Few studies have been made on the incidence of residual paralysis from non-depolarizing relaxants in people over 65 years old; however, estimating the number of cases and treatment thereof are both important.
ObjectiveTo study the incidence of residual paralysis with non-depolarizing relaxants in patients over 65 years of age and discuss treatment.
MethodologyAnalytical observational study based on a cohort design.
ResultsThe pre-extubation residual paralysis was estimated at 23.2% and at 9.2% at patient admission to the Recovery Suite. Pharmacological reversal showed 89.4% and 100% success rates with Neostigmine and Sugammadex respectively, with similar times at T4/T1>0.9.
ConclusionsThe incidence of pre-extubation residual paralysis was lower than the figure published worldwide. Pharmacological reversal therapies were typically highly effective.
La incidencia de Relajación Residual por relajantes no despolarizantes en mayores de 65 años ha sido poco estudiada, siendo relevante su calculo y su tratamiento.
ObjetivoEstudiar la incidencia de Relajación residual en pacientes mayores de 65 años con relajantes neuromusculares no despolarizantes y describir su tratamiento.
MetodologíaEstudio Observacional Analítico con Diseño de Cohorte.
ResultadosLa Relajación Residual pre-extubación fue del 23.2% y al ingreso a la Sala de Recuperación del 9.2%. La reversión farmacológica con Neostigmina exhibió un éxito del 89.4% y con Sugammadex del 100%, con similares tiempos a una T4/T1>0.9.
ConclusionesLas incidencias de Relajación Residual pre-extubación y en la Sala de Recuperación fueron mas bajas que las publicadas a nivel mundial. Las terapias de reversión farmacológica se distinguieron por su alta eficacia.
Neuromuscular relaxation is mainly used to facilitate orotracheal intubation, for improved visualization and manipulation of the surgical field, and to optimize the patient–ventilator interaction when appropriate.1,2
Notwithstanding the considerable benefits of these drugs, they may lead to post-operative residual paralysis (Rp).1–3 The recommendation is to make the diagnosis using quantitative criteria to assess the function of the neuromuscular junction through peripheral nerve stimulation that guides our choice of a safe neuromuscular relaxation therapy and helps to optimize the pharmacological reversal measures.1–3
According to the study, the incidence of residual paralysis ranges from 2% to 88%.1,4,5 In the opinion of Donati,4 this number is closer to 57%, averaging the results from the trials by Murphy, Naguib, Kumar, Butterly and Thilen, reported between 2007 and 2012. It should be stressed however, that based on this study, only 1% of anesthetists actually consider the problem.6–10
Residual paralysis is relevant because it may go unnoticed during the post-anesthesia recovery period, giving rise to severe respiratory post-operative complications associated with longer extubation times, risk of re-intubation, bronchoaspiration, extended recovery, delayed PACU discharge, and even more severe life-threatening conditions.1,3,5–7,11
There is a wide range of studies worldwide but usually they fail to consider the population over 65 years of age. These so-called “elderly” patients usually present an impaired organ ability to clear medications and increased sensitivity in terms of length and depth of the relaxation effects that may result in additional economic and healthcare burden.12 In fact, the Polish trial by Pietraszewski et al.,12 describes the higher Rp incidence in patients aged 65 through 89, in contrast with patients between 19 and 57 years old (44% vs. 20%), and even higher rates of hypoxia when comparing both groups (17.9% vs. 8.2%).
The incidence of Residual Paralysis is yet unknown, particularly when referring to people over 65 years of age, since the statistics reported for this age group are meager.
Based on the above statements and considering the major impact of evaluating both the incidence and current management of Rp in patients over 65 years old, we tried to respond the following question: “What is the incidence of Rp in patients over 65 years of age, exposed to nondepolarizing neuromuscular relaxants? Which are the characteristics of the current treatment for residual paralysis in patients aged 65 and older?
