Cardiac magnetic resonance imaging (MRI) is an important tool that makes it possible to evaluate patients with cardiovascular disease; in addition to infarction and alterations in myocardial perfusion, cardiac MRI is useful for evaluating other phenomena such as microvascular obstruction and ischemia. The main prognostic factors in cardiac MRI are ventricular dysfunction, necrosis in late-enhancement sequences, and ischemia in stress sequences. In acute myocardial infarction, cardiac MRI can evaluate the peri-infarct zone and quantify the size of the infarct. Furthermore, cardiac MRI's ability to detect and evaluate microvascular obstruction makes it a fundamental tool for establishing the prognosis of ischemic heart disease. In patients with chronic ischemic heart disease, cardiac MRI can detect ischemia induced by pharmacological stress and can diagnose infarcts that can be missed on other techniques.
La resonancia magnética cardíaca (cardiorresonancia magnética –CRM–) es una importante herramienta que permite evaluar en pacientes con enfermedad cardiovascular no solo el infarto y las alteraciones en la perfusión miocárdica, sino también otros fenómenos como la obstrucción microvascular y la isquemia. Los principales factores pronósticos en la CRM son la disfunción ventricular, la necrosis en las secuencias de realce tardío y la isquemia en las secuencias de estrés. En el infarto agudo de miocardio, la CRM puede evaluar la zona periinfarto y cuantificar el tamaño del infarto. Además, la capacidad de la CRM para detectar y evaluar la obstrucción microvascular la convierte en una herramienta fundamental para establecer el pronóstico de la cardiopatía isquémica. En los pacientes con cardiopatía isquémica crónica, la CRM puede detectar isquemia inducible con estrés farmacológico y diagnosticar infartos que pueden pasar desapercibidos con otras técnicas.
Ischemic heart disease still is the main cause of death in developed countries in spite of recent therapeutic and preventive improvements.1,2 Since it is so prevalent, it is very important to use the diagnostic means adequately, and cardiac magnetic resonance imaging (CMR) has established itself as a fundamental modality, not only to diagnose but also to establish these patients’ diagnosis. CMR has the advantage of assessing in the same examination all the parameters that are important to make clinical decisions in patients with ischemic heart disease, such as global and segmental ventricular function, perfusion and myocardial ischemia and the amount of viable myocardial tissue.3 Unlike echocardiography, it does not present the limitations of the acoustic window. It has more spatial and temporal resolution than nuclear medicine modalities and better tissue characterization. In addition, it does not use ionizing radiation which is another advantage in respect of other imaging modalities such as computed tomography (CT) or nuclear medicine.4
In this paper, we will review the basic physiopathological concepts of ischemic heart disease and the data that support the use of CMR to establish the prognosis and follow-up of patients with acute myocardial infarction (AMI) and chronic ischemic heart disease.
Physiopathologic bases of the ischemic heart diseaseKnowing the physiopathology of ischemic heart disease and the consequences that the absence of flow has on the cardiac muscle is essential to assess the prognosis of patients and the possibilities of success of the different therapeutic options. When a coronary artery narrows, the mechanisms of coronary self-regulation try to normalize the myocardial flow by reducing the resistance of the distal arterial bed to stenosis. In normal conditions, a stenosis must surpass 85–90% of the lumen diameter before the blood flow to the myocardium decreases significantly.5,6 After a coronary occlusion, a series of myocardial changes, known as ischemic cascade, take place. These modifications can be reversible or irreversible. Among the reversible changes are contractile dysfunction (it occurs a few seconds after occlusion) and intracellular edema (20–30min).5 Subsequently, irreversible changes occur both in the myocytes (30–60min) and in the endothelial vascular cells (60–90min) ending up in cellular necrosis and apoptosis.6 Ischemic damage progresses “in the form of a wave” from the subendocardium to the subepicardium in approximately 3–6h. Transmural damage is directly proportional to the ischemic time.7 There are multiple reasons why the endocardium is the most sensitive area to ischemia. Firstly, coronary circulation goes from the epicardium to the endocardium; therefore, because it is at the end of the trajectory, the endocardium is more susceptible to ischemia. In addition, the endocardium needs more oxygen than the epicardium, even in normal conditions, as it happens during the ventricular systole since when the intraventricular pressure increases the endocardial artery walls are compressed to the point that they might even collapse and decrease their capacity to vasodilate.8 When a coronary obstruction persists in time, not only the areas that are the most sensitive to ischemia (more subendocardial) will necrotize, but also other more borderline mesocardial and epicardial áreas will suffer from its effects. Those limits of the necrosis area are also part of the myocardium threatened by the ischemia. All the myocardial territory whose perfusion depends on the damaged artery is referred to as myocardium at risk.9
Restoring the epicardial artery flow to preserve most of the myocardium at risk is the basis of the current AMI therapeutic strategies. However, in spite of its beneficial effects, the process of cellular death can go on for the first few hours after reperfusion, which is known as myocardial reperfusion injury.10 The main manifestation of this complex phenomenon is microvascular obstruction (MVO) where a complete lack of myocardial tissue perfusion occurs despite the coronary epicardial repermeabilization.11
When a coronary occlusion is treated and the flow is reestablished, it may happen that the myocardium recovers its contractility early or late its contractility. The transient state after the ischemia in which contractile function is diminished, even though there is no infarction, is referred to as myocardial stunning or stunned myocardium12 (Fig. 1). It can take days or weeks to normalize though it can improve with the administration of inotropic agents.
Determination of saved myocardium in an acute myocardial infarction. Patient with acute coronary syndrome due to a significant lesion in the proximal anterior descending artery. (A) T2-weighted (STIR) image. Extensive anterior and anterior–lateral hyperintense area (white arrows) outlining the myocardium at risk. (B) Late-enhancement sequence. Necrosis is not shown in this area and there is only a slight hyperintensity caused by the edema itself (black arrows). One old non-transmural necrosis is identified incidentally in the circumflex artery territory (arrow head).
