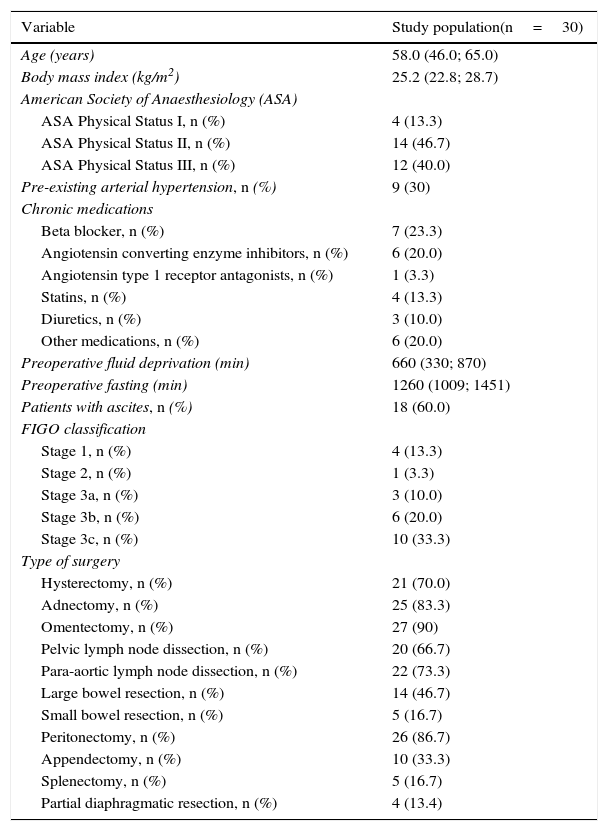
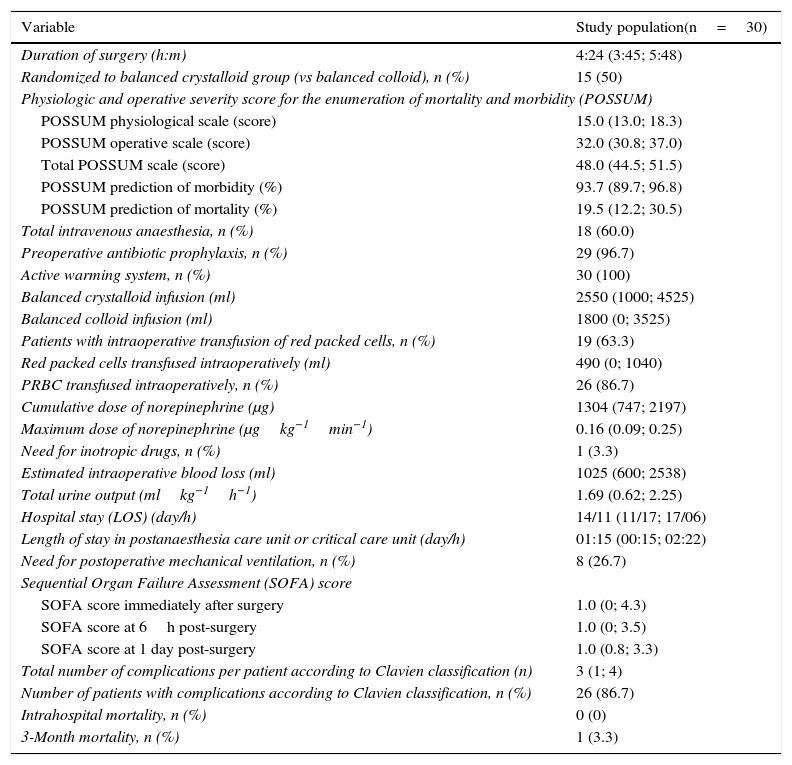
Demographic characteristics, tumour staging and surgery performed are shown in Table 1, and intra- and postoperative data are outlined in Table 2. Changes over time in haemodynamic variables are shown in Fig. 2A–F. Patients showed an increase in heart rate and need for norepinephrine administration to maintain mean arterial pressure during the first 3h of surgery. Stroke volume index decreased over the first 2h, and recovered before the end of surgery. Intraoperative central venous oxygen saturation, however, increased steadily (Fig. 2A–F).
