Secondary Raynaud's phenomenon is one of the primary cutaneous manifestations of various rheumatological diseases, which can significantly impact quality of life and whose systemic treatment can lead to several adverse effects. Therefore, this study was conducted to assess the effectiveness of glyceryl trinitrate (in transdermal patch form) in patients with secondary Raynaud's phenomenon (SRP), evaluated through vascular flow photoplethysmography.
Materials and methodsA comparative, analytical, open-label, non-randomized study was conducted to evaluate the efficacy of 4.5 mg of glyceryl trinitrate in a transdermal patch placed on the proximal region of one hand in patients diagnosed with SRP compared to the untreated contralateral hand. To determine the primary outcome, vascular flow photoplethysmography assessment was performed 30 days after daily patch use. Secondary results were obtained using an infrared thermometer to measure local temperature in both hands before patch application, 30 min after patch use, and at the end of the follow-up period. Additionally, various questionnaires, including the Visual Analogue Scale for pain, Raynaud's Condition Score (RCS), and Dermatology Quality of Life Index (DLQI), were administered on the first and last days of the study.
ResultsSixteen patients, all female, with a median age of 54 years, were included. Regarding the rheumatological diseases associated with secondary Raynaud's phenomenon, twelve patients (75% of the sample) were associated with systemic sclerosis, three patients (19%) with lupus erythematosus, and one (6%) with mixed connective tissue disease (MCTD). There was a significant increase in peripheral blood flow after 30 days of treatment with 4.5 mg of glyceryl trinitrate every 24 h, along with improvement in clinical symptom and quality of life questionnaires.
ConclusionsThis study supports the use of low-dose glyceryl trinitrate via transdermal patches to improve vascular flows, translating into short-term clinical symptom improvement associated with SRP.
El fenómeno de Raynaud secundario es una de las principales manifestaciones cutáneas de varias enfermedades reumatológicas, que puede condicionar una afección en la calidad de vida y cuyo tratamiento sistémico condiciona varios efectos adversos. Por tal razón, se realizó este estudio con el objetivo de evaluar la efectividad de trinitrato de glicerilo (en parches tópicos trasndermicos) en pacientes con fenómeno de Raynaud secundario (FRs), evaluado mediante fotopletismografía del flujo vascular.
Materiales y métodosSe llevó a cabo un estudio comparativo, analítico, abierto y no aleatorizado, en el que se evaluó la efectividad de 4,5 mg de trinitrato de glicerilo en un parche transdérmico puesto en la región proximal de una mano en los pacientes con diagnóstico de FRs, en comparación con la mano contralateral sin tratamineto. Para determinar el resultado principal se hizo una evaluación con fotopletismografía del flujo vascular 30 días después de su uso diario. Los resultados secundarios se obtuvieron mediante un termómetro infrarrojo midiendo la temperatura local en ambas manos antes de la aplicación y 30 minutos después del uso del parche, así como al final del seguimiento, además de cuestionarios de escala visual análoga de dolor, Raynaud’s Condition Score (RCS) y de calidad de vida dermatológico (DLQI), el primer y el último día del estudio.
ResultadosSe incluyeron 16 pacientes, todas del género femenino, la mediana de edad fue de 54 años. En cuanto a las enfermedades reumatológicas a las cuales se asociaba el fenómeno de Raynaud secundario, en 12 pacientes (75%) se asoció con esclerosis sistémica, en tres pacientes (19%) a LEG y en una (6%) a enfermedad mixta de tejido conectivo (EMTC). Hubo un incremento significativo en el flujo sanguíneo periferico después de los 30 días de tratamiento con 4,5 mg cada 24 h de trinitrato de glicerilo, además de mejoría en los cuestionarios clínicos de síntomas y calidad de vida.
ConclusionesEste estudio apoya el uso de bajas dosis mediante parches transdérmicos de trinitrato de glicerilo, a fin de que mejoren los flujos vasculares y que haya un mejoría clínica de los síntomas asociados a FRs a corto plazo.