The key objective in trying to answer these questions was to estimate the incidence of Rp in patients over 65 years old, exposed to nondepolarizing relaxants, and then to discuss the effectiveness of pharmacological reversal therapy.
Materials and methodsAnalytical, observational, cohort-based trial at the Samaritana University Hospital and the San Jose University Children's Hospital Foundation, between 2014 and 2015. The cohort inclusion criteria included: patients over 65 years old exposed to nondepolarizing neuromuscular relaxants. All patients discharged from the ICU with mechanical ventilation were excluded.
The patients enrolled in the trial were conveniently selected in consecutive order based on the surgical schedule of the research institution. Only the records that met the selection criteria and were fully compliant with the Stockholm Consensus were included in the analysis.13
Residual paralysis was defined as a train of four (TOF) ratio between the first and the last motor response (T4/T1) of less than 90%. This measurement was taken according to the parameters under the Stockholm Consensus13 using two different approaches: TOF-Watch SX (TOF-Watch XS Device, Organon, Oss, The Netherlands). Single-use disposable electrodes were placed over the skin on the cubital nerve after cleaning thoroughly a 2–3cm2 surface. The accelerometer was placed on the pulp of the first digit making sure that the hand movement was artifact-free for an unbiased accelerometer reading. A measurement was taken at the time of admission to the OR, at the end of surgery, prior to extubation, and when the patient was admitted to the recovery room. In case of reversal of the residual paralysis, the necessary TOF measurements were taken from the time of the initial administration of the agent, until a value of over 90% was obtained. This period of time was the time required for total reversal of the residual paralysis. It is important to highlight that the arm used for taking the measurements was immobilized during the measurements and the medications used were administered via a separate line. The measurements were taken by one of the three principal researchers participating in the trial and were masked and independent from medical care.
The clinical predictors for residual paralysis were evaluated at two different times: pre-extubation and at the time of admission to the recovery room. Some of the tests administered included the ability to hold the head up for more than five seconds, the presence of apnea, compliance with the ventilation extubation criteria, and the ability to speak easily.
Our primary objective was to measure the incidence of residual paralysis in the OR prior to extubation and at the time of admission to the recovery unit, in addition to evaluating the severity of the residual paralysis in association with clinical signs measurements for the recovery of the neuromuscular junction. In every case any residual paralysis-related complications were mentioned, in addition to the pharmacological reversal, including the need to admit the patient to the ICU.
Based on the world literature, we estimated that 228 records had to be assessed in order to obtain a 51.5% likelihood of Rp, with a 95% CI and a 6.5% accuracy.
The data recorded included demographics (age, gender, body weight, height), pre-surgical information (comorbidities, use of pre-anesthesia drugs, surgical diagnosis, and intra-operative variables such as IV anesthetic agents, inhaled anesthesia, antibiotics, GI protective agents, antiemetics, and divalent electrolytes, in addition to the neuromuscular relaxants studied at full and body weight estimated doses).
The TOF and TOF-T4/T1 protocol measurements were recorded and the latency time required for a value of less than 25% was measured. Similarly, the relaxation strategy (infusion or bolus), the need for additional doses, the time elapsed from the first to the last dose prior to extubation, were all analyzed. The clinical predictors for Rp were also considered and their concordance was evaluated using Kappas. Moreover, accuracy (sensitivity and specificity) and behavioral (positive and negative predictive value) tests were estimated, versus the TOF-based Rp diagnosis.
When reversal of the residual paralysis was used, a record was made of the pharmacological strategy used by the physician responsible for the patient in the recovery room or the OR, including the dose and recovery times. Any reversal-associated complications were monitored for at least 2h by one of the three principal researchers participating in this trial.
The additional confounding variables included trans-operative crystalloid volume in mL, trans-operative temperature and TOF measurements during residual paralysis reversal.
