Osteoporosis is a skeletal system pathology characterized by low bone mineral density and tissue structural deterioration. This condition is associated with high fracture risk that severely compromises quality of life. Osteoporosis incidence is becoming more significant with increasing lifespan worldwide.
Novel treatment strategies have been developed that aim to inhibit excessive bone resorption and/or increase bone formation. The most promising novel treatments include: denosumab, a monoclonal antibody for receptor activator of NF-κB ligand, a key osteoclast cytokine; odanacatib, a specific inhibitor of the osteoclast protease cathepsin K; and antibodies against the proteins sclerostin, GKS-3b and dickkopf-1, two endogenous inhibitors of bone formation. This review discusses these new therapies.
La osteoporosis es una patología que afecta el sistema esquelético y se caracteriza por una baja densidad mineral ósea y un deterioro estructural del tejido óseo. Esta enfermedad está asociada con un alto riesgo de fracturas que comprometen seriamente la calidad de vida. La incidencia de osteoporosis es actualmente mayor debido al aumento de la esperanza de vida en el mundo.
Numerosas investigaciones con respecto a nuevas estrategias de tratamiento han sido desarrolladas y tienen como objetivo inhibir la resorción ósea excesiva o aumentar la formación de hueso. Entre los tratamientos más prometedores están denosumab, un anticuerpo monoclonal que ejerce su acción contra el activador del receptor del ligando NF-kappa B, una citoquina clave de los osteoclastos; odanacatib, un inhibidor específico de la proteasa catepsina K de los osteoclastos; y anticuerpos monoclonales contra las proteínas antiesclerostina, la glucógeno sintasa quinasa-3b y dickkopf-1, dos inhibidores endógenos de la formación de hueso. En esta revisión se analizan y se revisan los conceptos actuales de las nuevas terapias.
It was conducted a literature review, searching articles of relevance on new therapies in osteoporosis, in order to perform subsequently a broad description of the topic. The review was carried out in the MEDLINE and EMBASE databases. The search strategy did not have date limits and was conducted using MeSH terms as it follows: “osteoporosis AND antiresorptive therapies”, “osteoporosis AND anabolic therapies”, “osteoporosis AND denosumab”, “osteoporosis AND cathepsin k inhibitors”, “osteoporosis AND odanacatib”, “osteoporosis AND relacatib”, “osteoporosis AND balicatib”, “osteoporosis AND Src kinase inhibitors”, “osteoporosis AND saracatinib”, “osteoporosis AND GLP-2 analogs”, “osteoporosis AND dickkopf-1 inhibitor”, “osteoporosis AND GSK-3b inhibitors”, “osteoporosis AND anti-sclerostin antibodies”, “osteoporosis AND blosozumab”, “osteoporosis AND romosozumab”.
In addition, in the search strategy was included “new therapies in osteoporosis”.
The inclusion criteria established that articles should have the following characteristics: systematic review or an article of interest for researchers that covers the subject of new therapies in osteoporosis; articles in a language other than English or Spanish were excluded.
After selecting the final articles, we proceeded to describe in a qualitative manner their most important findings.
IntroductionThe understanding of the intracellular control processes that regulate bone remodeling is essential for planning the therapy against osteoporosis.1
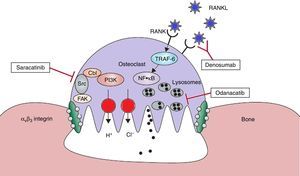
Bone remodeling consists in a strict coupling between bone resorption and bone formation, which continues throughout life; this process is necessary for the removal of damaged and obsolete bone, in addition to maintaining the normal bone structure. The process begins with the resorption of a volume of bone by the osteoclasts, followed by new bone formation by the osteoblasts.2–4 The new therapies are being developed on mechanisms of action that are different from those of the existing drugs. Some of these could offer an inhibition of the resorption without reducing the bone formation (Fig. 1).