Demographic characteristics and tumour staging of study patients.
| Variable | Study population(n=30) |
|---|---|
| Age (years) | 58.0 (46.0; 65.0) |
| Body mass index (kg/m2) | 25.2 (22.8; 28.7) |
| American Society of Anaesthesiology (ASA) | |
| ASA Physical Status I, n (%) | 4 (13.3) |
| ASA Physical Status II, n (%) | 14 (46.7) |
| ASA Physical Status III, n (%) | 12 (40.0) |
| Pre-existing arterial hypertension, n (%) | 9 (30) |
| Chronic medications | |
| Beta blocker, n (%) | 7 (23.3) |
| Angiotensin converting enzyme inhibitors, n (%) | 6 (20.0) |
| Angiotensin type 1 receptor antagonists, n (%) | 1 (3.3) |
| Statins, n (%) | 4 (13.3) |
| Diuretics, n (%) | 3 (10.0) |
| Other medications, n (%) | 6 (20.0) |
| Preoperative fluid deprivation (min) | 660 (330; 870) |
| Preoperative fasting (min) | 1260 (1009; 1451) |
| Patients with ascites, n (%) | 18 (60.0) |
| FIGO classification | |
| Stage 1, n (%) | 4 (13.3) |
| Stage 2, n (%) | 1 (3.3) |
| Stage 3a, n (%) | 3 (10.0) |
| Stage 3b, n (%) | 6 (20.0) |
| Stage 3c, n (%) | 10 (33.3) |
| Type of surgery | |
| Hysterectomy, n (%) | 21 (70.0) |
| Adnectomy, n (%) | 25 (83.3) |
| Omentectomy, n (%) | 27 (90) |
| Pelvic lymph node dissection, n (%) | 20 (66.7) |
| Para-aortic lymph node dissection, n (%) | 22 (73.3) |
| Large bowel resection, n (%) | 14 (46.7) |
| Small bowel resection, n (%) | 5 (16.7) |
| Peritonectomy, n (%) | 26 (86.7) |
| Appendectomy, n (%) | 10 (33.3) |
| Splenectomy, n (%) | 5 (16.7) |
| Partial diaphragmatic resection, n (%) | 4 (13.4) |
Data are shown as median (interquartile range) or as number of patients (%).
Intraoperative and postoperative characteristics of study patients.
| Variable | Study population(n=30) |
|---|---|
| Duration of surgery (h:m) | 4:24 (3:45; 5:48) |
| Randomized to balanced crystalloid group (vs balanced colloid), n (%) | 15 (50) |
| Physiologic and operative severity score for the enumeration of mortality and morbidity (POSSUM) | |
| POSSUM physiological scale (score) | 15.0 (13.0; 18.3) |
| POSSUM operative scale (score) | 32.0 (30.8; 37.0) |
| Total POSSUM scale (score) | 48.0 (44.5; 51.5) |
| POSSUM prediction of morbidity (%) | 93.7 (89.7; 96.8) |
| POSSUM prediction of mortality (%) | 19.5 (12.2; 30.5) |
| Total intravenous anaesthesia, n (%) | 18 (60.0) |
| Preoperative antibiotic prophylaxis, n (%) | 29 (96.7) |
| Active warming system, n (%) | 30 (100) |
| Balanced crystalloid infusion (ml) | 2550 (1000; 4525) |
| Balanced colloid infusion (ml) | 1800 (0; 3525) |
| Patients with intraoperative transfusion of red packed cells, n (%) | 19 (63.3) |
| Red packed cells transfused intraoperatively (ml) | 490 (0; 1040) |
| PRBC transfused intraoperatively, n (%) | 26 (86.7) |
| Cumulative dose of norepinephrine (μg) | 1304 (747; 2197) |
| Maximum dose of norepinephrine (μgkg−1min−1) | 0.16 (0.09; 0.25) |
| Need for inotropic drugs, n (%) | 1 (3.3) |
| Estimated intraoperative blood loss (ml) | 1025 (600; 2538) |
| Total urine output (mlkg−1h−1) | 1.69 (0.62; 2.25) |
| Hospital stay (LOS) (day/h) | 14/11 (11/17; 17/06) |
| Length of stay in postanaesthesia care unit or critical care unit (day/h) | 01:15 (00:15; 02:22) |
| Need for postoperative mechanical ventilation, n (%) | 8 (26.7) |
| Sequential Organ Failure Assessment (SOFA) score | |
| SOFA score immediately after surgery | 1.0 (0; 4.3) |
| SOFA score at 6h post-surgery | 1.0 (0; 3.5) |
| SOFA score at 1 day post-surgery | 1.0 (0.8; 3.3) |
| Total number of complications per patient according to Clavien classification (n) | 3 (1; 4) |
| Number of patients with complications according to Clavien classification, n (%) | 26 (86.7) |
| Intrahospital mortality, n (%) | 0 (0) |
| 3-Month mortality, n (%) | 1 (3.3) |
Data are shown as median (interquartile range) or number of patients (%).