Raynaud's phenomenon (RP) is characterized by acute, reversible peripheral ischemia following exposure to cold, predominantly affecting the digital arteries of the fingers. It progresses through three clinical phases: an initial phase of intense pallor, followed by cyanosis as arterial flow recovers, and finally erythema.1 Various factors have been implicated in its pathophysiology, including thermoregulation, controlled by mediators derived from the vascular endothelium, as well as peripheral and central neurogenic mechanisms.2,3 Central neurogenic control, in contrast to primary RP—where the problem is considered a local failure in thermoregulation and physiological changes in blood vessels—secondary RP involves acquired conditions that cause vascular lesions. These conditions may lead to structural deterioration and complete loss of cutaneous vessels, including the nutritional microvasculature. Additionally, the local cutaneous response to temperature involves several mechanisms, including the activation of the transient receptor potential melastatin 8 (TRPM8) and vanilloid 1 (TRPV1) ion channels and the release of vasoactive neuropeptides from sensory nerves.1,2,4
RP is considered a criterion for the early diagnosis of systemic sclerosis (SSc) (VEDOSS), a classification system developed by the European League Against Rheumatism (EULAR) and the European Scleroderma Trials And Research Group (EUSTAR). VEDOSS includes three red flags: RP, puffy fingers, and positivity for antinuclear antibodies, along with the presence of SSc-specific antibodies or abnormalities in nailfold capillaroscopy.5
Flow plethysmography (PPG) is an instrument primarily used to determine and record variations in blood volume or blood flow that occur with each heartbeat.6 It is assessed using a photoelectric plethysmograph, which works by shining a light onto the skin through a probe and measuring the variations in light intensity caused by changes in blood vessel volume. This technique has proven useful in analyzing skin perfusion in cases of vasospastic disease.7
The treatment of RP depends on its severity and the presence of any underlying diseases.8 Drugs commonly used include calcium channel blockers (such as nifedipine, amlodipine, or dihydropyridines), with a reported effectiveness rate of 35% in reducing the number of attacks.4 The second-line therapy involves phosphodiesterase 5 inhibitors.2 However, the systemic side effects of both therapies limit their use.8 Glyceryl trinitrate has been employed in the management of RP. One study reported improved skin perfusion, assessed by laser Doppler, in subjects who applied topical nitroglycerin compared with those who used a placebo, resulting in increased perfusion of ischemic areas.9 However, these studies are limited and mostly consist of clinical case reports. Additionally, it is important to consider that complications of RP, such as secondary digital ulcers, may have a potentially reversible ischemic etiology, which can be addressed with vasoactive therapies like topical glyceryl trinitrate.10
Given these considerations, this clinical study aimed to assess the effectiveness of topical glyceryl trinitrate (delivered through transdermal nitroglycerin patches) in individuals with secondary RP, using vascular flow PPG. The secondary objective was to evaluate dermatological quality of life using the DLQI questionnaire before and 30 days after applying topical glyceryl trinitrate patches in patients with secondary RP.
Materials and methodsAn open, non-randomized, comparative clinical study was conducted involving men and women over 18 years of age, diagnosed with secondary RP (according to the classification criteria for RP), with at least one year of evolution, and no secondary sequelae. Participants who had been treated with medications for RP (such as calcium channel blockers or phosphodiesterase inhibitors) or who did not provide informed consent were excluded. The study was approved by the Ethics and Research Committee of the institution.
The following procedures were carried out:
- 1
At the baseline visit (before starting treatment), participants were administered the DLQI11 and Raynaud's Condition Score (RCS)12 and asked to rate the degree of pain associated with RP using a visual analogue scale. A baseline PPG study was conducted on both hands at room temperature, followed by exposure to cold for 30 min using frozen gels covered by a blanket.
- 2
Participants were given one-quarter of an 18 mg glyceryl trinitrate transdermal patch (4.5 mg) to apply on the proximal region of one hand. A transdermal micropore patch (placebo) was placed on the contralateral hand. After 30 min, a PPG study was performed again on both hands.
- 3
The method for cutting the 18 mg glyceryl trinitrate patch into four equal parts (4.5 mg each) and its transdermal application in the proximal region of the chosen hand was explained. The patch was to be replaced every 24 h for 30 days.