Therapeutic targets in the osteoclast. With the help of avβ3 integrin, the osteoclasts adhere to the bone surface and form sealing zones. It is produced a highly acidic microenvironment which is essential for the catalytic activity of enzymes such as cathepsin K. Odanacatib inhibits cathepsin K, a lysosomal protease that degrades collagen. Src kinase plays a crucial role in the activity of osteoclasts and it can be inhibited by saracatinib. RANKL acts as essential regulator in the differentiation and activity of osteoclasts. Denosomab, a totally human monoclonal antibody, prevents RANKL from binding to its receptor. FAK: focal adhesion kinase; NF·kB: nuclear factor kappa B; PI3K: phosphatidylinositol 3-kinase; RANK: receptor activator of NF-kB; RANKL: RANK ligand; TRAF-6: tumor necrosis factor receptor associated alpha-6; Src: kinase which regulates cell growth. Adapted and modified from Rachner TD, Khosla D, Hofbauer LC. Osteoporosis: Now and the future. Lancet. 2011;377:1276–87.
That means that now are available drugs that allow to promote the formation of new bone, these therapies have been called anabolic therapies and have been generated thanks to the recent studies and discoveries in bone biology, which are promising through the identification of new molecular targets of this type of therapy.5–7
Anti-bone resorption therapyDenosumabIs a fully human monoclonal antibody that has a high affinity for RANKL, a molecule known for its prominent role in osteoclastogenesis.8,9
Its pharmacokinetic properties are superior, compared with other drugs, which results in a longer dosing interval. It is the most advanced of all the compounds under research and it has been approved in Europe for the treatment of osteoporosis and in the United States for the management of osteoporosis and bone metastases.10
It suppresses, in a dose-dependent manner, the N-terminal telopeptide of the urinary collagen type 1 (NTXu), a molecule that is also used as a biochemical marker of bone resorption, up to 81% in postmenopausal women.11,12
In women, denosumab increases the bone mineral density (BMD) in the lumbar spine, between 3.0%-6.7%, compared with the loss of 0.8% in the placebo group. In the total hip, the BMD increased by 1.9%-3.6% in the denosumab group, but it decreased by 0.6% in the placebo group.13,14
In a randomized, phase 3, placebo-controlled study (FREEDOM) the reduction in fractures with denosumab (60mg every 6 months) was assessed in 7868 women with postmenopausal osteoporosis, of which 24% had pre-existing vertebral fractures. After 3 years, denosumab had reduced the risk of new vertebral fractures by 68%, of hip fractures by 40% and of non-vertebral fractures by 20%. It is noteworthy that the risk for events of cardiovascular diseases, cancer and infections did not differ between the two groups. However, the incidence of eczema was 3.0% vs. 1.7%, and of cellulitis, including erysipelas, was 0.3 vs. <0.1%, being significantly higher in women who received denosumab. The complete evaluation of the immune status of the patients who received denosumab for 12 months did not show relevant changes in the target cells, T cells, B cells or natural killer cells (NK).15
The extension of the FREEDOM study is evaluating the long-term efficacy and safety of denosumab during a maximum of 10 years. In an open study of 4550 women are presented the results of the first 3 years of the extension, in representation of up to 6 years of exposure. The treatment with denosumab for 6 years remained well tolerated and the BMD continued to increase. The incidence of fractures remained low, with no increase in the incidence of adverse events.16
Monotherapy or combination therapy with teriparatide and denosumab were evaluated in the DATA study. The combination therapy increased the BMD in the spine and the femoral neck more than any other agent; therefore, it could be useful for the treatment of patients with high risk of fracture. The initial design of the study was not intended to detect the effects on the risk of fracture, being the open design another potential limitation of the study. Finally, although the safety profile of the combination therapy appears to be similar to the profiles of the groups of individual therapy, this study could not properly evaluate the long-term safety of any of the interventions. This would require a much larger number of patients in a clinical trial.17
After two years of extension of the DATA study, 94 women had a greater increase in BMD than in monotherapy. The combination of these agents could become an interesting treatment option in patients with high risk of fracture.18
The results show that denosumab, unlike bisphosphonates, is associated with a maximum suppression of bone resorption, even when administered with teriparatide.17,18
Two randomized, placebo-controlled studies have embarked on the use of denosumab in women who receive aromatase inhibitors for breast cancer and in men under androgen ablation therapy for prostate cancer.