The quantitative statistics were presented according to their respective area, using central trend measurements (median or mean values) and scatter (ranges or standard deviation) depending on their distribution; the qualitative variables were presented in absolute frequencies and percentages. The various hypotheses were compared based on the statistical requirements, using the Ji2 test to compare qualitative variables and Student-T test or U Mann–Whitney for quantitative variables. When required, the impacts and the 95% CI were estimated. The correlation coefficients were evaluated for ordinal data using RHO Spearman and statistical significance. The R2 was measured and reported as needed. The statistical significance was based on p<0.05, and the statistical package used for data processing was STATA 12.0.
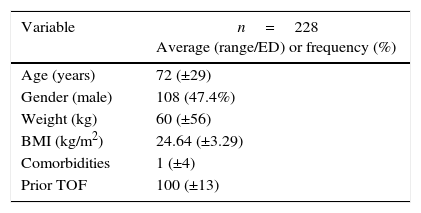
ResultsPrior authorization of the Technical Committee and of the Ethics Committee for research on human beings of the Samaritana University Hospital and the San Jose University Children's Hospital Foundation, in addition to the Research Sub-commission of the School of Medicine of the Sabana University, 228 complete records that met the selection criteria were included in the analysis; these are listed in Table 1.
Demographic information and TOF prior to exposure.
| Variable | n=228 Average (range/ED) or frequency (%) |
|---|---|
| Age (years) | 72 (±29) |
| Gender (male) | 108 (47.4%) |
| Weight (kg) | 60 (±56) |
| BMI (kg/m2) | 24.64 (±3.29) |
| Comorbidities | 1 (±4) |
| Prior TOF | 100 (±13) |
kg: kilograms; BMI: body mass index; TOF: train of four.
Source: Authors.
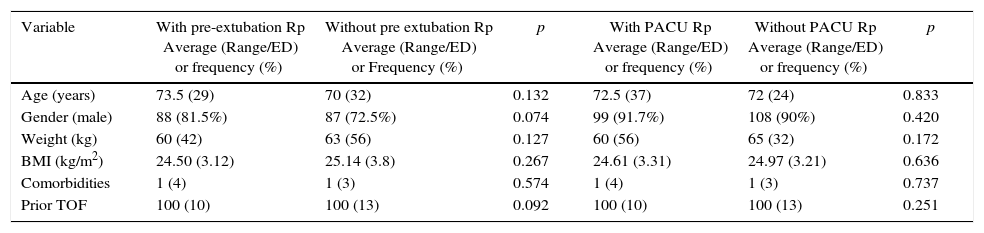
When describing the demographics of the patients with Rp prior to extubation and admission to the recovery room, vs. those with no residual relaxation, no statistically significant differences were identified between the two groups (Table 2).
Demographic data and TOF prior to exposure according to outcome.
| Variable | With pre-extubation Rp Average (Range/ED) or frequency (%) | Without pre extubation Rp Average (Range/ED) or Frequency (%) | p | With PACU Rp Average (Range/ED) or frequency (%) | Without PACU Rp Average (Range/ED) or frequency (%) | p |
|---|---|---|---|---|---|---|
| Age (years) | 73.5 (29) | 70 (32) | 0.132 | 72.5 (37) | 72 (24) | 0.833 |
| Gender (male) | 88 (81.5%) | 87 (72.5%) | 0.074 | 99 (91.7%) | 108 (90%) | 0.420 |
| Weight (kg) | 60 (42) | 63 (56) | 0.127 | 60 (56) | 65 (32) | 0.172 |
| BMI (kg/m2) | 24.50 (3.12) | 25.14 (3.8) | 0.267 | 24.61 (3.31) | 24.97 (3.21) | 0.636 |
| Comorbidities | 1 (4) | 1 (3) | 0.574 | 1 (4) | 1 (3) | 0.737 |
| Prior TOF | 100 (10) | 100 (13) | 0.092 | 100 (10) | 100 (13) | 0.251 |
kg: kilograms; BMI: body mass index; TOF: train of four.
Source: Authors.