- 4
The second visit took place 30 days later, during which a physical examination was performed (to check for adverse effects) and a PPG study was conducted on both hands (treated and untreated) at normal temperature and after exposure to local cold. The DLQI, RCS, and visual analogue scale for pain associated with RP were also administered again.
PPG, also known as photoplethysmography, is valued for its ease of assembly, comfort, simplicity, and cost-effectiveness when compared to other types of plethysmographs. It operates through a probe that contains a light source to illuminate the tissue (in this case, the skin) and a photodetector to measure small variations in light intensity associated with changes in blood vessel volume. These variations help identify the cardiovascular pulse wave as it propagates through the body. The PPG signal reflects the movement of blood through the vessels, from the heart to the fingertips, in a wave-like motion. In previous studies, PPG is a useful and user-friendly tool for assessing skin perfusion in vasospastic diseases.7
PPG typically consists of a light emitter and a light detector integrated into a single device. The light emitter sends light that penetrates the tissue. The light is absorbed by various tissue components, including red blood cells, while some of it is reflected or transmitted back to the detector. Changes in the amount of blood in the tissue affect the amount of light that is reflected or transmitted, and these changes can be recorded to assess blood perfusion and vascular activity.7
The curve displayed on the monitor has several phases:
- 1
Baseline: Under resting conditions or before any stimulus, the curve shows a baseline level representing normal blood flow.
- 2
Initial Phase or Rapid Increase: When a stimulus, such as cold application, is introduced (after establishing the baseline), the curve may show a rapid increase, potentially due to vasoconstriction in response to the stimulus.
- 3
Plateau Phase: After the initial increase, the curve may reach a plateau, indicating a stable state of blood flow.
- 4
Return to Baseline Phase: After the stimulus is removed or after some time has passed, the curve may show a gradual return to baseline, reflecting the vascular system's response and recovery following the intervention.
To identify these four phases, a baseline assessment is conducted with the application of local cold.
- •
Infrared Thermometer: Temperature was measured before starting the baseline measurement and after 30 min.
- •
Questionnaires Used to Assess the Objectives:
- o
RCS: This is a subjective, patient-reported scoring scale that evaluates the cumulative daily frequency, duration, severity, and impact of Raynaud's phenomenon (RP) attacks. Scores range from 1 to 10.13
- o
DLQI: The Dermatology Life Quality Index (DLQI) is a unidimensional scale consisting of a questionnaire with 10 questions related to the perceived impact of skin disease on quality of life in the past week. These questions cover aspects such as symptoms and feelings (questions 1 and 2), daily activities (questions 3 and 4), leisure activities (questions 5 and 6), work and school life (question 7), interpersonal relationships (questions 8 and 9), and side effects of treatment (question 10). Each item has four possible responses according to the Likert scale: 0) not at all/not relevant, 1) a little, 2) a lot, and 3) very much. The minimum possible score is 0, and the maximum is 30. High scores indicate a deterioration in quality of life. Interpretation of the DLQI scores: 0−1: no impact on the patient's life; 2−5: small effect; 6−10: moderate effect; 11−20: large effect; 21−30: extremely large effect on the patient's life.11,14
- o
A descriptive analysis of the variables of interest was conducted using frequencies and percentages for categorical variables and medians (minimum-maximum) for continuous variables. Comparisons of these variables were performed using the Mann–Whitney U test, and the Fisher F test was used for categorical variables. A two-tailed p-value ≤ 0.05 was considered significant. Statistical analysis was conducted using SPSS version 21 (International Business Machines Corporation [IBM], USA).
