Coronary perforation is a rare complication of percutaneous coronary intervention and is potentially catastrophic. Coronary perforation may lead to pericardial effusion with or without cardiac tamponade and if not diagnosed early on and treated properly it is life-threatening. We present a case of percutaneous coronary intervention complicated by coronary perforation, which was quickly treated by the reversal of anticoagulation, prolonged balloon inflation and a coated-stent, using the double guiding catheter technique.
Tratamento com a Técnica de Duplo Cateter-Guia de Perfuração Coronária Tipo III
A perfuração coronária é uma complicação rara da intervenção coronária percutânea e potencialmente catastrófica. Pode levar ao derrame pericárdico, com ou sem tamponamento cardíaco, e, se não diagnosticada com precocidade e tratada adequadamente, pode levar ao óbito. Relatamos o caso de uma intervenção coronária percutânea complicada com perfuração coronária, rapidamente tratada com reversão da anticoagulação, insuflação prolongada com balão e implante de stent recoberto pela técnica do duplo cateter-guia.
Coronary perforation (CP) is a rare complication in percutaneous coronary intervention (PCI), which can lead to pericardial effusion and cardiac tamponade. If undiagnosed and not treated promptly, it can lead to death. The incidence the CPs associated with PCI is approximately 0.1 to 0.6%, and its occurrence is associated with complex lesions, especially those with severe calcification and chronic occlusion, use of rotational atherectomy devices, and use of hydrophilic guidewires.
In 1994, Ellis et al.1 classified coronary perforations on a scale of 1 to 4, based on the angiographic criteria (Table 1). A subsequent study evaluated the in-hospital outcome of patients with CP and concluded that those with large perforations (type III) had a mortality rate of 21.4% vs. ≤ 1%, when compared to type I or II perforations.2
Modified Ellis classification1,3 for coronary perforations