Changes over time in heart rate (A), mean arterial pressure (B), systolic volume index (C), norepinephrine administration (D), temperature (E), and central venous saturation (F). Data are shown as median (interquartile range) during surgery and the nonparametric analysis of systemic changes over time in longitudinal data is shown as the corresponding p value.
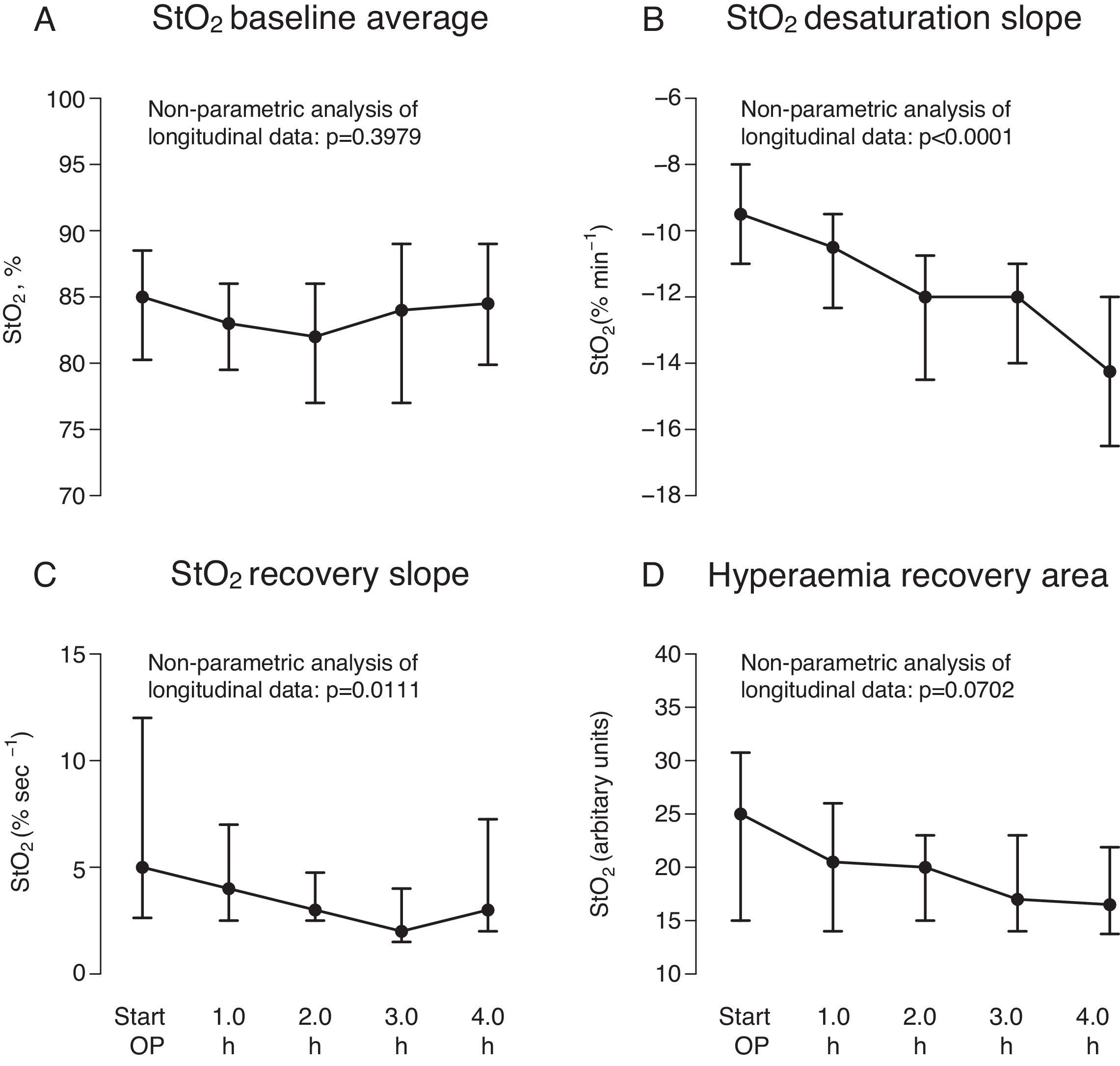
Median baseStO2 was 85% (79.5%; 89%), and remained constant during surgery (p=0.3979) (Fig. 3A) (Appendix Table 1, available online). However, baseStO2 showed a wide interquartile range, indicating high inter-patient variability.
Variables calculated during the four time points of the repeated vascular occlusion test (VOT): mean baseline pre-VOT StO2 (A), StO2 saturation slope during ischaemia (B), StO2 recovery slope during reperfusion (C), and the hyperaemic recovery area during hyperaemia (D). Data are shown as median (interquartile range) during surgery and the nonparametric analysis of systemic changes over time in longitudinal data is shown as the corresponding p value.
desStO2 and recStO2 progressively decreased over the first 4h of surgery (p<0.001 and p=0.0111, respectively) (Fig. 3B,C, Appendix Table 1, available online). recStO2 also showed a wide interquartile range at the start of