Finally, the term hibernating myocardium is used to describe the maintained state in which contractile function is reduced in an infraperfused but viable myocardium recovering completely after revascularization.13
Sequences of magnetic cardio-resonance for ischemic heart diseaseCMR can potentially assess everything involved in ischemic heart disease. The current technology – CT-coronariography is a better modality than CMR to identify coronary artery disease this is why in practice the CMR is used to assess the functional consequences of coronary artery disease.14 The sequences of the MR coronariography need electrocardiographic and respiratory synchronization, and this is why the acquisition time is long; also in patients whose heart or respiratory rate is very irregular, it is a modality that just cannot be applied. Also the spatial resolution of MR is lower than that of the CT and it is a more operator-dependent modality. For all of the aforementioned except in selected cases, coronary examination should be performed through a CT.15
Myocardial contractilityCine gradient echo sequence (SSFP or steady-state free precession) is an alternative to conventional echocardiography. It is reliable and reproducible to calculate volumes and ventricular function because it allows us to assess both global and segmental contractility.16,17 Although it has lower spatial and temporal resolution, when it is not possible to perform this sequence, due to serious arrhythmias or incapacity for an adequate apnea, cine sequences in real time can be used. Due to its lower resolution, it is only possible to make a qualitative assessment of contractility, not a quantitative one.18
The volumetric and functional analysis of the heart are performed by acquiring contiguous images (between 8 and 12) usually on a short axis. Through the outlining of the endocardium borders in diastole and systole and those of the epicardium in diastole we can obtain function parameters such as the telediastolic and telesystolic volumes, the ejection fraction, the stroke volume, the cardiac output of both ventricles and the left ventricle myocardial mass. The ejection fraction thus calculated is based on Simpson's method. Dilated, asymmetrical ventricles or those with altered segmental contractility do not affect the method's accuracy. Its foundation claims that the left ventricular volume equals the sum of the volumes of different contiguous discs perpendicular to the larger axis of the left ventricle. Since the method's main limitation is defining the endocardial borders, and because this is always better achieved with CMR than with echocardiography, the left ventricle ejection fraction is calculated more accurately through the CMR.19
Myocardial ischemia is detected in CMR with pharmacological stress, which can be carried out with vasodilating drugs (adenosine or dipyridamole) and perfusion sequences, or with inotropic drugs (dobutamine) and cine sequences for the analysis of contractility.20,21 Cine sequences during the pharmacological stress allow us to assess both ischemia (with high doses of dobutamine) and feasibility (with low doses of dobutamine). The dobutamine administration protocol is similar to that of stress echocardiography. It begins with 5μ/kg/min and it is gradually increased to 10, 20, 30 and 40μ/kg/min every 3min, until 85% of the maximum heart rate (220 – age) is reached. If it is not reached with the maximum dose of dobutamine, 0.5–1mg of atropine is added. The cine images are acquired at rest and at each of the dobutamine levels and the images are studied immediately. Depending on the machines, 3 short-axis projections can be performed and then one or two long-axis projections can be added (2 and 4 chambers) both at rest and at each stage of infusion. There is ischemia in a myocardial segment when its contractility worsens with increasing doses of dobutamine.20
Myocardial perfusionCoronary circulation and myocardial perfusion have their physiopathological correlate with CMR. In basal conditions, myocardial perfusion does not alter until coronary stenosis reaches approximately 85–90%. Instead, during the stress, the myocardium distal to a coronary lesion ranging from 50 to 85% may suffer ischemia and a coronary lesion can be considered hemodynamically significant.21
The sequences used to assess myocardial perfusion are the first-step sequences. They are T1-weighted gradient echo sequences, with a high temporal resolution acquired as gadolinium is being injected; in these sequences it is possible to see how the contrast is disseminating throughout the myocardial tissue. The perfusion studies are performed at rest and with pharmacological stress administering vasodilating drugs (the most frequently used are adenosine and dipyridamole). Dipyridamole is used in 0.56mg/kg doses in 4min. Adenosine is used in doses of 140μg/kg/min for 3–6min depending on the patient's response. Dipyridamole is an adenosine precursor that metabolizes in the liver; therefore, its vasodilating capacity may depend on each patient.22 The injection of both dipyridamole and adenosine produces an extracellular increase of adenosine that dilates the arterioles and precapillaries (coronary resistance vessels) increasing the regional myocardial flow between 3 and 5 times. The increase is greater in healthy coronary vessels than in stenotic ones and it causes a phenomenon known as coronary steal. Due to the fact that adenosine has a much shorter half-life than dipyridamole (10s as opposed to 30–40min), it is necessary to use a continuous infusion pump and inject it as the images are being acquired (injection stops after the first-step perfusion is studied). With dipyridamole, the images are acquired after the injection has finished. The usual gadolinium dose is 0.2mmol/kg with a 3–5ml/s debit. The ischemic myocardium is hypointense and it follows a given coronary anatomical territory in respect of the normally perfused myocardium.20,21
Myocardial edemaFast spin-echo or FSE black blood T2-weighted sequences are used (double inversion-recovery, triple inversion-recovery, or short-tau inversion recovery (STIR) in the ventricular short axis. The cellular membrane patency increases in the infarcted myocardium and the increase of the free extracellular water in these sequences is seen as a usually transmural hyperintensity following the coronary territory of the artery responsible. It is accepted that in AMIs the hyperintense territory in the T2-STIR sequences reflects the myocardium at risk.23 Hyperintensity decreases with time in such a way that it can only be assessed in the acute–sub-acute phases (approximately 7–10 days).24 In the last few years T2-mapping sequences have been introduced to assess myocardial edema quantitatively and not qualitatively like T2-STIR sequences.25
Late enhancementThese are T1-weighted 2D/3D gradient echo sequences with a previous inversion-recovery pulse. They are performed 10–15min after injecting gadolinium. The previous inversion pulse is intended to cancel the signal of a healthy or normal myocardium. This inversion time must be adjusted individually for each patient and it is usually at 200–300ms. Modern machines often have a “look-locker” sequence to do tracking with different inversion times to select it. The time must be increased gradually as the time goes by during image acquisition due to the kinetics of gadolinium. In acute infarctions, cellular death and loss of cytoplasmic membrane integrity allows the gadolinium to penetrate the myocyte, which explains the late enhancement in the acute phase. In chronic infarction, the necrosis occurring after the infarction increases extracellular space due to the increase of extracellular collagen and the fact that gadolinium builds up in scar or fibrosis regions. The correlation between the size of the infarction detected through CMR and the anatomopathologic studies is excellent.26 The necrotic areas appear as hyperintense, subendocardial and more or less intramural areas based on the extension of the infarction. The myocardium saved through percutaneous revascularization can be determined by subtracting from the T2 sequence edema area the necrosis area of the late-enhancement sequence (Fig. 1).