| Type I | Extraluminal crater without linear extravasation of contrast suggesting dissection |
| Type II | Pericardial or myocardial blush, with exit orifice < 1mm |
| Type III | Clear contrast extravasation into the pericardium through orifice > 1mm in diameter |
| Type IV | Perforation with spillage of contrast directly into the left ventricle, coronary sinus or other vascular chamber, excluding the pericardium |
The purpose of this case report was to describe the particular management of a CP case that occurred in a patient submitted to elective PCI in an interventional cardiology service with a large number of procedures, unexpectedly, after pre-dilation with balloon (balloon: artery ratio < 1).
CASE REPORTMale patient, 66 years old, with a history of systemic arterial hypertension and dyslipidemia, had grade II stable angina, having been referred for elective angioplasty after coronary angiography assessment.
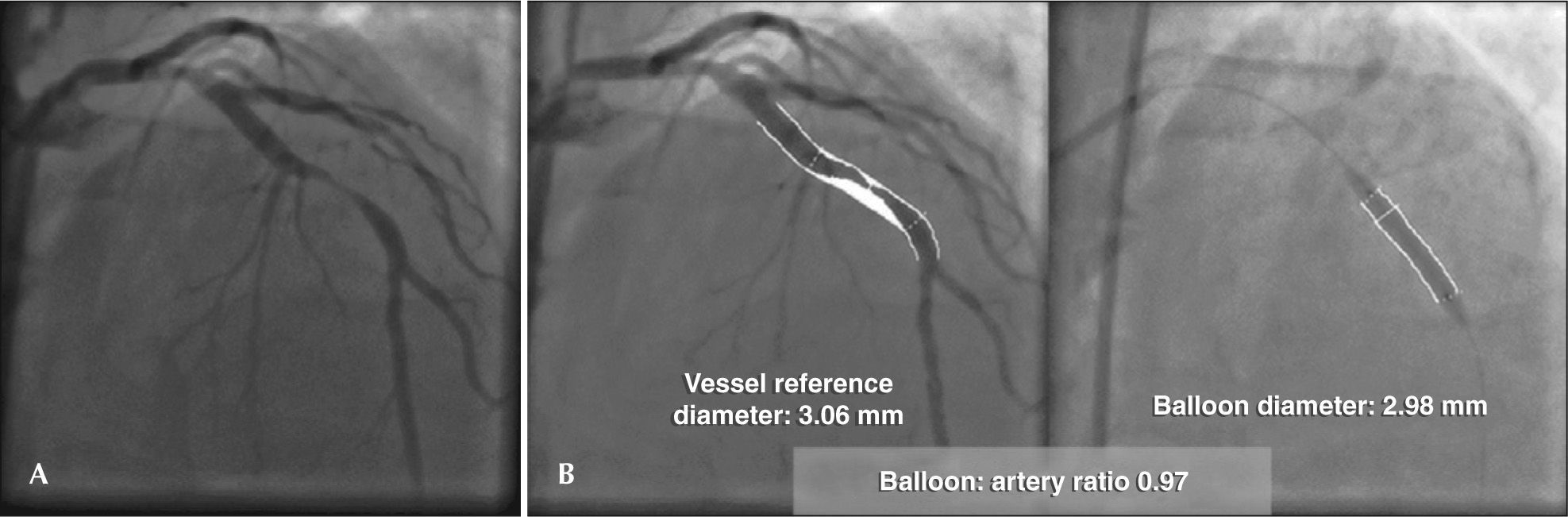
The coronary angiography showed no lesions in the right coronary and left circumflex arteries; the left anterior descending (LAD) artery had a mild proximal lesion (30%) and a 90% eccentric segmental lesion in its middle third (Figure 1A). A quantitative coronary angiography (QCA) was performed offline (CAAS II; Pie Medical Imaging, Maastricht, the Netherlands), which showed that the reference diameter of the target vessel was 3.06mm (Figure 1B).
Laboratory tests at the clinical assessment pre-PCI were within normal limits. The patient received 300mg of clopidogrel the day before the procedure and was already taking 100mg/day of acetylsalicylic acid (ASA).
The PCI planning included pre-dilation, followed by implantation of a bioresorbable vascular scaffold (BVS, Abbott Vascular, Temecula, California, United States), according to a specific clinical trial protocol. The procedure was performed through the right femoral approach with 6F sheath, and 100 IU/kg of unfractionated heparin were administered during the procedure, aiming for activated clotting time (ACT) > 250/s.
