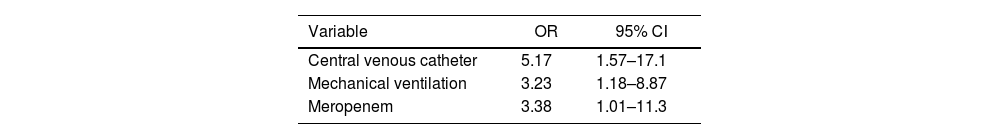
This study aimed to identify factors associated with neonatal sepsis due to Serratia marcescens in a Neonatal Intensive Care Unit (NICU) in Mexico. A case–control study was conducted, matched by gestational age and birth weight. The analysis was performed using logistic regression. Seventy newborns with S. marcescens sepsis were identified; the median gestational age was 34.4 weeks, and the median birth weight was 1680g. Microbial isolates were identified in the bloodstream in 98.6% of cases, and 88.6% of these were diagnosed after 72h of life. Independent variables associated with infection were the presence of a central venous catheter (OR 5.17; 95% CI 1.57–17.1), mechanical ventilation (OR 3.23; 95% CI 1.18–8.87), and prior use of meropenem (OR 3.38; 95% CI 1.01–11.3). The case fatality rate of the infection was 32.9%.
El objetivo del estudio fue identificar los factores asociados a sepsis neonatal por Serratia marcescens en una unidad de cuidados intensivos neonatales (UCIN) de un hospital de México. Se realizó un estudio de casos y controles pareado por edad gestacional y peso al nacer; el análisis se efectuó mediante regresión logística. Se identificaron 70 recién nacidos con sepsis por S. marcescens, los que tuvieron una mediana de edad gestacional de 34,4 semanas y una mediana de peso al nacer de 1680 gramos. Los aislamientos microbianos se identificaron en el torrente sanguíneo en el 98,6% de los casos; el 88,6% se diagnosticaron después de las 72 horas de vida. Las variables independientes asociadas a la infección fueron la presencia de catéter venoso central (OR 5,17; IC 95% 1,57 – 17,1), la ventilación mecánica (OR 3,23; IC 95% 1,18 – 8,87) y el uso meropenem durante la estancia hospitalaria (OR 3,38; IC 95% 1,01 – 11,3). La letalidad de la infección fue del 32,9%.
In Neonatal Intensive Care Units (NICUs), outbreaks of healthcare-associated infections are mainly caused by Enterobacterales, with implications for neonatal morbidity and mortality9.
Bacteria of the genus Serratia are aerobic Gram-negative bacilli belonging to the order Enterobacterales. More than 23 species are known within this order, 6 of which are related to human infections, namely, S. marcescens, S. plymuthica, S. liquefaciens, S. rubidaea, S. odorifera, and S. fonticola. S. marcescens is an opportunistic bacterium that mainly infects patients with immunodeficiency or who have received antibiotics. It is commonly found in hospital settings15.
S. marcescens colonizes and infects newborns during their passage through the birth canal. Outbreaks of this infection in NICUs are caused by horizontal transmission, and its control is challenging because these bacteria can persist on inanimate surfaces, including disinfectants and soaps2.
Factors associated with neonatal S. marcescens infections include prematurity, low birth weight, and the use of invasive devices, primarily central venous catheters (CVC) and mechanical ventilation (MV). Some studies have suggested a higher risk of infection in patients who had received antibiotics prior to the event1,5,13.
This study aimed to analyze the risk factors associated with neonatal sepsis due to S. marcescens in newborns hospitalized in an NICU in Mexico.
A case–control study matched by gestational age and birth weight was conducted at the Nuevo Hospital Civil de Guadalajara “Dr. Juan I. Menchaca” (NHCGJIM) in Guadalajara, Mexico. The institution offers care to the general population, with a focus on individuals without social security coverage. The neonatal care unit comprises an NICU with 27 beds and an intermediate care unit with 67 beds. The obstetrics and gynecology unit includes a Maternal-Fetal Medicine Unit that treats and monitors high-risk pregnancies.