In the women with aromatase inhibitors for non-metastatic breast cancer, denosumab (60mg every 6 months, for 12 months) increased BMD in the lumbar spine by 5.5% compared with placebo; this study was not designed to evaluate the reduction in fractures.19
In the HALT study, men under androgen deprivation therapy for prostate cancer were evaluated; denosumab (60mg every 6 months, for 24 months) also increased BMD in the lumbar spine by 6.7%, in the total hip by 4.8% and in the distal third of the radius by 5.5%, compared with placebo. The incidence of new vertebral fractures was reduced by 62% at 36 months compared with placebo (1.5% vs. 3.9%).20
Regarding the safety of denosumab, it has been proposed that it could be associated with an increased risk of infections, since RANKL is also expressed on B and T lymphocytes. However, in the clinical trials, the mortality rates associated with infections were similar in the women treated with denosumab and in those assigned to placebo.21
Treatment with denosumab is contraindicated in hypocalcemia, hypoparathyroidism, surgery of the thyroid or parathyroid glands, malabsorption or resection of the small bowel, since there is a higher risk of hypocalcemia; in patients who receive immunosuppressive agents or with a history of immunosuppression and in patients who have severe infections in the context of the treatment. Also, a complete dental examination is indicated before treatment with denosumab.22,23
Osteonecrosis of the jaw (ONJ) associated with denosumab has been described in patients with osteoporosis after a dental extraction, existing a temporal association between the use of the drug and ONJ, as well as between its withdrawal and improvement.24
In addition, in several cases described in the literature, ONJ often appears after a tooth extraction. Although the main risk factors that have been found for ONJ include the use of glucocorticoids, multiple myeloma, chemotherapy, intravenous bisphosphonates and dental pathology or extraction.25
The advantages offered by denosumab include the absence of gastrointestinal side effects and the possibility of use in patients with chronic kidney disease with GFR <30ml/min, with a regular monitoring of vitamin D and calcium levels.26
Cathepsin K inhibitorsOdanacatibBased on the concept that the protease cathepsin K plays an important role in the enzymatic degradation of the bone, the use of cathepsin K inhibitors has emerged as a new therapeutic approach. Cathepsin K is a key lysosomal enzyme of the activated mature osteoclasts, which inhibits the function of osteoclasts, but preserves their viability.27,28
Odanacatib is, currently, the most potent inhibitor of cathepsin K and the most advanced in clinical research. The oral bioavailability of cathepsin K inhibitors depends on the formulation, being higher than the oral bioavailability of bisphosphonates. It is metabolized by cytochrome P450 enzymes and it can interact with other medications.29,30
Phase 1 studies of odanacatib show that oral doses of 50mg and 100mg, once a week, reduce serum concentrations of the C-terminal telopeptide of collagen type 1 (CTXs), a marker of bone resorption, by 62%. Daily administration of odanacatib (10mg) reduces serum concentrations of CTXs by 81%.31
In a Phase 2 study (OCEAN study), weekly oral doses of odanacatib were evaluated in 399 women with postmenopausal osteoporosis. After 24 months, odanacatib (50mg) increased BMD in the lumbar spine by 5.7% and in the total hip by 4.1%, compared with placebo. The suppression of the bone resorption markers was dose-dependent. It is noteworthy that a subgroup of 32 women underwent bone biopsies, followed by histomorphometry, showing as a result a modest and transient reduction of bone formation markers without suppression of the formation rate. The adverse reactions with odanacatib were similar to those with placebo, standing out scleroderma-like lesions.32
The extension of the OCEAN study for 2 years reveals that the inhibition of cathepsin K with odanacatib resulted in a decrease in most resorption markers but did not decrease the bone formation markers. This was associated with an increase in BMD; the effect on the biochemical markers was rapidly reversible after discontinuation of treatment.33