After selective catheterization of the left coronary ostium with JL 3.5 6F guide catheter (Figure 2A), the lesion was crossed without difficulty with a 0.014-inch BMW® guidewire (Abbott Vascular, Temecula, California, United States). Then, pre-dilation was performed using a 3.25 × 20mm noncompliant NC Trek® balloon catheter (Abbott Vascular, Temecula, California, United States) up to 10 atm, aiming at a balloon: artery ratio close to 1 (ratio of 0.97, measured offline at QCA), according to technical recommendations for BVS implant (Figure 2B).
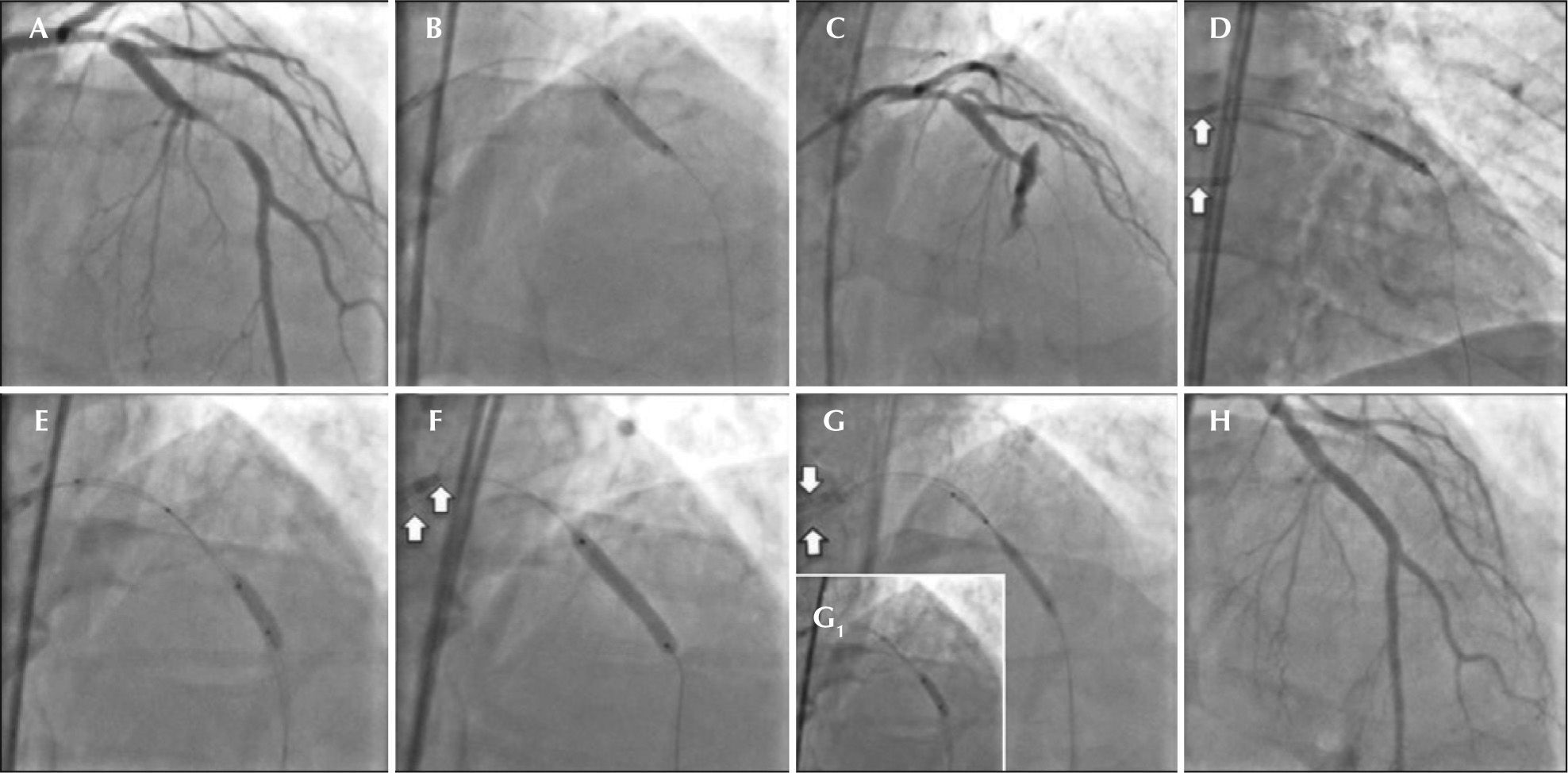
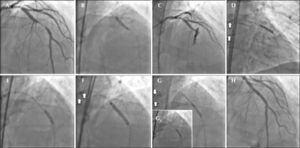
– (A) Coronary angiography in left anterior oblique view showing subocclusive lesion in the mid-third of left anterior descending artery. (B) Pre-dilation with noncompliant balloon catheter Trek NC (Abbott Vascular, Inc.). (C) Injection of control after pre-dilation showing type III coronary perforation with contrast extravasation and distal TIMI flow grade 0. (D) Prolonged inflation (20 minutes) of the balloon catheter at the perforation site. (E) Second guide catheter, through which the drug-eluting and coated stents will be advanced. (F) Release of drug-eluting stent, covering the entire segment of the lesion in the mid-third of the left anterior descending artery. (G and G1) Positioning and release of coated stent at the perforation site with fast balloon catheter deflation maneuver, which remained inflated. (H) Final control injection (white arrows indicate the two guide catheters).
The control angiography after pre-dilation showed clear contrast extravasation into the pericardium, through an orifice ≥ 1mm, indicating type III CP associated with distal vessel occlusion (Figure 2C). Immediately, the orifice area was sealed by means of prolonged inflation at low pressure (4 atm) using the same balloon catheter used in pre-dilation, for about 20 minutes. Simultaneously, anticoagulation reversal was performed with 50mg of intravenous protamine in order to achieve ACT < 150 seconds.