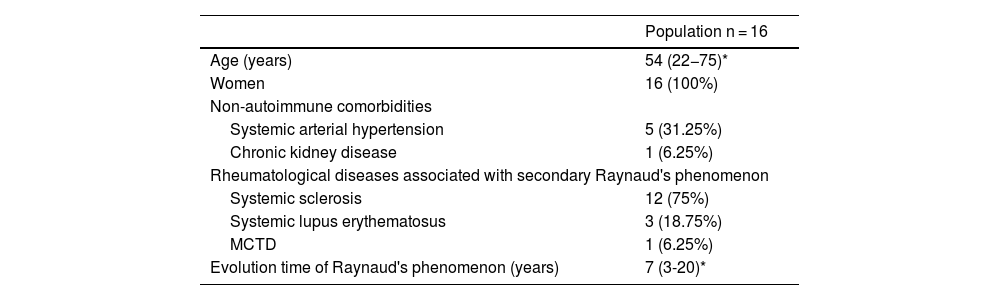
ResultsSixteen patients were included, all female, with a median age of 54 years (range: 22−75). Demographic characteristics are shown in Table 1. Regarding the rheumatic diseases associated with secondary RP, 12 subjects (75%) had systemic sclerosis (SSc), three (19%) systemic lupus erythematosus (SLE), and one (6%) mixed connective tissue disease (MCTD).
Demographic characteristics of the study population.
| Population n = 16 | |
|---|---|
| Age (years) | 54 (22−75)* |
| Women | 16 (100%) |
| Non-autoimmune comorbidities | |
| Systemic arterial hypertension | 5 (31.25%) |
| Chronic kidney disease | 1 (6.25%) |
| Rheumatological diseases associated with secondary Raynaud's phenomenon | |
| Systemic sclerosis | 12 (75%) |
| Systemic lupus erythematosus | 3 (18.75%) |
| MCTD | 1 (6.25%) |
| Evolution time of Raynaud's phenomenon (years) | 7 (3-20)* |
MCTD: mixed connective tissue disease.
In terms of complications associated with RP, the most common issue at the time of the initial examination was pain, which was present in all patients. The median pain score on the visual analogue scale (0–10) was 5 (minimum: 2, maximum: 9). This was followed by punctate digital ulcers on the fingertip pulp (n = 9; 56%), and larger atrophic ulcers (>3 mm) on the fingers (n = 7; 43%). Distal necrosis in the fingers was documented in only two patients (12%).
Changes in venous volume by photoplethysmographyAnalysis of blood flow changes in the palms of both hands, comparing baseline data from the first visit and measurements 30 days after the application of the 4.5 mg glyceryl trinitrate transdermal patch, showed a significant increase in final venous volume (p < 0.023). This was confirmed by assessing the PPG curves at 30 days, with a percentage change of more than 40% compared to the baseline curves of the treated hands.
Dermatological quality of life indexThe median DLQI score at the initial visit was 7 points (range: 2−20), indicating moderate impairment in quality of life. Thirty days after treatment, the median DLQI score decreased significantly to 4 (range: 0−15), reflecting a small effect on quality of life. At the baseline visit, nine subjects (60%) had moderate impairment, and three patients (18%) had severe impairment. After 30 days of treatment, the percentage of individuals with moderate impairment decreased to 25% (n = 4), and those with severe impairment decreased to 12.5% (n = 2) (Table 2).
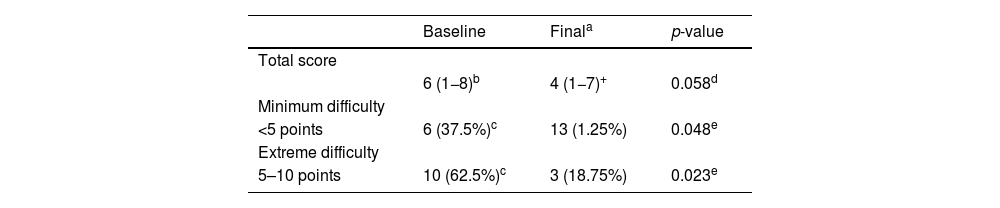
Raynaud’s condition scoreThe median RCS at the initial visit was 6 points (minimum: 1, maximum: 8). At the final visit, the median score decreased to 4 points (range: 1−7), showing a trend towards statistical significance (p = 0.058). When the population was divided into those with minimal or extreme difficulty based on the RCS, at the baseline visit, six subjects (37.5%) had minimal difficulty, and ten individuals (62.5%) had maximum difficulty. After treatment, the proportions changed significantly: 13 patients (81.25%) had minimal difficulty, while only three subjects (18.75%) continued to experience maximum difficulty (Table 3).