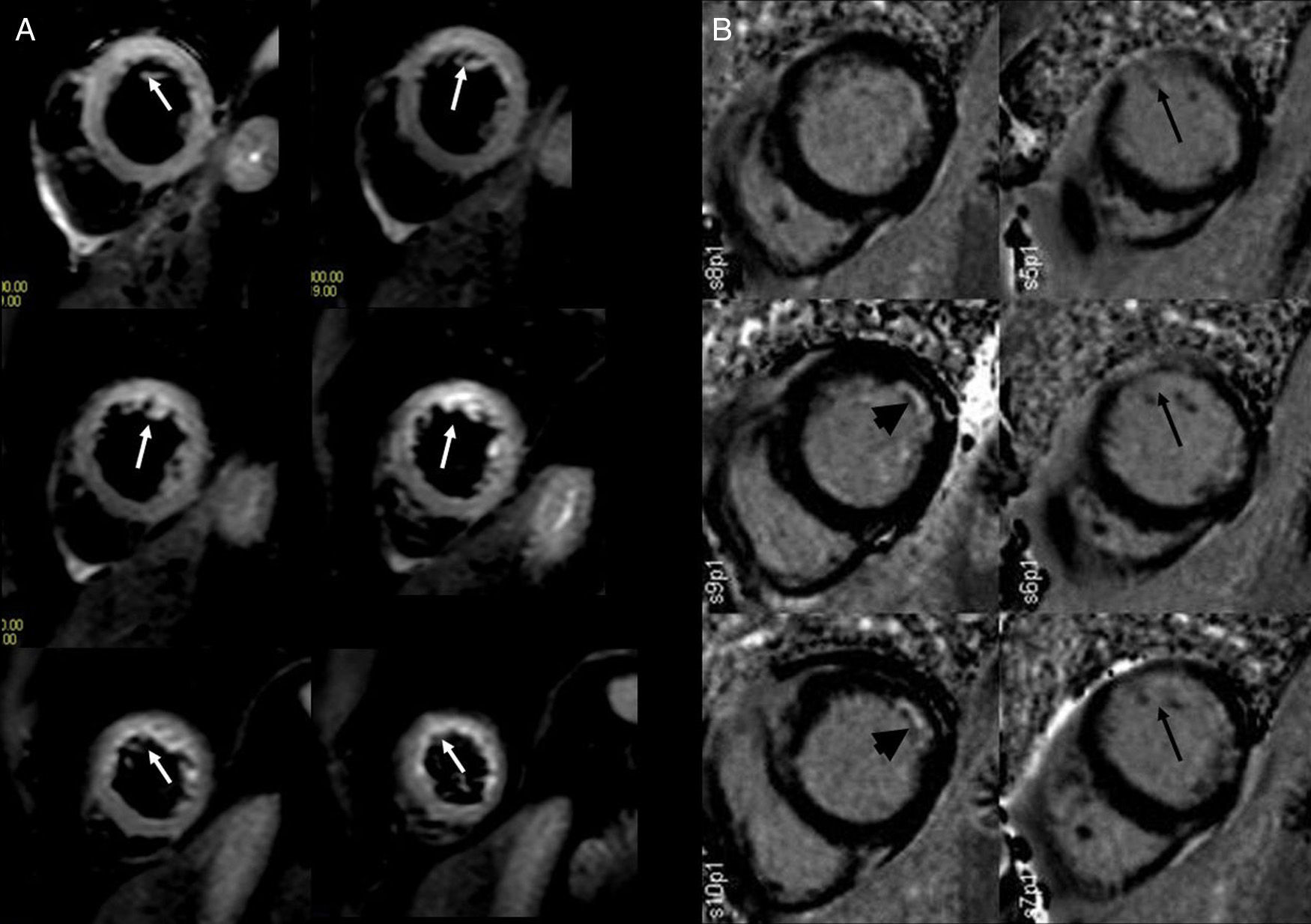
Prognostic value of magnetic cardio-resonance in the acute myocardial infarctionPeri-infarction areas and ventricular arrhythmiasVentricular arrhythmias and sudden death are very important causes of morbidity and mortality in the post-infarction period. The peripheral area of an infarction is made up of viable myocardium areas intertwined with infarcted tissues or fibrosis areas that presuppose a substrate to develop reentry circuits and electric conductivity disorders that may develop ventricular tachycardias (Fig. 2). It has been pointed out that these pre-infarction areas can be seen in the late-enhancement sequence as intermediate signal areas surrounding the necrotic area.27 In an electrophysiological study through CMR of 40 patients before implantation of pacemakers or implantable automatic defibrillators, the size of the pre-infarction area in the CMR predicted ventricular tachycardia.28 In another study, the size of the pre-infarction area in the CMR predicted any causes of cardiac mortality in the next 2.4 years.29
Several authors have also shown that the size of the pre-infarction area is better than classical factors (left ventricle ejection fraction, total size of the infarction) to predict ventricular tachyarrhythmia and the lesser response to electrophysiological therapy.30–32 Therefore, many authors accept today that assessing the characteristics and extension of the pre-infarction area can be a bloodless tool to establish the risk of malignant ventricular tachyarrhythmias and the probability of success of electrophysiological therapies.