The Phase III (LOFT), randomized, blind, placebo-controlled study, with provisional pre-planned analysis to allow the early termination if there were significant differences, had three primary objectives that were to determine radiological vertebral and hip fractures and non-vertebral clinical fractures. The secondary objectives included increase in BMD, bone turnover markers, and safety and tolerability, including bone histology. The participants were women aged 65 years or older, with a BMD T-score≤−2.5 in the hip or in the femoral neck or with a vertebral fracture and a T-score≤−1.5. They were randomized for tablets of odanacatib or placebo.34
After a planned interim analysis, an independent data monitoring committee recommended to stop the study early, due to the robust efficacy and a favorable risk/benefit profile.34
Regarding the safety profile, the most commonly reported adverse events include headache, influenza-like symptoms, odynophagia, eating disorders and abdominal discomfort.35,36
Odanacatib is a lipophilic compound with poor solubility, the dosage of fat in the diet increases the secretion of bile, which can further increase the dissolution of the drug.37 Pharmacokinetic studies showed that foods with high fat content increase the area under the curve (AUC) of the cathepsin K inhibitor by approximately 110%. A high-fat breakfast before dosing, for doses of 25–300mg, produced an approximately two-fold increase in plasma concentrations in relation with fasting.38,39
Odanacatib is a CYP3A4 substrate, which implies a risk of drug interactions. One study evidenced that concomitant use with steroids (prednisone) does not cause significant effects on the exposure of the sensitive CYP3A4 substrates in vivo at therapeutic doses. Concomitant administration of prednisone 10mg, once a day, had no effect on the pharmacokinetics of odanacatib 10mg.40
Another study evaluated the administration in conjunction with warfarin evidencing that the pharmacokinetics and the pharmacodynamics were not affected by the administration of multiple doses of odanacatib, indicating that this is not a clinically important inhibitor of CYP 2C9, 3A4, 2C19 or 1A2. Just like what was reflected in another study of administration in conjunction with digoxin.41,42
RelacatibIt acts non-selectively on cathepsins K, L and V. Clinical trials with this drug were stopped, after Phase I, due to the drug-drug interactions with commonly prescribed medications such as acetaminophen, ibuprofen, and atorvastatin.43
BalicatibHighly selective cathepsin K inhibitor, but it is not so selective in the cells because of its high concentration in the lysosomes.44
Phase II studies were carried out in subjects with osteoarthritis and osteopenia/osteoporosis. During one year, four daily oral doses (5, 10, 25 o 50mg) were compared against placebo in 675 postmenopausal women. The treatment with 25 and 50mg reduced bone resorption markers (CTXs 61%, NTXu 55%) with no change in bone formation markers. It was associated with a dose-related increase in BMD, reaching 4.5% in the lumbar region and 2.2% in the total hip with the weekly dose of 50mg.44
Despite these results, it was discontinued due of adverse events related to the skin, including eruptions and scleroderma-like, morphea-like lesions.45
Src kinase inhibitorsSaracatinibSrc kinase belongs to the family of tyrosine kinase and it is essential for the function of osteoclasts and the bone resorption. It plays a role in many of the signaling pathways responsible for the survival, the motility and the activation of osteoclasts by RANKL.46
Saracatinib inhibits the Src kinase and the Abl kinase, implied in the cell proliferation, differentiation and response to oxidative stress, but it has very little activity against it.47
Its effect on bone turnover in healthy men was investigated in a randomized, double blind, placebo-controlled, with multiple ascending dose, phase I trial of saracatinib. 59 healthy men with an average age of 34.6 years participated in the study, they were randomly divided into 5 cohorts; 4 with 12 subjects and one with 11 subjects, within each cohort in the proportion of 3:1 to receive a single dose of saracatinib or placebo, respectively, followed 7–10 days later by daily doses for further 10–14 days. The markers of bone turnover were measured before and 24 and 48h after the initial single dose; and immediately before, 24 and 48h and between 10 and 14 days after the final dose.