Local temperature changesLocal temperature measurements taken with an infrared thermometer on both hands, before, and after the follow-up period, showed an improvement of nearly 1 °C with the use of glyceryl trinitrate (Table 4).
DiscussionSecondary RP is a common cutaneous manifestation of rheumatic diseases, leading to various long-term local complications and negatively impacting the quality of life of patients. This is primarily due to the associated pain and the resulting inability to perform daily or work activities.12 The therapeutic options recommended in the guidelines are based on expert consensus, case reports, and unreported professional experience.15 These options predominantly involve systemic drugs such as calcium channel blockers, angiotensin-converting enzyme inhibitors, alpha-blockers, and selective serotonin reuptake inhibitors.15 However, their routes of administration often result in multiple adverse effects.8,15
Topical glyceryl trinitrate has been reported in clinical cases through a gel vehicle, showing favorable clinical responses.16 However, this presentation is unavailable in our environment. Consequently, a pilot study was conducted with 16 subjects with secondary RP, using low-dose (4.5 mg) transdermal patches. More objective methods were employed to quantify the response in peripheral blood flow (using photoplethysmography), changes in temperature, associated symptoms, and the effect on dermatological quality of life, as well as documenting any adverse effects. This study demonstrated an improvement in digital blood flow 30 days after topical use of 4.5 mg glyceryl trinitrate via transdermal patches, with an increase in local temperature and improved clinical function (assessed by the RCS and DLQI). One advantage of using patches was the ability to control the application time and exact dosage, which is not possible with other vehicles (such as gel).
Topical nitrates applied locally increase the concentration of cyclic guanosine monophosphate in smooth muscle. Once absorbed, they are degraded by the enzyme aldehyde dehydrogenase into nitrous oxide, which provides an exogenous supply of vasodilators, increasing blood flow, influencing temperature regulation, and promoting its rise.17
In the current study, we did not observe any effects beyond changes in temperature on physical examination (e.g., healing of digital ulcers). However, the increase in perfusion suggests that this treatment could complement therapeutic approaches to prevent digital ischemia in patients with RP. It is important to note that the follow-up period was not sufficient to assess this fully.
Using scores such as the RCS and DLQI, validated questionnaires that evaluate the level of difficulty caused by RP and health-related quality of life in skin diseases, respectively, we demonstrated that the transdermal treatment was highly effective. It led to a reduction in symptoms and an improvement in the ability to perform daily activities, thus enhancing work or school performance and interpersonal relationships. This is one of the major strengths of the study, as these subjects often experience significant disability due to the cutaneous manifestations associated with this syndrome.
No adverse effects were documented in any of the participants. Published case reports commonly note headache as the main adverse effect, occurring around two weeks after the first dose, typically a few hours after topical application of nitroglycerin, followed by hypotension, which is often associated with the concurrent use of other vasodilators.10 We believe that the low dose of glyceryl trinitrate (4.5 mg), as well as the application site (the wrist region), may explain the absence of such symptoms. It should also be noted that the concomitant use of systemic vasodilators was an exclusion criterion in this study.
LimitationsThe follow-up period was short (30 days). Larger studies with longer follow-ups are needed to assess the long-term benefits, including those involving individuals at different stages of the disease (early courses to assess effects on complications such as ulcers and distal necrosis, and late stages to assess recovery from sequelae).
ConclusionsWe demonstrated that a low dose (4.5 mg) of topical glyceryl trinitrate via transdermal patches was objectively effective in improving blood flow, increasing local temperature, and enhancing dermatological quality of life. Additionally, it led to improved performance in daily activities in patients with secondary Raynaud's phenomenon. This presentation is easily accessible in our setting, easy to apply, and ensures the correct dosage and application time. Moreover, there were no reported local or systemic adverse effects.
Ethical considerationsThe study has the approval of the Committee on Medical Research and Ethics of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. The authors also have the informed consent of the participating patients for the publication of data relevant to the results of the research.
FinancingThis work received no funding.