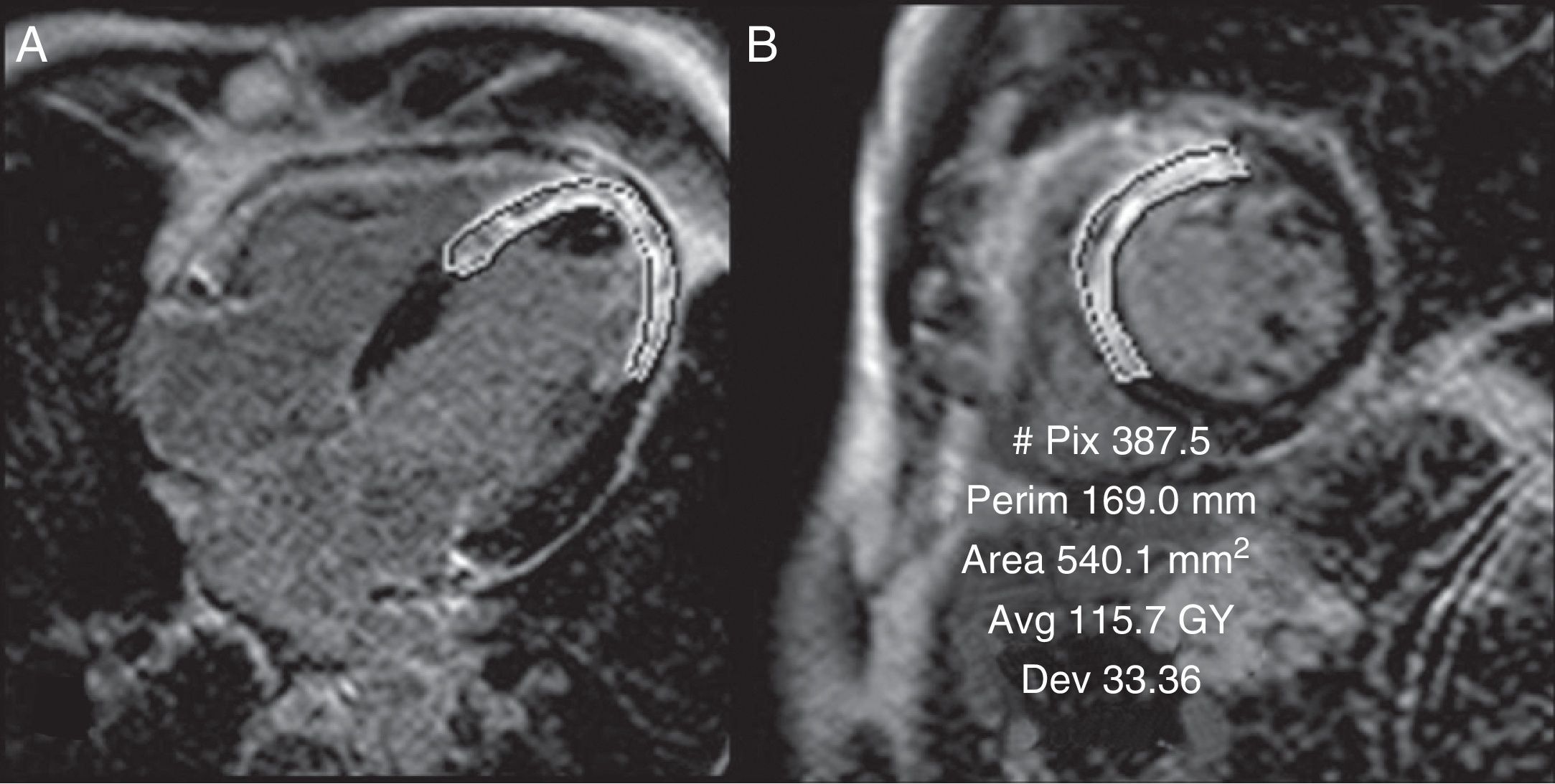
Infarction sizeThe CMR's capacity to quantify the infarction size in the late-enhancement sequence has been widely validated.33 Infarction size is a very powerful predictive factor for cardiac episodes during follow-up34,35 (Fig. 3). Several studies have proved that it is even better than other classic prognostic factors such as the left ventricle ejection fraction, the ventricular tele-systolic and tele-diastolic volumes, or heart failure-related symptoms.36–41 In a study with 103 patients with ST-elevation acute coronary syndrome (STEACS) in whom an CMR was performed during the first 12h and then 6 months after revascularization, who were also followed for an average of 2.4 years, late enhancement measured right after revascularization independently predicted the recovery of the left ventricle ejection fraction at 6 months as well as major cardiovascular episodes, even better than the initial left ventricle or other clinical parameters.42 It is also important to assess not only the amount of necrosis but also the extent of the ischemia.43 The relationship between the infarction size and the extent of ischemia seen in the first-step sequence with adenosine infusion was significantly greater in STEACS patients with Q wave than in patients with non-Q STEACS or NSTE-ACS.44
Quantification of infarction size. In order to quantify infarction size, the gadolinium uptake area perimeter is outlined in each ventricular cut showing enhancement. The values obtained though this way and multiplied by the cut's thickness and the myocardial density (1.05g/cm3) are equivalent to the infarcted myocardial mass. Although