The study was conducted from October 2021 to January 2024. The cases included patients born and hospitalized at the NHCGJIM with neonatal sepsis due to S. marcescens. Sepsis was diagnosed in patients with ≥one of the following manifestations: fever, hypotonia, seizures, oral intolerance, apnea, cyanosis, respiratory distress, and at least one bottle containing a blood culture and/or cerebrospinal fluid (CSF) with S. marcescens growth. For each case, a control was selected with the following characteristics: hospitalized newborn without neonatal sepsis and of similar gestational age (±one week) and weight (±200g). Each patient born immediately after a case and who presented these characteristics was assigned as a control.
For the cases and controls, information on the clinical and demographic characteristics of the newborns, as well as maternal medical history, was collected from medical records: antibiotic and/or corticosteroid use, diagnosis of urinary tract infection and/or rupture of membranes, and the presence of fever and/or leukocytosis. We also recorded whether the newborns had received antibiotics or surfactant factor, if they had prior sepsis due to bacteria other than S. marcescens, the length of fasting and hospital stay, as well as the use and duration of CVC, MV, and total parenteral nutrition. In the case group, these variables were considered present only if they were implemented before the diagnosis of S. marcescens infection. Antibiotic use was considered present if they had been prescribed for events occurring prior to the onset of S. marcescens sepsis.
At the NHCGJIM, the empirical regimen for the treatment of neonatal sepsis is ampicillin+gentamicin in early infection events (<72h of life) and vancomycin+amikacin in late sepsis (≥72h).
Specimens for blood and CSF cultures were collected in accordance with the neonatal sepsis diagnostic protocol of the Mexican Ministry of Health. Blood and CSF were obtained using aseptic techniques and inoculated into BacT/ALERT PF Pediatric FAN® vials. Microorganism growth was monitored using the automated Bact/ALERT®3D system, and samples showing growth were plated on blood agar, chocolate agar, and McConkey agar. The VITEK®2-bioMérieux platform was used for bacterial identification. Antimicrobial susceptibility testing breakpoints were interpreted according to the Clinical and Laboratory Standards Institute (CLSI).
In the descriptive analysis, frequencies and percentages were estimated for qualitative variables and medians and interquartile ranges (IQRs) for quantitative variables. The cases and controls were compared using nonparametric tests for related data: McNemar's test for qualitative variables and Wilcoxon's test for quantitative variables. Variables showing significant associations in the bivariate analysis were subjected to a multivariate analysis using logistic regression. To obtain the final model, each variable was entered step by step and excluded based on its statistical significance and influence on the remaining variables.
During the study period, 70 newborns with neonatal sepsis due to S. marcescens were identified. The median gestational age was 34.4 weeks (maximum, 41.6; minimum, 26.4; IQR, 6), and the median birth weight was 1680g (maximum, 4210; minimum, 710; IQR, 1387). A total of 85.7% (n=60) of the isolates were identified from the bloodstream, and 12.8% (n=9) from CSF and blood. In one patient, the isolates came only from CSF. The highest incidence of the infection occurred from January to April 2022 and from December to February 2024.
The median age of the patients at the time of infection was 12 days (maximum, 96; minimum, 0; IQR, 35.7); in 11.4% (n=8) of patients, infection occurred within the first 72h of life, and in 88.6% (n=62), 72h or later. Persistently positive cultures were recorded in 41.4% (n=29) despite effective treatment for ≥48h.