surgery, indicating that this time point also varied considerably between patients. In contrast, intraoperative hyperaemia recovery area did not change significantly (p=0.0702) (Fig. 3D, Appendix Table 1, available online).
The GEE regression analysis of the correlation between preoperative variables and changes over time in StO2 variables showed that a higher score on the nutritional risk screening system17 is associated with an intraoperative decreases in desStO2 and increase in recStO2. Higher desStO2 values were associated with a longer period of preoperative fluid deprivation (Appendix Table 2, available online).
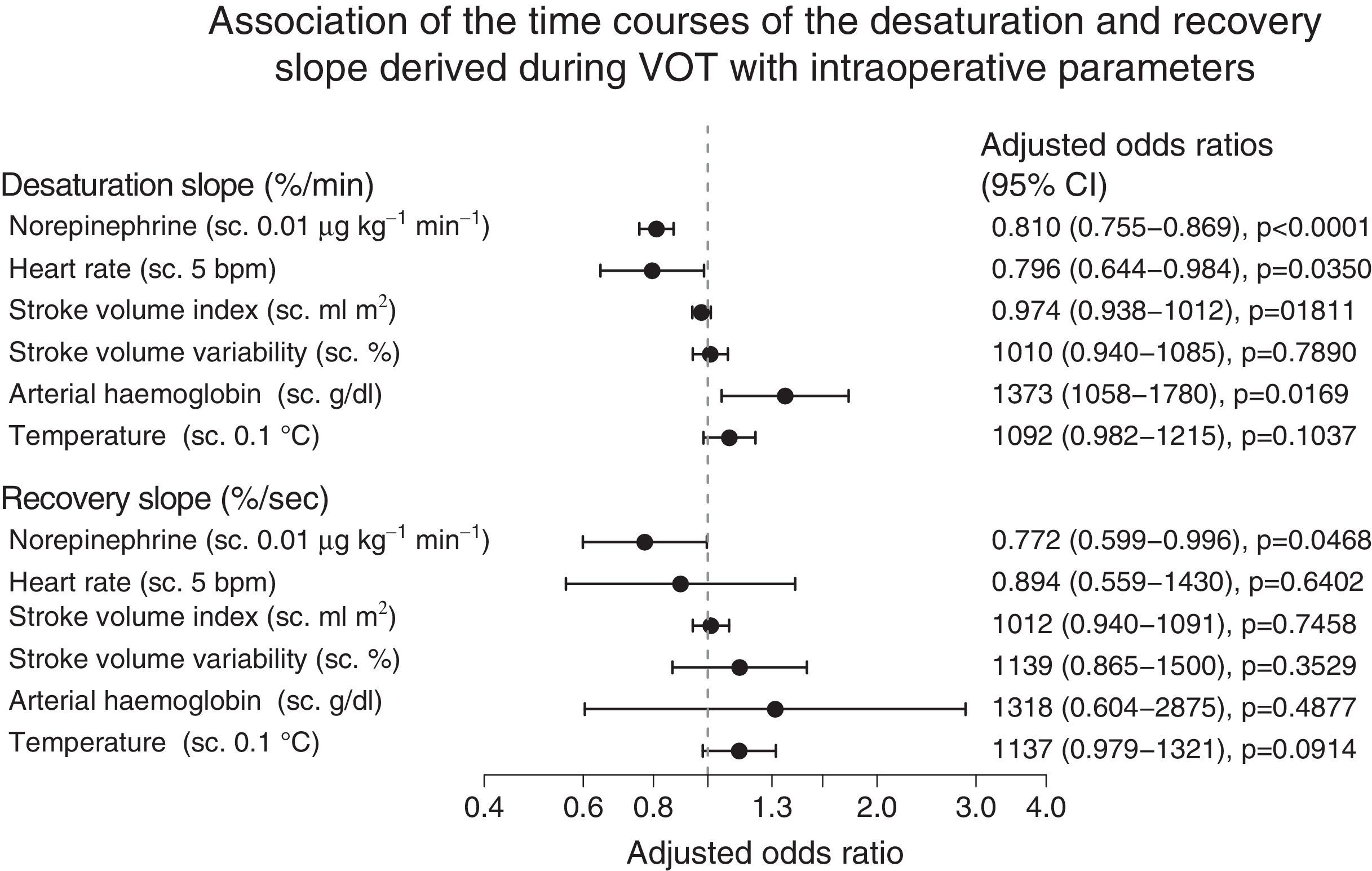
The GEE analysis of the association between intraoperative variables and mean baseline StO2 levels showed that administration of high-dose norepinephrine was associated with lower (negative) intraoperative desStO2 values, a sharper decline in StO2 during vascular occlusion, and lower recStO2, all of which were associated with slower recovery of baseline values (Fig. 4). Furthermore, increased heart rate and lower arterial haemoglobin levels were associated with lower (negative) intraoperative desStO2 values; in other words, a steeper decline in StO2 during vascular occlusion implied an impaired StO2 response (Fig. 4). None of the VOT variables were associated with body temperature in any study patients.
Forrest plot showing the correlation between the intraoperative changes over time in saturation and recovery slopes and intraoperative variables, showing the odds ratio obtained by GEE. The odds ratio with a 95% confidence interval (95% CI) was plotted on a logarithmic scale and adjusted for the remaining variables. The odds ratios of norepinephrine, heart rate, and temperature were stepped (s.c.) at intervals of 0.01μgkg−1min−1, 5bpm and 0.1°C, respectively.
In the case of postoperative variables, lower (negative) desStO2 values during surgery, in other words, a steeper decline in StO2 during VOT, was associated with a longer hospital stay, both in the intensive care unit and on the ward, and higher score in the sequential organ failure assessment at 1 and 6h post-surgery, and the first postoperative day. RecStO2 was not related to any postoperative patient variables (Fig. 1). Even though intraoperative temperature remained steady, there was considerable inter-patient variability, and cooling of the body surface interfered with StO2 measurements due to peripheral vasoconstriction.16 Therefore, it seemed important to us to include temperature in the regression analysis to adjust all other covariables, although in itself it was not significant.