in selected cases it is possible to use planes like that of four chambers (A), short-axis planes are generally used (B). Short-axis planes are generally used though in selected cases other planes can be used (in this case 4 chambers) for verification purposes. The total size of the necrosis area was 30g accounting for 25% of the total mass of the left ventricle.
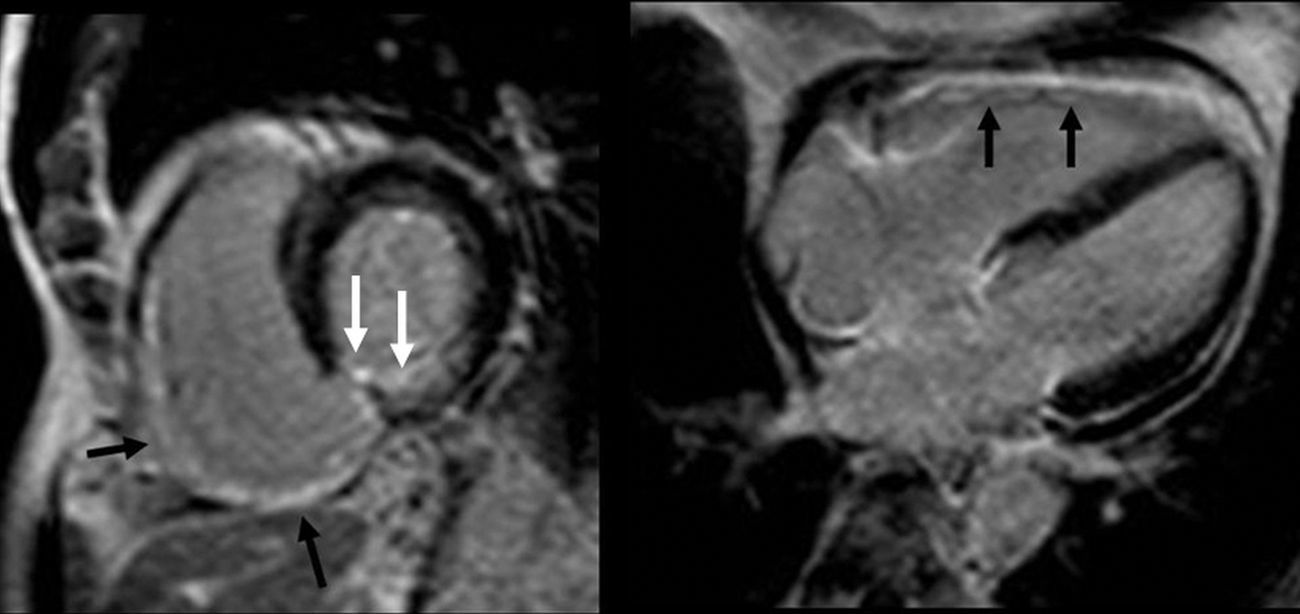
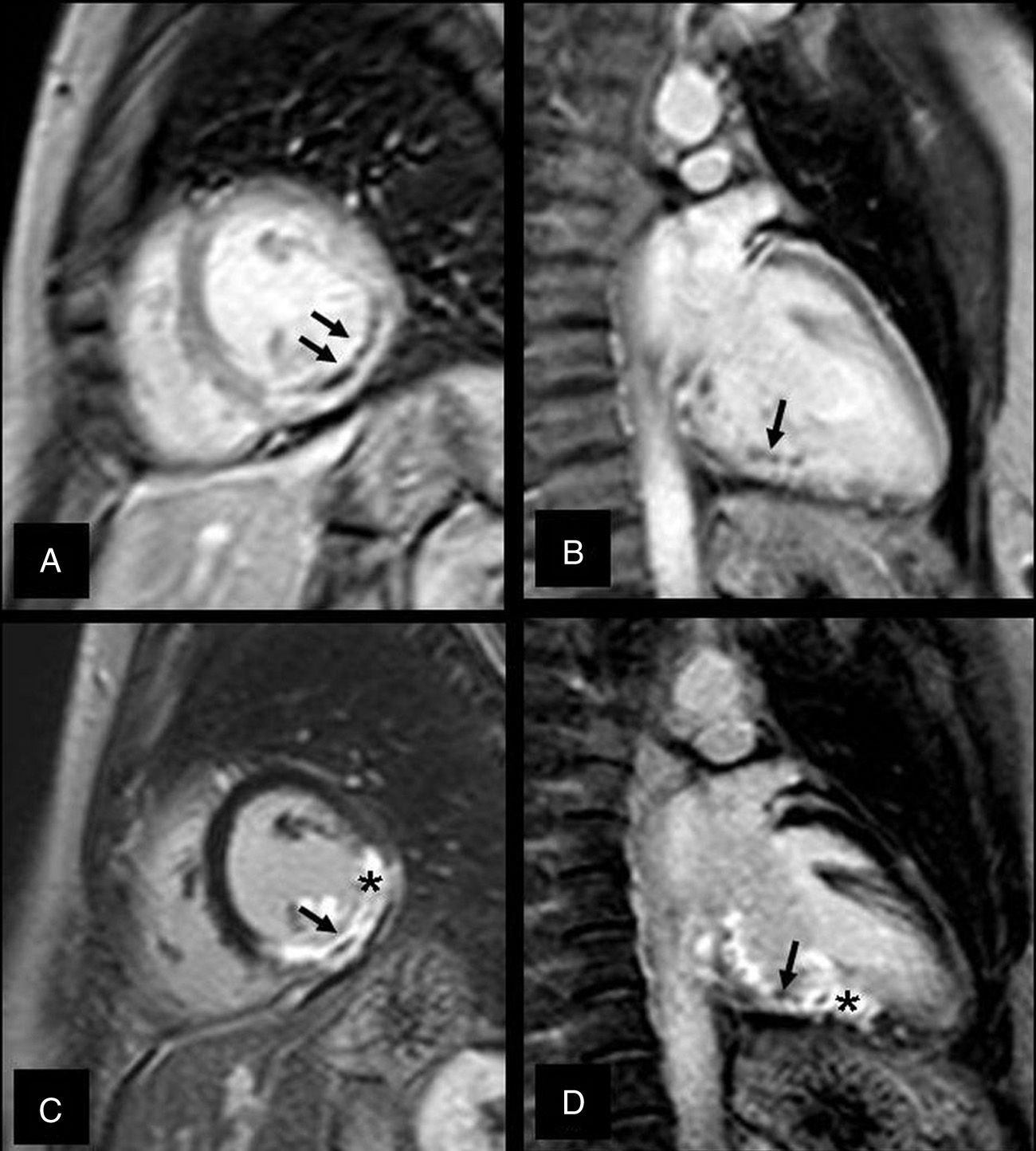
The right ventricle infarction is usually associated to a left ventricle inferior infarction due to the proximal right coronary artery occlusion (Fig. 4). The CMR has proved to be much more sensitive to detect small right ventricle infarctions than physical examinations, ECGs or echocardiograms.45,46 The literature is contradictory when it comes to establishing the prognostic value of this infarction. Whereas some authors believe that the right ventricle damage is a powerful predictor of major cardiovascular episodes during follow-up, even after the infarction size has been normalized in the left ventricle–which can have a significantly greater mortality46,47; others have not shown any short-term prognostic relations in the infarction of the right ventricle.48,49 In sum right ventricle AMI can be reliably detected through CMR yet the prognostic meaning of late enhancement in the right ventricle remains unclear.
Right ventricle infarction. The short-axis, late-enhancement, four-chamber images show transmural enhancement in the inferior side and part of the left ventricle free wall (black arrows). We can also observe an inferior septal infarction and at the inferior side of the left ventricle (white arrows).