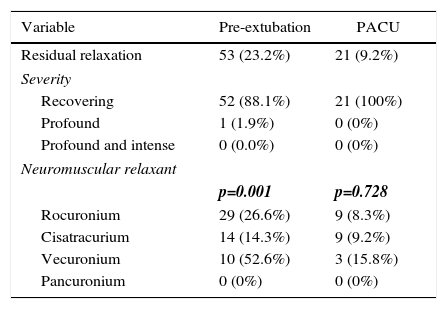
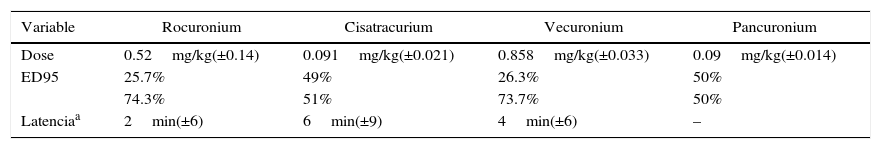
The incidence of residual paralysis in each situation and its intensity are described in Table 3, together with the stratified Rp incidences for each neuromuscular relaxant used. Table 4 illustrates the mean dose of each relaxant used, the percentage use with a 95 ED and the percentage of use at doses slightly below 95 ED. Additionally, the latency measured under the protocol parameters is shown for 156 valid records.
Residual relaxation.
| Variable | Pre-extubation | PACU |
|---|---|---|
| Residual relaxation | 53 (23.2%) | 21 (9.2%) |
| Severity | ||
| Recovering | 52 (88.1%) | 21 (100%) |
| Profound | 1 (1.9%) | 0 (0%) |
| Profound and intense | 0 (0.0%) | 0 (0%) |
| Neuromuscular relaxant | ||
| p=0.001 | p=0.728 | |
| Rocuronium | 29 (26.6%) | 9 (8.3%) |
| Cisatracurium | 14 (14.3%) | 9 (9.2%) |
| Vecuronium | 10 (52.6%) | 3 (15.8%) |
| Pancuronium | 0 (0%) | 0 (0%) |
Neuromuscular relaxants (95ED: percentage use at a 95% effective dose,
| Variable | Rocuronium | Cisatracurium | Vecuronium | Pancuronium |
|---|---|---|---|---|
| Dose | 0.52mg/kg(±0.14) | 0.091mg/kg(±0.021) | 0.858mg/kg(±0.033) | 0.09mg/kg(±0.014) |
| ED95 | 25.7% | 49% | 26.3% | 50% |
| 74.3% | 51% | 73.7% | 50% | |
| Latenciaa | 2min(±6) | 6min(±9) | 4min(±6) | – |
a Estimated Latency of 68.42% of the data, n=156.
Source: Authors.
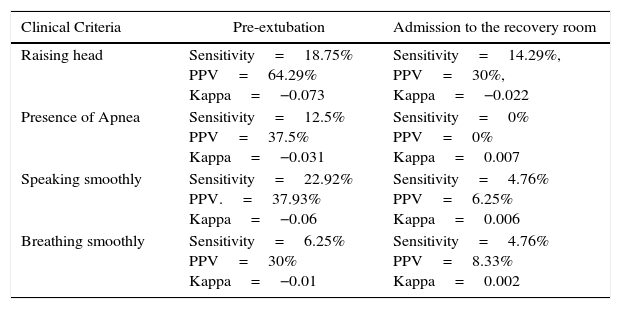
The clinical evaluation of Rp showed a very poor correlation between the TOF value and the presence of clinical predictors for Rp. When evaluating based on Searman Rho coefficient, clinical pre-extubation criteria and the presence of Rp diagnosed by TOF the result was Rho=0.137, while in terms of the presence of clinical criteria at admission to the recovery room and the presence of Rp diagnosed by TOF the Rho=−0.052. Furthermore, when evaluating the accuracy and the behavior of each individual criterion both at the time of pre-extubation and at admission to the recovery room, we evidenced poor diagnostic accuracy in each case (Table 5 shows the sensitivities, the PPV (positive predictive value), and the Kappa coefficients for each test).