The echocardiography showed mild pericardial effusion, without hemodynamic repercussions. The BVS implantation was then contraindicated due to the complication.
It was decided to employ the double guide-catheter technique4−7 in order to implant a long drug-eluting stent, covering the entire lesion segment, followed by implantation of a short in-stent polytetrafluoroethylenecoated (PTFE) stent only in the CP site. For that, a second arterial access was attained through a left femoral artery puncture, followed by the placement of another 6F sheath. Then, a JL 3.5 6F guiding catheter was positioned in parallel to the first, in the left coronary ostium. A 0.014-inch guidewire (ChoICE® Floppy, Boston Scientific, Natick, United States) was advanced until the proximal border of the balloon catheter that was inflated. Quickly and synchronously, the balloon was deflated; the second guidewire was introduced to a distal point in the LAD artery, and the balloon catheter was once more inflated (Figure 2D). A 3 × 24mm Endeavor® drug-eluting stent (Medtronic Inc., Minneapolis, United States) was advanced through the second guidewire to the proximal border of the balloon catheter inflated at up to 10atm in the perforation area. In a quick maneuver, the balloon was deflated and retracted, and the stent was advanced and implanted at the perforation site, covering the entire segment of the lesion in the middle third of the LAD (Figures 2E and F2). Then, a 3.5 × 12mm coated Jostent GraftMaster® stent (Abbott Vascular – Temecula, California, United States) was implanted (released with 14 atm) in-stent at the perforation site, using the same technique of rapid deflation of the balloon and stent implantation (Figure 2G and 2G1).
The control angiography showed complete CP sealing and no residual lesion (Figure 2H).
Echocardiograms during the procedure and three hours later showed minimal pericardial effusion; there was no significant increase in troponin. The echocardiography was repeated on the following day and showed no additional changes. The patient was referred to the coronary care unit, and was discharged on the day after the procedure and prescribed dual antiplatelet therapy for 12 months.
DISCUSSIONDuring PCI, CP can occur as a consequence of advancement of the guidewire, balloon, or stent; stent release; oversizing of stents/balloons; and stent fracture or subintimal passage of balloon/stent, leading to severe dissections and perforation. It has been reported that perforation after stent implantation is mainly caused by excessive dilation or implantation of a stent oversized for the treated vessel.
The main cause of CP is the improper handling of the guidewire, either when crossing the lesion, by positioning it inadvertently out of the arterial bed, or at the distal arterial bed, beyond the ideal point, pushing and perforating. Guidewires with hydrophilic coating, particularly those with a polymer-coated tip, which are indicated in severe coronary lesions, occlusions, and severe tortuosity, cause increased risk of perforation due to their low coefficient of friction and easy distal migration.8−13 Although more frequent, trauma-related CP by guidewire is generally easily well-controlled, and the occurrence of significant pericardial effusion with cardiac tamponade is rare.14
The oversizing of stents/balloons brings increased risk of CP during PCI. Ajluni et al.15 reported that perforations were more frequent when the balloon: artery ratio was 1.3 ± 0.3, when compared to PCIs in which the balloon: artery ratio was 1.0 ± 0.3 (P < 0.001). Similarly, Ellis et al. 1 published a registry of patients undergoing PCI in which those who suffered CP had a balloon: artery ratio of 1.19 ± 0.17 vs. 0.92 ± 0.16 in those without this complication (P = 0.03). This observation was confirmed in another large randomized study in which a balloon: artery ratio > 1.1 increased the risk of severe dissection and perforation by two to three fold, when compared to a balloon: artery ratio < 1.1.3 In the present case, the balloon: artery ratio was 0.97 and therefore, it did not justify the complication that occurred (Figure 1).