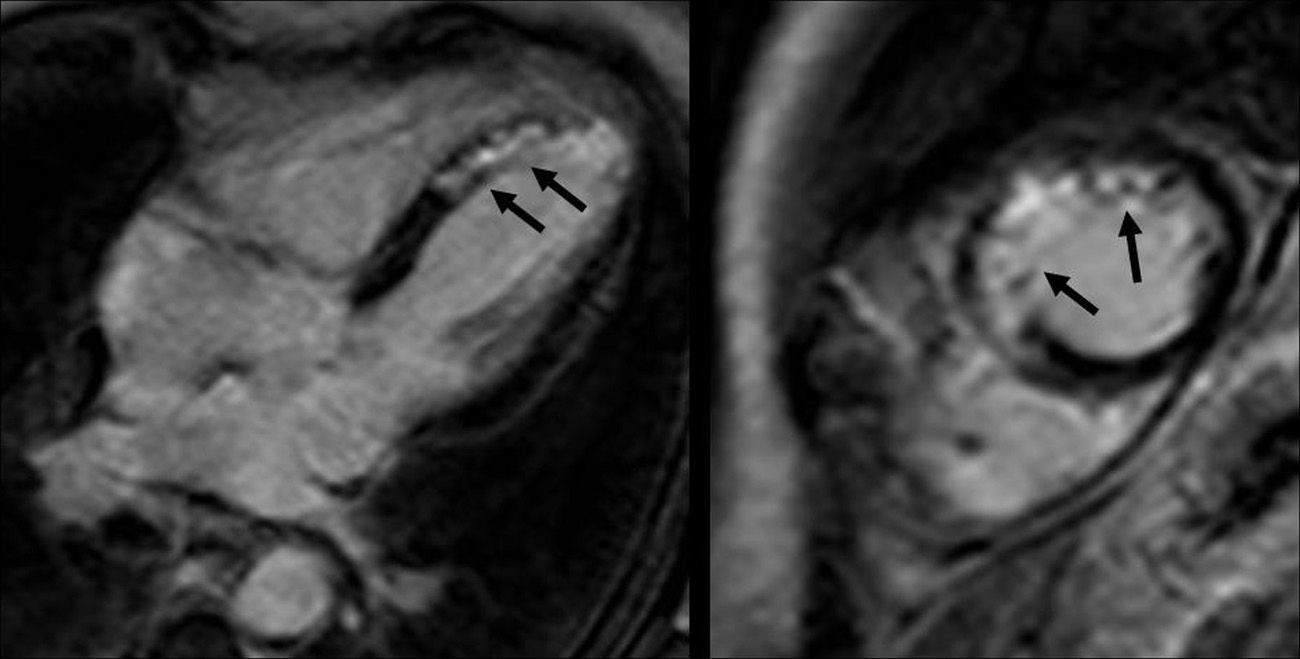
In approximately 20–50% of AMI one microvascular occlusion (MVO) occurs in the infarcted myocardium despite the modern percutaneous revascularization techniques.50 The MVO can be detected through CMR using three different techniques: first-step perfusion, early enhancement and late enhancement.51,52 The early-enhancement technique consists of a late-enhancement sequence 1–2min after the injection of gadolinium with a generally high inversion time (400–600mseg) that does not cancel the normal myocardium. The MVO area is a hypointense area that clearly differentiates from both the normal and the infarcted myocardium. An early MVO can also be detected in the basal first-step sequence. The latter technique consists of performing a late-enhancement sequence 10–15min after the injection of contrast. This late MVO is a hypointensity area within the infarcted myocardium hyperintense area, often extending from the subendocardium. There is no consensus yet whether the MVO should be assessed through early or late enhancement (Fig. 5). The extent of early MVO correlates well with anatomopathologic findings.11 Several studies have proved that early MVO is useful to predict both the left ventricle function and the ventricular remodeling after one year, as well as the size of the infarction and transmural character.53–55 However, it does not have a good correlation with TIMI (thrombolysis in myocardial infarction)54 angiographic parameters. Other authors have described a similar prognostic value for late MVO,55 and for some it is a better predictor than early MVO53 is a phenomenon that can accompany MVO is myocardial hemorrhage. In several experimental models it has been demonstrated that hemorrhage occurs within MVO areas and that its extent is proportional to the duration of the ischemia.56 On the other hand, it correlates with the size of the infarction and increases proportionally with the occlusion time prior to perfusion.57 Myocardial hemorrhage can be detected through T2 and T2* sequences. In the T2 sequences it is identified as a hypointense area within the hyperintense edema area. Given MVO also presents this behavior, it is difficult to differentiate them; therefore so to diagnose myocardial hemorrhage we rather use T2* sequences which are also more sensitive due to the paramagnetic effect of hemoglobin degradation.58–62 The size of the hemorrhage is always smaller than that of the MVO and is identified in the T2* sequence as a hypointense area.56–58
Microvascular occlusion in sequences of (A) and (B) early enhancement and (C) and (D) late enhancement. The early-enhancement images, (A) short axis and (B) two chambers show the microvascular occlusion (MVO) as hypointense areas (black arrows). The late-enhancement sequence in (C) short axis and (D) two chambers of the same patient shows the hyperintense infarction area (asterisk) and in the inside hypointense areas signaling the MVO. The MVO is more easily detectable in the early-enhancement sequence.
In sum early or late MVO in one AMI has a worse prognosis. Therefore, detecting it will make us consider more modern, strict medical treatments and a closer monitoring of patients.59,60