Sensitivity, positive predictive value (PPV), and kappa coefficient for each Rp clinical criterion, based on the time of measurement.
| Clinical Criteria | Pre-extubation | Admission to the recovery room |
|---|---|---|
| Raising head | Sensitivity=18.75% PPV=64.29% Kappa=−0.073 | Sensitivity=14.29%, PPV=30%, Kappa=−0.022 |
| Presence of Apnea | Sensitivity=12.5% PPV=37.5% Kappa=−0.031 | Sensitivity=0% PPV=0% Kappa=0.007 |
| Speaking smoothly | Sensitivity=22.92% PPV.=37.93% Kappa=−0.06 | Sensitivity=4.76% PPV=6.25% Kappa=0.006 |
| Breathing smoothly | Sensitivity=6.25% PPV=30% Kappa=−0.01 | Sensitivity=4.76% PPV=8.33% Kappa=0.002 |
Additionally, prior to extubation the following Kappa coefficients were estimated: raising head (−0.073), apnea (−0.031), speaking (−0.06), and ability to ventilate properly (−0.01). At admission to the recovery room, the following Kappa coefficients were estimated: raising head (−0.022), apnea (0.007), speaking (0.006), and ability to ventilate properly (0.002).
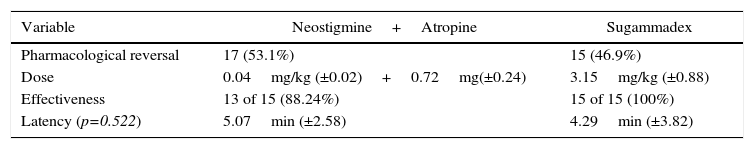
Finally, the use of each pharmacological reversal therapy, their mean doses, effectiveness achieved, and latency minutes required for satisfactory reversal in each group were specified (Table 6).
DiscussionThe study of post-operative residual paralysis (Rp) has become increasingly relevant during the past decade; the growing use of neuromuscular junction function monitors and the publication of several studies stressing the importance thereof, have given rise to a broad diagnostic spectrum for preventing Rr-associated post-operative complications.4
The information on the incidence of residual relaxation and its associated factors prior to the advent of neurostimulation lacked a strong analytic foundation to establish direct causality. After the introduction of relaxation monitoring with TOF in the 1970s, the incidence of Rp apparently declined, and although the analytic methods measuring TOF gained validity, the considerably varying incidences identified by different authors have failed to provide global and reliable data applicable to all populations.
Three different techniques may be used to monitor the neuromuscular junction: electromyography, mechanomyography, and acceleromyography. The latter is the foundation for modern anesthesia monitoring which is convenient, economic, and highly correlated with other more sensitive evaluation approaches such as the results from mechanomyography.3,11,14,15 It is important to state that the method used in our trial was acceleromyography, since this is the standard measurement method in the OR.
Multiple studies have shown that qualitative monitoring is not associated with quantitative measurements. In fact, the latter have been described as measurements that are independent from the clinical signs of complete neuromuscular relaxation. In our study we found no correlation between TOF monitoring and the clinical criteria often used in anesthesiology to predict adequate performance of the junction at the end of surgery (RHO Spearman=0.137), and the same parameters with regards to the TOF value measured at the time of admission to the recovery room (RHO Spearman=−0.052). These are proven facts of very poor sensitivities, PPV and Kappa for every clinical predictor. (See Table 6).
In terms of the patients enrolled in this analysis, the incidence of residual paralysis prior to extubation was 23.2%, and 9.2% at the time admission to the recovery room. These values are lower than those measured by Fortier et al.5 (Metaanalysis – 2015); in this case the incidence of residual paralysis was 63.5% prior to extubation and 56.5% at the time of admission to the recovery room.