Plasma interleukin 6 (IL-6) levels increased slightly during the first 2h of surgery, and then sharply 1h post-surgery. They did not return to baseline levels until the third postoperative day (Fig. 2 of the Appendix, available online). Changes over time in the endothelial marker ICAM-1 decreased 2h after the start of surgery. Postoperatively, ICAM-1 increased 6h after surgery, and unlike IL-6, reached a peak on the first postoperative day. It did not normalize until the third postoperative day (Fig. 2 of the Appendix available online).
DiscussionOur findings evaluate the dynamic StO2 response reflected by changes in StO2 measured with NIRS during a vascular occlusion test (VOT) during major non-cardiac surgery.
The main findings of the study are: (1) At the start of surgery, desStO2 and recStO2 values are similar to those measured in healthy volunteers, but during surgery dynamic muscle StO2 variables, indicative of microvascular reactivity, progressively declined; (2) surgery-induced systemic inflammation was found in all study patients. This was evidenced by increased administration of norepinephrine and increased heart rate during the first 3h of surgery, and a peak IL-6 level immediately after surgery; and (3) decline in the dynamic StO2 response was associated with the rate of norepinephrine administration, heart rate and arterial haemoglobin rates, thus confirming the validity of intraoperative NIRS monitoring.
Non-invasive NIRS has already been used to investigate changes in StO2 in trauma patients during initial care,9,18 and in patients with severe sepsis or septic shock.7,8,19–21 This, however, is the first study to assess detailed changes in intraoperative dynamic StO2 response measured by NIRS combined with a vascular occlusion test (VOT) during major non-cardiac surgery, and their association with clinical characteristics. The patients in this trial were treated within a goal-directed haemodynamic algorithm to optimize stroke volume according to the Frank–Starling mechanism by bolus administration of intravenous fluids while maintaining mean arterial blood pressure by norepinephrine administration and using inotropes to prevent low cardiac output.12 Despite this, patients showed a steady deterioration in dynamic StO2 variables caused by changes in StO2 during the different VOT periods.
desStO2 and recStO2 showed significant intraoperative decline, and the values measured at 3 and 4h after the start of surgery were similar to those reported in studies in patients with severe sepsis or septic shock. In the case of recStO2 our study patients showed values of 5% (2.5%; 14.5%)/s at the beginning of surgery, comparable to the 5.20±1.19%/s values found by Gomez et al. in healthy volunteers. During surgery, our study patients showed a steady decrease in recStO2 to 3% (2.5%; 5.0%) and 2% (1.25%; 4.5%)/s after the second and third hour of surgery. These values are consistent with those reported by Mesquida et al. in septic patients (3.02±1.7%/s), by Creteur et al. in patients with severe sepsis and septic shock (3.2% [1.8%; 4.5%]/s and 2% [1.2%; 2.9%]/s, respectively)7 and by Skarda et al. in septic patients (2.3±1.0%/s).21 These results, combined with the substantial increase in heart rate and need for norepinephrine during the first 3h of surgery, and a significant perioperative increase in IL-6, indicate that our patients transitioned from a state similar to healthy controls to a state of surgery-induced systemic inflammation at a microvascular level at 3h after start of surgery.
Basically, a change in desStO2 during occlusion could be due to a rise or fall in local oxygen consumption (VO2). However, it could also by caused by impaired local oxygen delivery due to microcirculatory changes.6 We found a steep intraoperative decline in StO2 following vascular occlusion, suggesting a gradual increase in local oxygen uptake and/or local impairment of oxygen delivery during surgery. In septic patients, other authors have shown that an initial increase in metabolic activity seems to be fuelled by an increase in cellular respiration. Prolonged sepsis leads to mitochondrial dysfunction and damage, and a down-regulation in mitochondrial protein expression.5 recStO2, meanwhile, seems to derive from the interaction of perfusion pressure and endothelial integrity.6 In line with these findings, we also found a correlation between more gentle recStO2 and greater need for norepinephrine administration. Our findings are further supported by a recent study, showing that microcirculation in septic patients is predominantly characterized by a proportional decrease in perfused capillaries along with increased flow heterogeneity, which could explain the rapid decrease of desStO2 and slower increase in recStO2.22 This hypothesis is based on the observation that higher arterial haemoglobin levels are related with a more gentle decrease in desStO2 and higher heart rate, while greater need for norepinephrine is correlated with a steeper decline in desStO2 and a slower recStO2. In our surgical patients, therefore, progressive deterioration in dynamic StO2 response during VOT could also correlate with an alteration in microperfusion. Nevertheless, the association between alteration in dynamic muscle StO2 variables and intraoperative haemodynamic variables mainly support the validity of intraoperative NIRS monitoring.