Silva et al.16 reported their experience in the occurrence of coronary perforations at Instituto Dante Pazzanese de Cardiologia in São Paulo (Brazil) from December 2007 to January 2012, in which 5,585 patients were submitted to PCI and 18 had CP (0.32%). In that group, the LAD was the most often treated vessel (61.1%), as well as Type C lesions (61.1%). Chronic occlusions were treated in 27.8% of cases. Most coronary perforations had lower complexity according to the modified Ellis classification. The device with the balloon catheter was responsible for the CP in 61.1% of cases. Prolonged inflation with a balloon catheter and heparin reversal with protamine was performed in 72.2% and 88.9% of cases, respectively. There were no deaths associated with CP. Female gender and chronic occlusions were predictors of CP, according to the multivariate analysis.
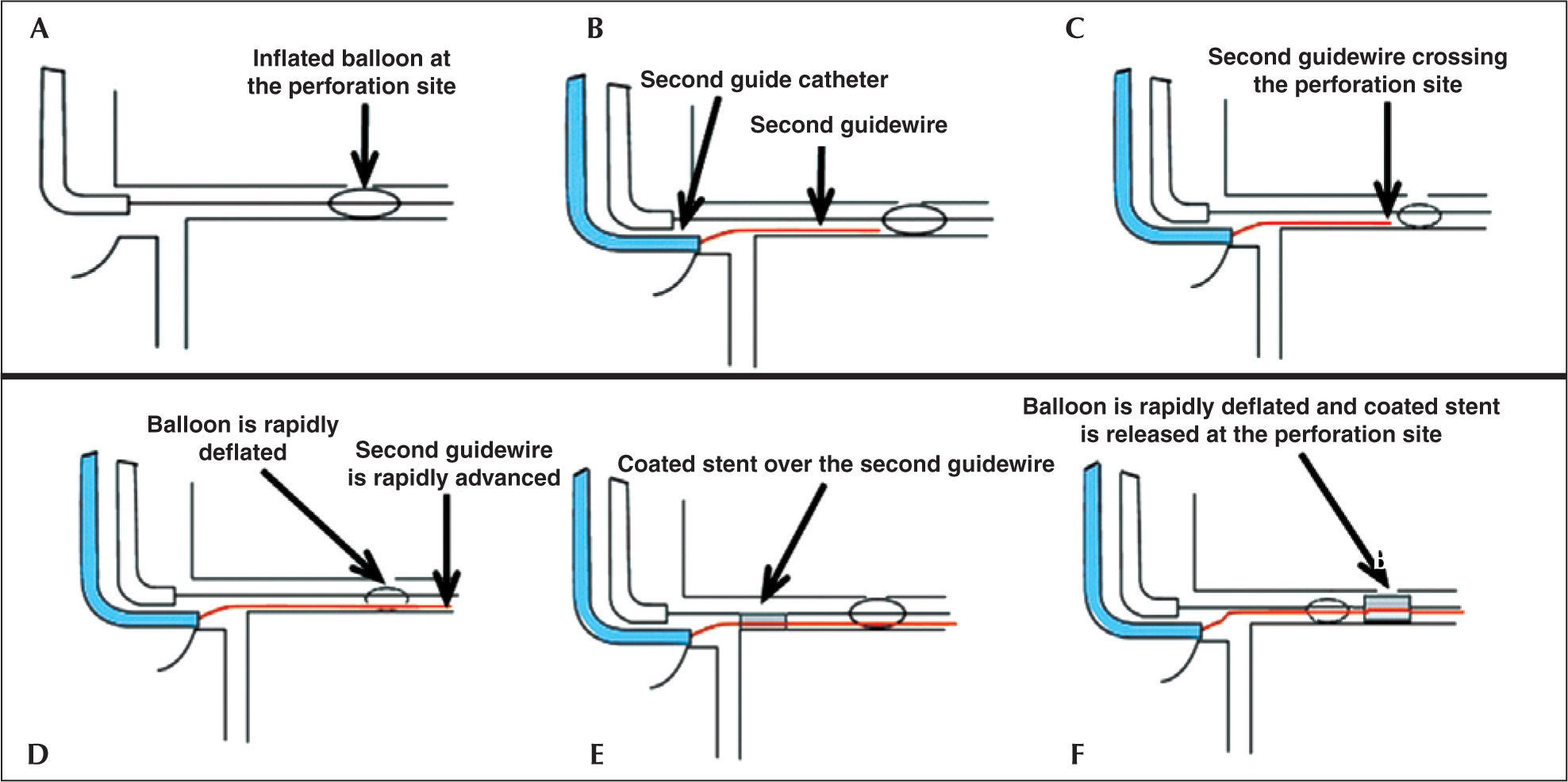
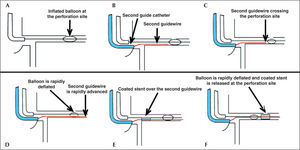
In the present case, in order to minimize the time between the inflated balloon withdrawal at the CP site and introduction and coated stent implantation, it was decided to use the double guide-catheter technique (Figure 3). This technique was first described by Silver et al.6 for the treatment of type III CP, sealed with a stent coated with PTFE, which was released through a second guide catheter inserted into the contralateral femoral artery, while the perforation site was controlled and sealed with the angioplasty balloon. Ben-Gal et al.5 have recently described the first series of patients treated by double catheter technique. The number of patients included in this study was not great enough to generate definitive conclusions, but the observation of a relatively lower rate of adverse events using the double guide-catheter technique appears to favor this approach.