Prognostic utility of magnetic cardio-resonance in the chronic ischemic heart diseaseInducible myocardial ischemiaStress CMR with inotropic drugs such as dobutamine, or vasodilators such as adenosine or dipyridamole, are techniques used to diagnose myocardial ischemia. Myocardial stress is not only useful to detect coronary stenosis with functional repercussion, but also to establish its prognosis.
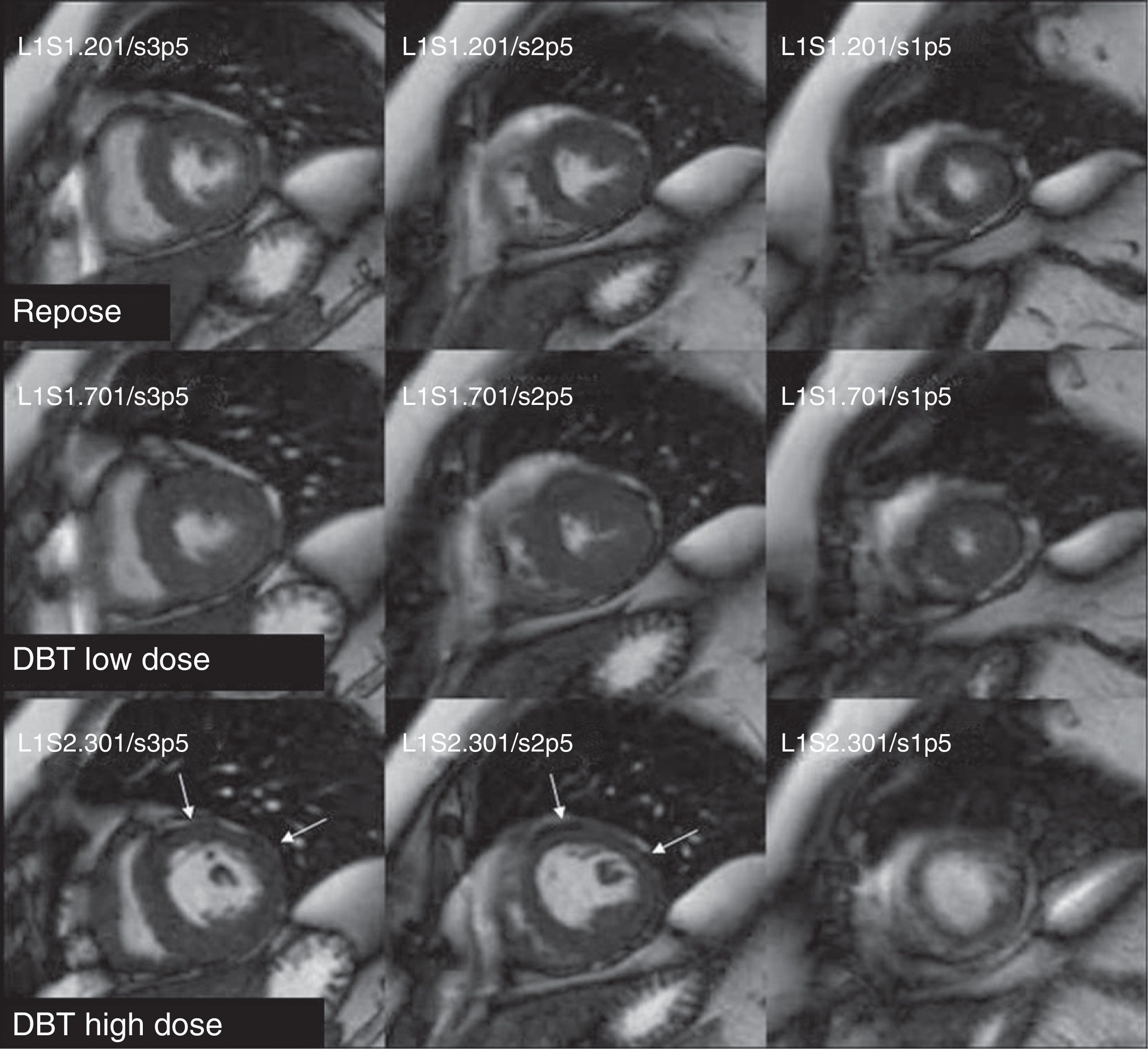
Dobutamine stress CMR has been used to stage risk in patients with intermediate risk before non-cardiac surgical interventions. A positive CMR–dobutamine can independently predict infarction, death or heart failure during or after intervention.63 In a study with dobutamine and adenosine stress CMR in patients with chest pain or dyspnea and suspicion of coronary artery disease, the survival free of cardiac episodes after 3 years was significantly greater in patients with normal CMR–dobutamine or CMR–adenosine.64 In addition, a normal stress test predicted better the episodes that the cardiovascular risk factors or the segmental contractility defects. Other studies have proved the usefulness and prognostic value of CMR–dobutamine when it is not possible to perform stress echocardiography examinations.65 Dobutamine-induced ischemia was significantly associated with a future myocardial infarction or heart-related death, irrespective of the existence of cardiovascular risk factors or significant coronary artery disease (Fig. 6).
Detection of ischemia though stress dobutamine cardiovascular magnetic resonance. The systole image of the left ventricle with low-dose dobutamine (20μg/kg/min) is correct. With high-dose dobutamine (40μg/kg/min) hypokinesia is observed in the anterior, lateral mid-basal side and in all of the left ventricle apex (white arrows) in a female patient with significant lesion of the common trunk.
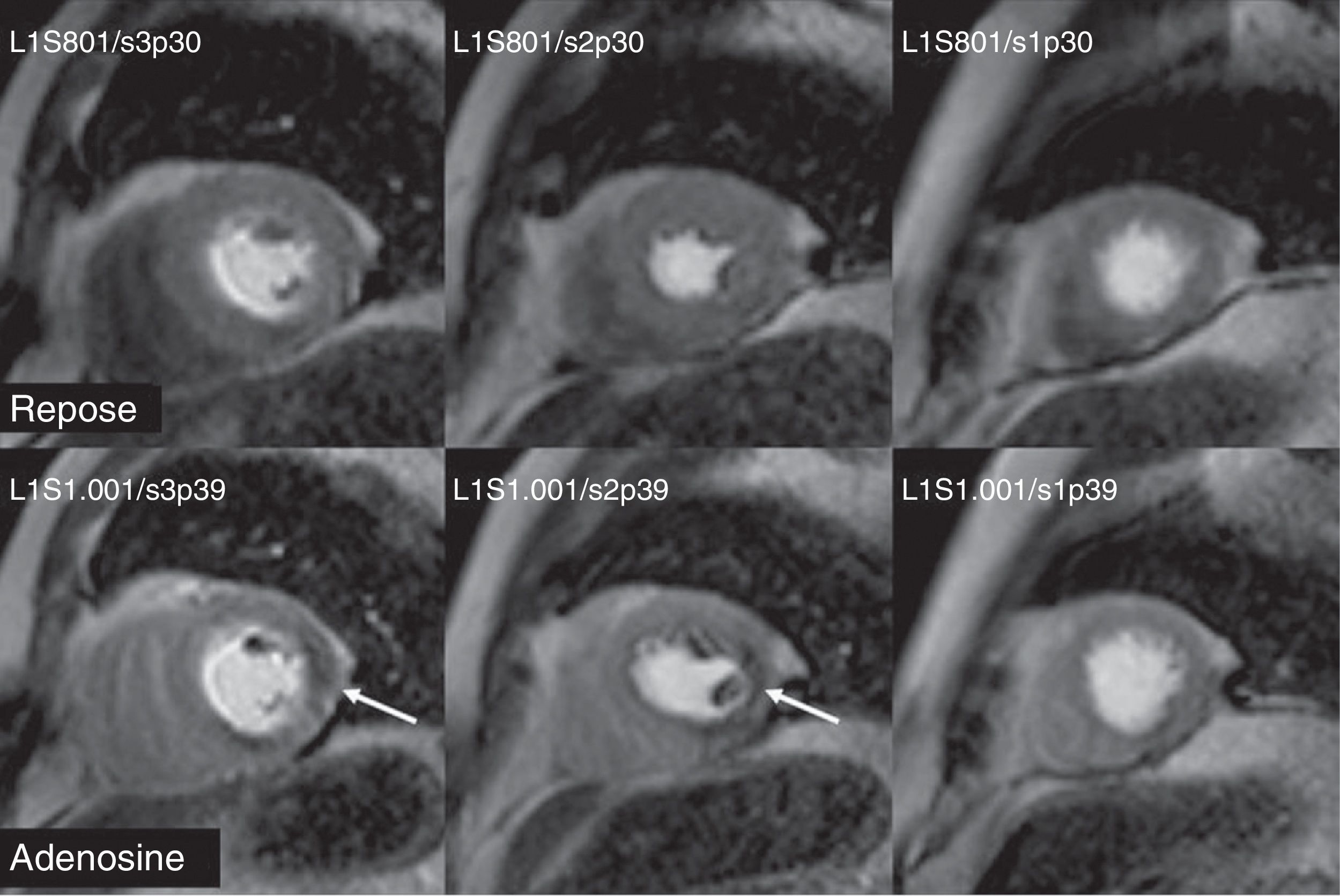
The prognostic value of CMR with vasodilating drugs has been proven in several studies64 (Fig. 7). In one of them with 218 patients with suspicion of coronary artery disease and negative CMR–adenosine, the rate of major cardiovascular episodes after 12 months was 2/218, without cases of death or infarction. The negative predictive value of CMR–adenosine was 99.1%. Therefore, a negative test can reduce the number of unnecessary invasive coronary angiographies.66 In another paper with dipyridamole stress CMR with 420 patients with chest pain and known or suspected coronary artery disease, the rate of major cardiovascular episodes, after a 420-day-follow-up, was significantly greater in patients with induced perfusion defect. In addition, in the multivariant analysis, the extent of segmental contractility disorders induced during the dipyridamole infusion was independently associated with the rate of major cardiovascular episodes.67 CMR with adenosine has also been useful to stage risk in patients with acute chest pain in the ER.68 In patients with a negative stress test, the free of episodes-survival rate after a year of follow-up was significantly greater if there was a perfusion defect in the CMR–adenosine. Therefore, in patients with suspicion of coronary artery disease or known coronary disease, the fact of not finding myocardial ischemia with CMR–dobutamine or vasodilating drugs is a sign of good prognosis.69