At a dose of 250mg (maximum tolerated dose), the CTX decreased by 88% (CI 95% 84–91%) and NTXu decreased by 67% (CI 95% 53–77%) of the baseline 24h after the final dose. There was no significant effect on bone formation markers. Although papular eruption occurred in 30% vs. 6% and the patients had diarrhea in 24% vs. 0%, these reactions were more frequent in the men with saracatinib than in those who received placebo.48
It is currently being tested in Phase 2 studies for solid tumors and bone metastases, but not for osteoporosis.49
Then, the conclusion is reached that the inhibition of Src kinase reduces osteoclastic bone resorption in human beings. Saracatinib is a potentially useful treatment for diseases characterized by increased bone resorption, such as metastatic bone disease and osteoporosis.50
Glucagon-like peptide analogsThe glucagon-like peptide (GLP-2) can decouple the bone resorption process from the bone formation at night; that is how it reduces the nocturnal resorption without affecting the bone formation. Since GLP-2 has a short plasma half-life (7min), the effect only occurs at night and in normal postprandial, reducing bone resorption at breakfast. This could change the balance of the remodeling in favor of bone formation, resulting in a net increase in BMD after repeated administrations.51
GLP-2 applied at 10p.m. in postmenopausal women, for 14 days, results in a dose-dependent decrease of nocturnal bone resorption, according to the evaluation of CTXs.52
A 4-month, double blind, placebo controlled therapeutic trial compared 3 different doses of GLP-2 (0.4mg, 1.6mg and 3.2mg, administered at night) against an injection of saline solution as control. It was evidenced an increase in BMD in the hip of 1.1% from baseline, but not in the lumbar spine; it does not suppress or stimulates the bone formation markers. The overall rates of adverse events in the 4 treatment groups were similar and there were no signs of tachyphylaxis or antibodies against GLP-2. The results indicate that GLP-2 produces a substantial decrease in bone resorption without suppression of bone formation.53
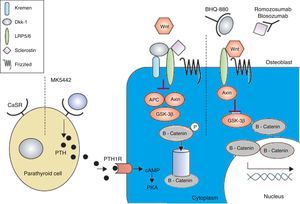
Anabolic therapyAnabolic drugs improve bone formation rather than avoiding a greater loss of bone mass, and the result is a faster increase in bone mass. Special attention is being paid to the activation of the canonical Wnt bone-forming signaling pathway to achieve an anabolic effect on the bone. The canonical Wnt signaling pathway offers several objectives that may be suitable for pharmacological intervention. The main purpose of this interventions is to increase signaling in order to increase the bone mass (Figs. 2 and 3). This has been achieved in animal models by the inhibition of dickkopf-1 (DKK-1), glycogen synthase kinase-3b (GSK-3b), or sclerostin, as described below.54,55
The calcium-sensing receptor is antagonized by MK-5442 and triggers short bursts of PTH secretion. The binding of PTH to its receptor improves the functions of osteoblasts and bone formation. The presence of Wnt antagonists – DKK-1 and sclerostin inhibits signaling. DKK-1 is required to form a complex with Kremen and subsequently it binds to LRP5/6, while sclerostin binds directly to LRP5/6. BHQ-880 and AMG-785 are anti-DKK-1 and anti-sclerostin antibodies, respectively. After neutralizing DKK-1 and sclerostin, the Wnt can bind to LRP5/6, resulting in the degradation of GSK-3. As a consequence – catenin is stabilized, then, it accumulates and translocates to the nucleus where it regulates the transcription of osteoblastic genes. cAMP: cyclic adenosine monophosphate; APC: adenomatous polyposis coli; CaSR: calcium-sensing receptor; DKK-1: dickkopf-1; GSK: glycogen synthase kinase 3 receptor related protein; PKa: protein kinase A; LRP: low density lipoprotein; PTH: parathyroid hormone; PTH1R: PTH1 receptor. Adapted and modified from Rachner TD, Khosla D, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–87.
The low density lipoprotein (LRP5) can form a complex with DKK-1, which triggers a rapid internalization and depletion of LRP5, leading to inhibition of the canonical Wnt signaling pathway.
Genetic studies with mice lacking a single allele of DKK-1 showed a marked increase in the volume of trabecular bone and in the bone formation rate.56
The neutralization of DKK-1 is still limited in preclinical trials. The blockage of DKK-1 inhibits bone loss in a model of rheumatoid arthritis and of multiple myeloma; the inhibition of DKK-1 prevents the formation of osteolytic lesions and increases the bone formation rate.