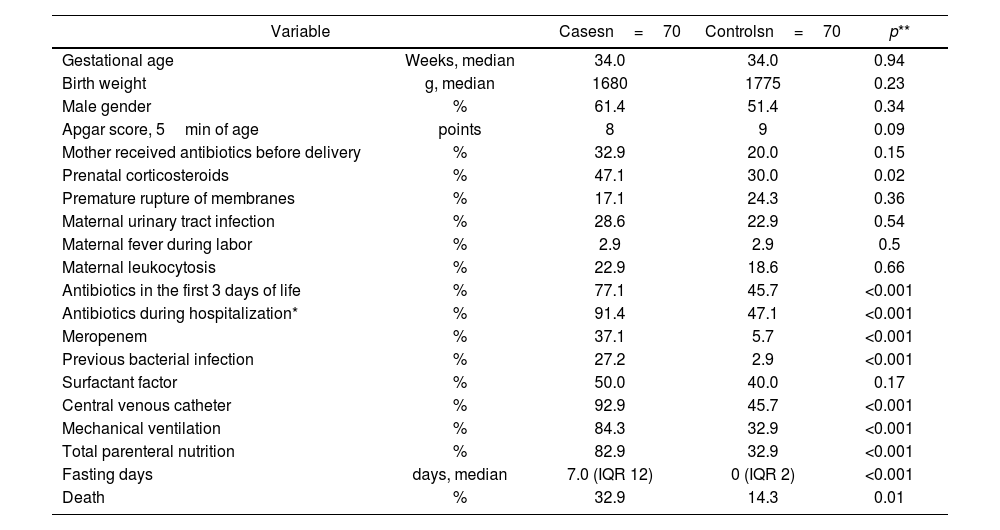
When comparing the study variables between the cases and controls, we observed a greater frequency of prenatal corticosteroid use, prior bacterial infection, CVC use, MV, total parenteral nutrition, and fasting in the former. Regarding antimicrobial exposure, a statistically significant difference was observed in the administration of antibiotics in the first three days of life (77.1% vs. 45.7%, p<0.001), antibiotic exposure at any time during hospitalization (91.4% vs. 47.1%, p<0.001), and meropenem use (37.1% vs. 5.7%, p<0.001) (Table 1).
Bivariate analysis of clinical and demographic characteristics between cases and controls.
| Variable | Casesn=70 | Controlsn=70 | p** | |
|---|---|---|---|---|
| Gestational age | Weeks, median | 34.0 | 34.0 | 0.94 |
| Birth weight | g, median | 1680 | 1775 | 0.23 |
| Male gender | % | 61.4 | 51.4 | 0.34 |
| Apgar score, 5min of age | points | 8 | 9 | 0.09 |
| Mother received antibiotics before delivery | % | 32.9 | 20.0 | 0.15 |
| Prenatal corticosteroids | % | 47.1 | 30.0 | 0.02 |
| Premature rupture of membranes | % | 17.1 | 24.3 | 0.36 |
| Maternal urinary tract infection | % | 28.6 | 22.9 | 0.54 |
| Maternal fever during labor | % | 2.9 | 2.9 | 0.5 |
| Maternal leukocytosis | % | 22.9 | 18.6 | 0.66 |
| Antibiotics in the first 3 days of life | % | 77.1 | 45.7 | <0.001 |
| Antibiotics during hospitalization* | % | 91.4 | 47.1 | <0.001 |
| Meropenem | % | 37.1 | 5.7 | <0.001 |
| Previous bacterial infection | % | 27.2 | 2.9 | <0.001 |
| Surfactant factor | % | 50.0 | 40.0 | 0.17 |
| Central venous catheter | % | 92.9 | 45.7 | <0.001 |
| Mechanical ventilation | % | 84.3 | 32.9 | <0.001 |
| Total parenteral nutrition | % | 82.9 | 32.9 | <0.001 |
| Fasting days | days, median | 7.0 (IQR 12) | 0 (IQR 2) | <0.001 |
| Death | % | 32.9 | 14.3 | 0.01 |
Variables showing a significant association (p<0.05) were subjected to multivariate analysis using logistic regression. Independent risk factors for neonatal S. marcescens infection included the use of a CVC, MV, and meropenem administration (Table 2).
Higher mortality was observed (32.9% vs. 14.3% in newborns with S. marcescens infection, p=0.01); similarly, the rate was higher in patients with a gestational age <37 weeks (27.0% vs. 10.3%, p=0.04), a very low birth weight (<1500g) (35.6% vs. 14.8%, p=0.004), or an Apgar score <8 at 5min of life (50.0% vs. 18.6%, p=0.01). These factors were subjected to multivariate analysis, and the independent factors associated with death were as follows: very low birth weight <1500g (OR 2.64; 95% CI 1.11–6.29), an Apgar score <8 at 5min of life (OR 3.03; 95% CI 1.10–8.32), and neonatal sepsis due to S. marcescens (OR 2.83; 95% CI 1.18–6.81).