Two other factors also valuable to predict functional recovery are myocardial thickness and contractile reserve. Parietal telediastolic thickness of the left ventricle ≥5.5mm and systolic thickness ≥1mm in the CMR are related with feasibility determined through SPECT or PET.70 Nevertheless we must remember that parietal thickness per se has high sensitivity (95%) but low specificity (41%) to establish unfeasibility. Contractile reserve is studied through CMR and low dobutamine doses (5–20μg/kg/min). One parietal thickening ≥2mm during the injection of dobutamine predicts the recovery of segmental contractility after revascularization way better than the preservation of the telediastolic myocardial thickness.70
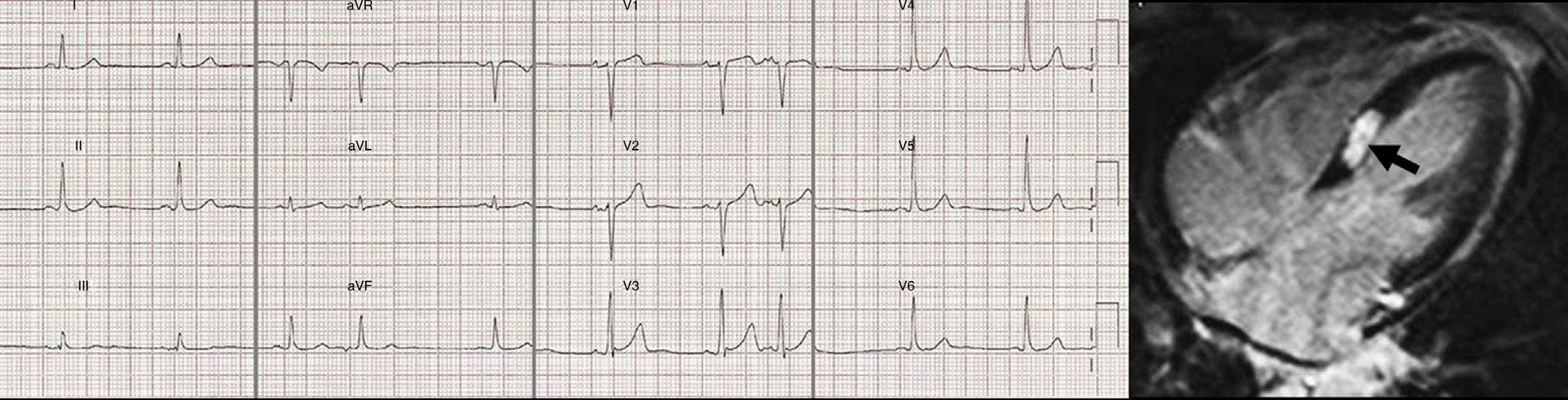
Hidden myocardial necrosisLate-enhancement sequence shows excellent sensitivity and specificity to detect even very small myocardial scars.71 In addition, there are data indicating that late-enhancement CMR can identify misdiagnosed myocardial infarctions whose existence has important prognostic implications.72 In a study of a sample of patients over 70 years old, late-enhancement sequence detected nearly 20% of necrosis in patients without a history of prior infarctions (Fig. 8). The prevalence of hidden myocardial necrosis seems to be greater among patients with high cardiovascular risk.71 In a study of 195 patients with suspicion of coronary artery disease but without a known history of AMI 44 showed late enhancement. Even with little late enhancement, the risk was high and the prognostic value of major cardiovascular episodes and cardiac death was greater than that of the conventional functional and angiographic predictors.73 Similar findings have been described in diabetic patients without a history of prior infarctions. Therefore, late enhancement in patients with suspicion of coronary artery disease can improve risk stratification.71–73
ConclusionThe CMR is an essential tool in patients with cardiovascular disease and it provides morphological (ventricular volumes, presence and extent of myocardial necrosis and microvascular occlusion) and functional parameters (global and segmental function, ischemia) in the same examination. All these factors have proved to predict not only survival but also the occurrence of future cardiovascular episodes.
Ethical responsibilitiesProtection of people and animalsAuthors confirm that no experiments have been performed on human beings or animals.
Data confidentialityAuthors confirm that there are no personal data from patients in this article.
Right to privacy and informed consentAuthors confirm that in this report there are no personal data from patients.
Authors- 1.
Manager of the integrity of the study: AH.
- 2.
Original idea of the study: AH.
- 3.
Study design: AH.
- 4.
Data mining: AH and GPLl.
- 5.
Data analysis and interpretation: AH and GPLl.
- 6.
Statistical analysis: N/A.
- 7.
Reference search: AH.
- 8.
Writing: AH.
- 9.
Critical review of the manuscript and intellectually relevant remarks: AH and GPLl.
- 10.
Approval of final version: AH and GPLl.
The authors declared no conflicts of interest.
Please cite this article as: Hidalgo A, Pons-Lladó G. Utilidad de la RM cardíaca en el pronóstico y seguimiento de la cardiopatía isquémica. Radiología. 2015;57:201–212.