Currently, it is being investigated in patients with refractory multiple myeloma. However, the effects of neutralization of DKK-1 have not been investigated yet in osteoporosis.57
Heterocyclic inhibitors of glycogen synthase kinase-3bInhibition of GSK-3b could prevent the phosphorylation of b-catenin, which leads to stabilization of the interactions of the b-catenin complex with Wnt.58
The mice treated with lithium chloride as an inhibitor of GSK-3 indicated an increase in the bone formation and in the bone mass. The treatment of oophorectomized rats with an active inhibitor of GSK dual a/b, orally for 2 months resulted in an increase in the number of trabeculae as well as in the trabecular area and thickness. BMD increased in the sites of spongy bone and this was associated with an increase in bone formation in histomorphometry.59
Anti-sclerostin monoclonal antibodies (romosozumab–blosozumab)Sclerostin is a product of the SOST gene that inhibits osteoblastogenesis. SOST mutations result in absent expression of sclerostin and are responsible for sclerosteosis and van Buchem disease. Both skeletal dysplasias are characterized by marked increases in bone mass.60
Anti-sclerostin humanized monoclonal antibodies cause improvement of the Wnt signaling and an increase of the bone mass in rodents and non-human primates. In a model of postmenopausal rats with osteoporosis due to oophorectomy, the treatment with an anti-sclerostin monoclonal antibody increased the bone mass and prevented the bone loss associated with estrogen defficiency.61
In a further stage in humans, it was demonstrated that anti-sclerostin antibodies can increase BMD and the biochemical markers of bone formation in human beings.61
In a Phase 1, randomized, double blind, placebo controlled, single dose study, 72 healthy subjects received AMG 785 or placebo subcutaneously (0.1–0.3–1–3–5–10mg/kg) or intravenously (1 or 5mg/kg) and were followed-up for a maximum of 85 days; it was well tolerated and increased the bone formation markers between 60% and 100% on day 21.
There was a statistically significant increase in BMD by up to 5.3% in the lumbar spine and by 2.8% in the total hip compared with placebo.61
Romosozumab increased the Type 1 amino-terminal propeptide from 66% to 147%, decreased serum C-telopeptide (sCTX) from 15% to 50%, and increased the lumbar spine BMD between 4% and 7%. The rates of adverse events were well balanced between the groups with no significant safety findings. These data support the inhibition of sclerostin in disorders that could benefit from an increased bone formation.62
A Phase II, multicenter, randomized, placebo-controlled, parallel study of 8 groups was conducted over a period of 12 months in 419 postmenopausal women between 55 and 85 years of age, with low BMD (T-score between −2.0 and −3.5 in the lumbar spine, total hip of femoral neck). The participants were randomly assigned into one of the 8 groups: placebo every 3 months or monthly, romosozumab 140mg SC every 3 months, romosozumab 210mg SC every 3 months, romosozumab 70mg SC monthly, romosozumab 140mg SC monthly, romosozumab 210mg SC monthly, alendronate 70mg weekly and teriparatide 20 mcg SC daily. The participants were followed-up during 12 months, BMD was evaluated at the beginning and at 3, 6 and 12 months and laboratory tests were performed at the beginning of the study, at week 1, and at months 1, 2, 3, 6, 9 and 12. Of the 419 participants enrolled in the study, 383 completed the 12-month visit, while 36 withdrew from the study. The main variable of the study was the percentage change from the beginning in BMD at lumbar spine at month 12, and the secondary objectives included the percentage change from the beginning in BMD in the total hip, femoral neck and distal third of the radius at month 12. Compared with placebo, all groups treated with romosozumab had significant increases in BMD at the lumbar spine, total hip and femoral neck at 12 months. The greatest gains were observed with monthly doses of romosozumab (210mg) with an increase of 11.3% in lumbar BMD, an increase of 4.1% in the total hip BMD and the increase of 3.7% in the femoral neck BMD, which were greater than the increase seen in those who were treated with teriparatide and alendronate. There were no significant differences in the distal third of the radio at 12 months in any group. At 6 months, the BMD of lumbar spine and total hip significantly increased in all groups treated with romosozumab, compared with the treatment with placebo, while the femoral neck BMD was higher in the groups that received 140mg of romosozumab monthly, 210mg monthly or 210mg every 3 months, compared with the treatment with placebo.63
Bone turnover markers were also measured as secondary endpoints in months 1, 3, 6, 9 and 12. In all romosozumab groups, the bone resorption marker, CTXs, fell from the baseline, with the largest decrease in the first week, and in the groups that received monthly doses of romosozumab, remained below the baseline at month 12.63