With regard to the sensitivity of the study strains, we observed sensitivity to ceftriaxone in 95.7% (n=67), cefepime in 95.7% (n=67), amikacin in 100% (n=70), and meropenem in 100% (n=70). Molecular studies were not performed; therefore, it was not possible to confirm whether they were from the same clonal group; however, only three isolates showed phenotypically distinct antibiograms.
Similar to our results, Adamson et al. found that the factors associated with neonatal colonization and infection by S. marcescens were gestational age, the presence of an arterial catheter, and the use of antibiotics1.
In Mexico, several reports of neonatal S. marcescens outbreaks7,14 have described an association with invasive devices such as gastric tubes or peripheral catheters, with a mortality rate of 7–14%. In patients at the NHCGJIM, the infection was associated with a higher risk of death and was independent of birth weight and Apgar score at 5min of age.
A significant effect following antibiotic administration in newborns is intestinal dysbiosis, characterized by an imbalance in the integration and development of the intestinal microbiota, which could promote the acquisition of nosocomial infections1. In the patients from the NHCGJIM, 77.1% of cases and 45.7% of controls received antibiotics in the first three days of life.
CVCs in hospitalized newborns are essential for infusing parenteral nutrition, antibiotics, and solutions; however, infections associated with these devices account for a significant proportion of healthcare-associated infections. Recent studies have identified S. marcescens as one of the leading causes of these infections8,11.
Among the virulence mechanisms of S. marcescens, its ability to adhere to vascular devices and form biofilms protects the microorganism from phagocytic cells and antibiotics. Studies have been conducted to determine whether antibiotics can reduce the biomass of these biofilms, but the results have not been satisfactory; even antibiotics such as gentamicin and ceftriaxone have increased this biomass12.
Similar to other Enterobacterales, such as Enterobacter spp., Citrobacter freundii, and Morganella morganii, S. marcescens can present inducible resistance to beta-lactam antibiotics through AmpC-type beta-lactamases in up to 5% of strains, mainly when exposed to broad-spectrum cephalosporins4. Other mechanisms of antimicrobial resistance that S. marcescens can show are extended-spectrum beta-lactamases and/or carbapenemases6,15.
In our study, the independent factors associated with neonatal S. marcescens infection were invasive devices capable of forming biofilms and the administration of meropenem. In line with our results, Li et al. found that the risk factors associated with neonatal S. marcescens infection were very low birth weight, a long NICU stay, prolonged antibiotic use, and the use of invasive devices such as MV and CVC.9
Concerning the phenotype of the bacteria, although it was similar in 95.7% of cases, we cannot be certain that it was the same clonal group, as genotype studies were unavailable. In a study of an outbreak of S. marcescens in a NICU, Merla et al.10 identified different circulating clones and observed no relationship between the bacteria identified in the environment and those that infected newborns. Conversely, in a similar study, Caggiano et al.3 identified different clonally related strains in different wards of a university hospital.
During the study period, various infection control measures were implemented, such as training and the implementation of a handwashing program and action packages to prevent healthcare-associated infections, the screening and isolation of patients with intestinal colonization, and environmental and hand cultures taken from staff. The NICU was closed in April 2022 and January 2024 due to increased cases. None of these bacteria were identified in the surface, solution, or hand cultures collected from the staff.
In this study, we concluded that there is an association between neonatal sepsis due to S. marcescens and the use of invasive devices such as MV and CVC, as well as the prior administration of meropenem. The limitations of this study included a small number of patients and an absence of molecular studies to determine whether the bacterial clones were distinct. Patients with intestinal colonization who did not present with sepsis were also excluded.
The protocol was authorized by the NHCGJIM Research and Ethics Committee under registration number 17CI14039116-COFEPRIS. This work was conducted in accordance with the 2013 version of the Declaration of Helsinki, which safeguarded the safety of the participants. Furthermore, the provisions of the General Health Law on Health Research in Mexico were followed.
FundingThis research has not received any specific grants from public sector agencies, commercial sectors, or non-profit organizations.
Conflicts of interestThe authors have no conflicts of interest related to the development or results obtained from this research.
The research was presented as a poster at the “International Congress on Advances in Medicine 2024” in Guadalajara, Jalisco, April 2024.