Debaene et al.1 determined the percentage of residual paralysis in the recovery room, and found that the incidence of patients with TOF<0.9 (acceleromyography) following a single dose of intubation (equivalent to two effective 95 doses, ED95) at the tie of admission to the recovery room was 45%, and 37% 2h later (in the same unit).
Murphy et al., based on clinical and acceleromyography criteria, estimated the residual paralysis at 88% of the patients prior to extubation or immediately after extubation and 32% of these patients in the recovery room with TOF<0.9, despite meeting the clinical criteria.
It is striking that none of the trials considered to be of clinical value and previously referred to in this discussion, weigh the severity of the paralysis measured before extubation and that measured in the recovery room. In our records based on the classification published in 2014 by Lien and Kopman16 and in 2007 by Fuchs-Buder et al.13, we classified the Rp into intense (no response even following a tetanic impulse); profound (TOF<1 but with post tetanic Twitch response); moderate (TOF between 1 and 3); and recovering (any TOF-T4/T1 value). So in our patients in the OR prior to extubation, only 1.9% were classified as moderate and the remaining 98.1% recovering, whilst in the recovery room 100% of the patients had a TOF>3 (indicative of recovering).
When analyzing the booster doses for adequate intraoperative relaxation and the cases of Rp prior to extubation and recovery room, no statistically significant differences were identified for either scenario (p=0.29 prior to extubation and p=0.16 in the recovery room). Apparently, the impact of these booster doses was not necessarily associated with an increased Rp frequency. The time elapsed since the last dose did not show a significant correlation when compared against the TOF values prior to extubation and at admission to the recovery room (RHO Spearman=0.184 and 0.187 respectively). These findings suggest that the time between the last dose and the quantitative TOF-T4/T1 value are not associated but are independent when weighing the risk of Rr in our patients.
An important consideration is that in our setting, despite the frequent use of nondepolarizing neuromuscular relaxants, a protocol is not followed and exclusively depends on the anesthetist's skill. However, it should be kept in mind that practically in every case the dose in these patients did not exceed the level indicated for intubation (twoED95); on the contrary, it was even slightly lower than the estimated level (see Table 4).
When evaluating the NMR used by subgroup, the incidence of pre-extubation Rp was 26.6% for Rocuronium; 14.3% for Cisatracurium; and 52.6% for Vecuronium, differing significantly from one-another (p=0.001). In contrast, the incidence of Rp in the recovery room was of 8.3% for Rocuronium; 9.2% for Cisatracurium; and 15.8% for Vecuronium; these differences however are not statistically significant (p=0.728).
When contrasting the incidences with Rocuronium versus Cisatracurium, at the time of extubation we found that the values were significantly different (p=0.022); a similar situation occurred when comparing the incidence of Rp with Cisatracurium and Vecuronium (p=0.001). Apparently the incidence of Rp with Cisatracurium is more beneficial for patients than with the other agents. While we may suggest that Cisatracurium was associated with a lower number of patients with Rp, we cannot conclude that the quality or the depth of relaxation during surgery were superior since that appreciation was beyond the scope of our study but these outcomes are indeed relevant when deciding which agent should be used.
The Colombian regulations governed by INVIMA ensure the equivalence of the molecules and apparently similar efficacy for our population. However, it should be mentioned that different brand names for each pharmaceutical product were used in this trial, except for Sugammadex; this fact may contribute to the difference in the values reported in the world literature that questions the pharmacokinetics of generics versus original products. Consequently, the results from our research, in addition to being novel, may represent evidence of a potential non-variation in the expected results for our patients, and are different from the outcomes reported by the pharmaceutical companies. This may attest to the clinical similarity of the various molecules regardless of their origin, production, or manufacturing methods, endorsing the technical opinion initially stated and consistent with the publications for other researchers such as Reyes et al.17.