The treatment with romosozumab was associated with a dose-dependent decrease in the calcium levels between 1.30% and 2.68% from the beginning, with a nadir in month one and a compensatory increase in PTH. The serum calcium returned to baseline in the follow-up visits and there were no adverse events associated with the change in the laboratory value. There was no difference in the proportion of participants who reported adverse events between the placebo group (90%) and the romosozumab groups (87%), and there was no apparent relationship between the dose. However, injection site reactions were more frequent with romosozumab than with placebo.63,64
Based on the promising results observed in the initial studies, there are several studies of additional Phase I and II, the studies that have been completed or are ongoing, include a study evaluating the safety of romosozumab in patients with end-stage chronic kidney disease and studies evaluating the effects of romosozumab on fracture healing. In addition, there are 5 ongoing Phase III studies, 4 investigating the use of romosozumab in postmenopausal women with osteoporosis and one that investigates the use of romosozumab in men with osteoporosis.65
Even though research on romosozumab continues, other humanized monoclonal anti-sclerostin antibodies are being developed. More specifically, blosozumab has been investigated in Phase I and Phase II studies. In the Phase I study, conducted in healthy postmenopausal women, the researchers found that there were dose-dependent responses in sclerostin, P1NP, BSAP, osteocalcin, CTXs, and BMD with single and multiple doses of blosozumab. At day 85, there was an increase by up to 3.41% and an increase by up to 7.71% of the baseline in the lumbar spine BMD with the single dose and with multiple doses, respectively.66
In the Phase II study, 120 postmenopausal women with a low BMD received blosozumab or placebo SC in varying doses for 12 months. At the end of the study, the BMD at the lumbar spine increased by 17.7% and the total hip BMD increased by 6.2% in the highest dose group. In addition, the bone formation markers initially increased and then they tended to the pretreatment levels, while the bone resorption markers remained decreased. In general, the drug was well tolerated; therefore, blosozumab also looks promising as a treatment for osteoporosis.67
Based on our understanding of the physiology of sclerostin and on data from studies in humans, there are several potential complications and adverse effects to consider. Firstly, patients with sclerostosis and van Buchem disease develop thickening of the skull and facial bones, that leads to entrapment of cranial nerves; these patients are also at risk for the development of spinal stenosis. It could be speculated that similar complications may occur with long-term treatment, due to excessive accumulation of bone in undesirable locations. However, the positive regulation of sclerostin can regulate the gain of bone mass as suggested by Stolina et al., and this can prevent the excessive accumulation of bone mass.68 Such effect, however, remains to be determined in human beings. The Phase I and Phase II studies have demonstrated that romosozumab is generally well tolerated with mild adverse effects. However, in the Phase I study, one patient developed transient hepatitis. It is not known if there was a causal relationship with the drug. A decrease in serum calcium was seen with the initial dosage. The patients had compensatory increases in PTH and the serum calcium levels returned to the baseline, these decreases in serum calcium could be clinically significant in patients with vitamin D deficiency or chronic kidney disease.69
Both in Phase I and Phase II studies some subjects developed neutralizing antibodies. Although this does not appear to affect the pharmacokinetics or the pharmacodynamics of the drug, with a long-term treatment it could affect the efficacy and potency of the drug.69
ConclusionsThe number of available anti-osteoporotic drugs will increase considerably in the coming years. Many of the new drugs combine efficacy with convenient administration, which could result in a better adherence. Odanacatib and to some extent saracatinib represent a different class of antiresorptive agents that inhibit the activity of osteoclasts rather than undermining their viability. The successful integration of these new compounds is based on the evidence of osteoporosis therapy that requires simple and applicable tools for clinical decision-making.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Posada AF, Aguirre HD, Casallas JCG, Patiño JDL, Oñate RV. Nuevas terapias en osteoporosis. Rev Colomb Reumatol. 2016;23:34–43.