– Schematic diagram showing the double guide-catheter technique. (A) Balloon used in the angioplasty inflated at low pressure (4 atm) at the perforation site. (B) Careful withdrawal of the first guide-catheter and catheterization of the coronary ostium with the second guide-catheter. (C and D). Advancement of the second guide-catheter, crossing the perforation site with quick balloon deflation, allowing its passage. (E) Coated stent advancing over the second guide catheter. (F). Rapid deflation maneuver, balloon withdrawal and coated stent implantation at the perforation site.
The technique allows for the preparation and simultaneous parallel insertion of another system including catheter-guide, guidewire, and coated stent, while the temporary sealing of the perforation is performed through the system used for the PCI that resulted in the CP. Due to the high profile, there are limitations in the maneuvering of stents coated with PTFE, and it is sometimes difficult to reach the perforation site. Furthermore, the inner lumen of the guide catheters commonly used in PCI (6F, 7F) is not sufficient for the passage of a balloon and a stent coated with PTFE in parallel.
This technique of double-guiding catheter requires some considerations. First, in case of impending cardiac tamponade, in which a coated stent coated can be easily and quickly implanted at the perforation site, the surgeon may consider pericardiocentesis, and then remove the balloon and implant the coated stent, keeping the continuous drainage of the pericardium. For such patients, the double guide-catheter technique might not be required. Secondly, the surgeon must be aware of when to stop the procedure and indicate emergency surgical intervention. The hemodynamic stability can hardly be maintained in patients with large perforations involving extensive areas of viable myocardium. In such cases, if a stent coated with PTFE cannot be easily implanted, circulatory support and surgical intervention should be considered immediately.
Successful treatment of this serious complication is associated with the rapid control of pericardial effusion, as mortality is closely linked to the development of cardiac tamponade. Treatment requires early detection and angiographic perforation classification; immediate balloon inflation at the perforation site; heparin neutralization; platelet transfusion in cases associated with the use of IIb/IIIa glycoprotein inhibitors, and coated stent implantation, when necessary. For that purpose, upon suspicion of CP during balloon/stent inflation with a balloon: artery ratio > 1.3, the balloon should be deflated, and without removing it from the site, an angiography should be rapidly performed for CP confirmation; if confirmed, the balloon should be immediately reinflated with low pressure, while checking the vessel occlusion/CP with a small contrast injection.
The double guide-catheter technique may be considered in some situations, especially when the first guide catheter is being used for persistent balloon inflation in order to temporarily seal the perforation. Great difficulty is expected to implant the coated stent, due to tortuosity, marked calcification, or previous stent in the proximal segment. Thus, surgeons will save time and find it easier to implant a stent coated with PTFE, providing a more safe procedure, avoiding a surgical intervention, and decreasing the mortality associated with this complication of PCI.
CONFLICT OF INTERESTSThe authors declare no conflicts of interest.