Different complications from residual relaxation due to poor airway protection, declining response to hypoxia, upper airway obstruction and decompensation of the genio-glossus muscle have been identified; this is all associated with extended intubation, risk of re-intubation, or bronchoaspiration, delayed recovery, and delayed discharge from the recovery room, inter alia.5–7,3,18 In this trial, notwithstanding the large number of patients who presented Rp in the recovery room, only one of them experienced a complication (laryngospasm) that represented 4.76% of the cases, but was not associated to any other outcomes or ICU requirements.
In order to prevent all these complications in clinical practice, we have medications available that can revert any residual paralysis. The most commonly used at the national level are the acetylcholinesterase inhibitors (Neostigmina) and Sugammadex.
The decision to administer one or the other is based on the severity of the Rp. The current literature states that the principal disadvantage of Neostigmine is its failure to effectively treat intensive and profound residual relaxation; this is contrary to the results reported with Sugammadex, of proven efficacy for any Rp condition, regardless of intensity, but with a higher cost and no proven effect over the benzilisoquinolone-like nondepolarizing relaxants such as Cisatracurium.4,19
In our study, the decision to reverse the residual paralysis was made individually by each anesthetist; the efficacy evaluation of the strategy used was 89.4% success rate in patients treated with Neostigmine plus Atropine, and of 100% in patients treated with Sugammadex. For Neostigmine and Atropine, the mean recovery time to T4/T1>90% was 5.07min (ED=2.58min) while for Sugammadex was 4.29min (ED=3.82min). The differences were not statistically significant (p=0.522), and we feel there of no clinical relevance. However, one of our patients experienced a waiting time to achieve TOF>0.9 with Sugammadex of 17min. In the light of our current knowledge this event is beyond our understanding, despite the good clinical practices and pharmacovigilance of the institution where the event happened.
Although nausea and vomiting have been consistently reported immediately after the administration of Neostigmine and Atropine, only one patient in the trial experienced this side effect, which could be associated not just with exposure to the agent, but with multiple other uncontrolled factors. It is important to note that in contrast to other publications on the subject, none of our patients experienced any hemodynamic complications or arrhythmias requiring further intervention.
ConclusionsThe incidence of residual paralysis associated with nondepolarizing neuromuscular relaxants in patients over 65 years old, prior extubation and admission to the Recovery Room was below the number reported worldwide. Residual paralysis reversal was equally effective when comparing Neostigmine versus Sugammadex, with the latter exhibiting a 10% higher clinical latency. We do not discuss any side effects requiring ICU care or additional interventions. We only focused on the quantitative monitoring of the neuromuscular junction function as the key for the safe management of the nondepolarizing neuromuscular relaxant, both to evaluate recovery and reversal therapy, given the significant non-correlation between the clinical values and the data of this monitoring exercise.
Although Sugammadex exhibits multiple advantages as compared to Neostigmine,20 our data may suggest that the major benefit is in situations of profound or intense blockade where the use of Neostigmine is controversial. However, for reversal of recovering patients, the administration of Neostigminemay offer similar results and safety as Sugammadex, except for patients with contraindications or controversial use. Rp and reversal-associated cardiac or respiratory complications were not relevant for the patients in this trial. It should be said however that not all patients underwent pharmacological reversal for Rp; in fact, 46.15% of the cases did not receive any medication since clinical monitoring and oxygen therapy were enough to revert the Rp with no complications. So the question arises: Is reversal of Rp with nondepolarizing neuromuscular relaxants really necessary in patients over 65 years of age, when residual relaxation presents during the recovery phase of neuromuscular relaxation?
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflicts of interest to declare.
FundingThis study was funded by the researchers and their research groups.
Please cite this article as: González-Cárdenas VH, Salazar-Ramírez KJ, Coral-Sánchez GT. Relajación residual postoperatoria en pacientes mayores de 65 años en la Unidad de Cuidado Posanestésico. Rev Colomb Anestesiol. 2016;44:209–215.