Our study has some limitations. The dynamic StO2 response combined with VOT can measure microvascular reactivity, and is a feasible method that can easily be used at the bedside, and even during limited access surgical procedures. However, the main limitation is that the dynamic StO2 response combined with VOT cannot clarify the important link between deterioration in microvascular activity and microcirculation and/or tissue oxygenation and tissue oxygen consumption.4 Although the level of hypnosis remained constant (bispectral index 40 and 55), the anaesthesia technique varied between patients, a factor that could have biased our results. These issues should be addressed in future studies. Furthermore, we observed that body surface cooling affects StO2 measurements due to peripheral vasoconstriction. In our study, an oesophageal temperature probe was used to measure intraoperative temperature. Although intraoperative temperature did not vary and was not associated with VOT variables in the regression analysis, both the considerable length of surgery and the use of vasoconstrictors could have affected peripheral temperature without a corresponding change in central temperature. In addition, the impact on microvascular reactivity of repetitive 3-min VOTs to recreate a situation of ischaemia-reperfusion is unclear. Finally, our sample was limited to 30 patients; therefore our findings should be confirmed in a larger study group.
The use of NIRS combined with VOT during major non-cardiac surgery showed progressive deterioration in microvascular reactivity measured by dynamic StO2 variables in patients with optimal haemodynamic monitoring. These data, along with a substantial increase in intraoperative heart rate and need for norepinephrine, and observation of intraoperative inflammatory markers, indicate that study patients transitioned to a surgery-induced inflammatory response accompanied by deterioration of microvascular reactivity.
Authors contributionStudy conceived and designed by: A.F., C.S.
Biometrics: K.-D.W.
Data collected by: A.F., O.H., L.K., J.K., H.S, J.S.
Data interpreted by: A.F., O.H., J.S., C.S.
Statistical analysis performed by: K.-D.W., A.F., O.H., C.S.
Manuscript written by: A.F., O.H., R.C.F., C.S.
Critical review of salient points: all authors.
Final revision: C.S.
Funds obtained by: C.S.
Study supervised by: A.F., K.-D.W., J.S., C.S.
Conflict of interestAll conflicts of interest have been declared. The ICMJE Conflict of Interest form was submitted to the editorial committee.
This project was designed by the researchers and received unlimited funding from Fresenius Kabi, Bad Homburg, German and Hutchinson Technology, Inc., MN, USA. The sponsors had no control over or access to the design of the study, data collection, analysis and interpretation, drafting the manuscript, or the decision to submit the manuscript for publication.
We would like to thank the researchers involved in the BalaCriCo study for their help with this study; also, Ansgar Jones, M.D., Holger Krebbel, M.D., Karin Weimann, M.D., Velizara Pavlova, M.D. and Olga Müller, M.D. for their help in recruiting patients for the study, and Mandy Koch, M.D., Jean-Philipp Zallet, M.D., Kathrin Solzbach, M.D., Alexander Giebels, M.D. and David Liehre, M.D. for their help with data acquisition.
Please cite this article as: Feldheiser A, Hunsicker O, Kaufner L, Köhler J, Sieglitz H, Casans Francés R, et al. La respuesta de la saturación dinámica muscular en cirugía no cardiaca se altera pese a la terapia hemodinámica dirigida por objetivos. Rev Esp Anestesiol Reanim. 2016;63:149–158.