Cartilaginous tumours are a large and heterogeneous group of neoplasms characterised by the presence of a chondroid matrix, with lobular growth and arcuate, ring-like or popcorn-like calcification patterns. MRI shows hyperintensity in T2-weighted sequences and a lobulated or septal relief in postcontrast images.
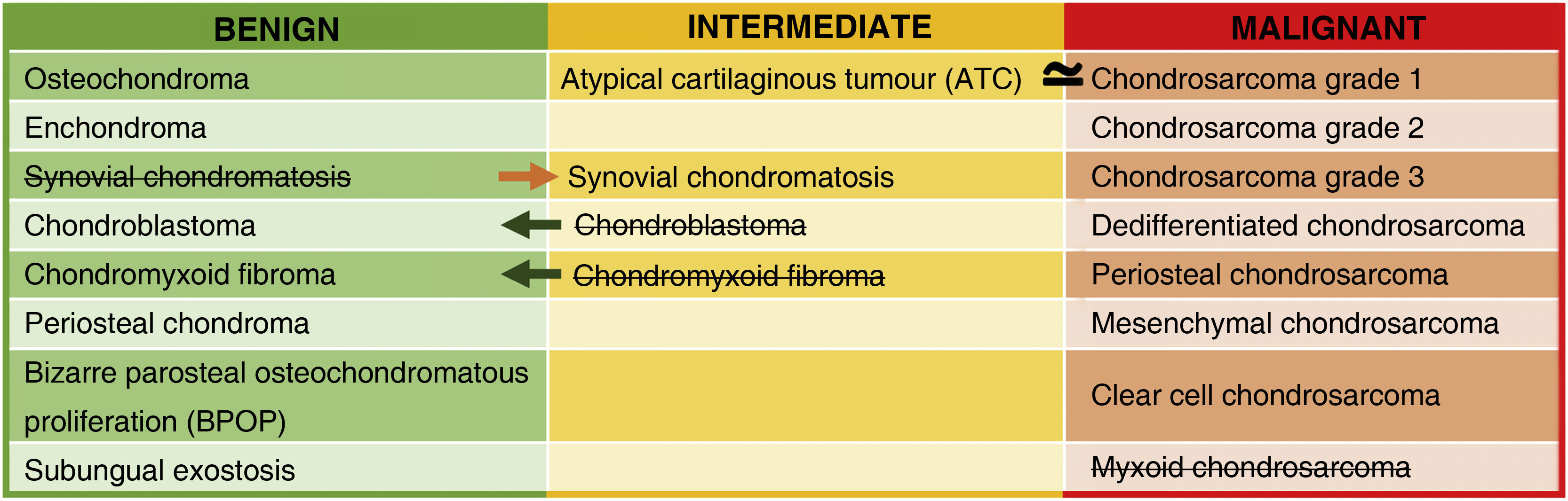
In the WHO 2020 classification, chondral tumours are classified as benign, intermediate or malignant. Despite technological advances, they continue to pose a challenge for both the radiologist and the pathologist, being the main difficulty the differentiation between benign and malignant tumours, which is why they require a multidisciplinary approach.
This paper describes the main changes introduced in the 2020 update, describes the imaging characteristics of the main cartilaginous tumours and provides the radiological keys to differentiate between benign and malignant tumours.
Los tumores cartilaginosos son un grupo amplio y heterogéneo de neoplasias caracterizadas por la presencia de una matriz condroide, que presenta crecimiento lobular y patrones de calcificación en arcos y anillos o en palomita de maíz. En RM destaca su hiperintensidad en las secuencias potenciadas en T2 y en las imágenes postcontraste un relace lobulado o septal.
En la clasificación de 2020 de la OMS, los tumores de estirpe condral se clasifican en benignos, intermedios o malignos. A pesar de los avances tecnológicos siguen suponiendo un reto tanto para el radiólogo como el patólogo, siendo la principal dificultad la diferenciación entre los tumores benignos y malignos, razón por la que requieren de un abordaje multidisciplinar.
Este trabajo recoge los principales cambios introducidos en la actualización de 2020 describe las características de imagen de los principales tumores cartilaginosos y proporciona las claves radiológicas para diferenciar entre tumores benignos y malignos.
Cartilaginous tumours, also known as chondrogenic or cartilage tumours, are a heterogeneous group of neoplasms characterised by the presence of a chondroid matrix and include a wide range of tumours.1
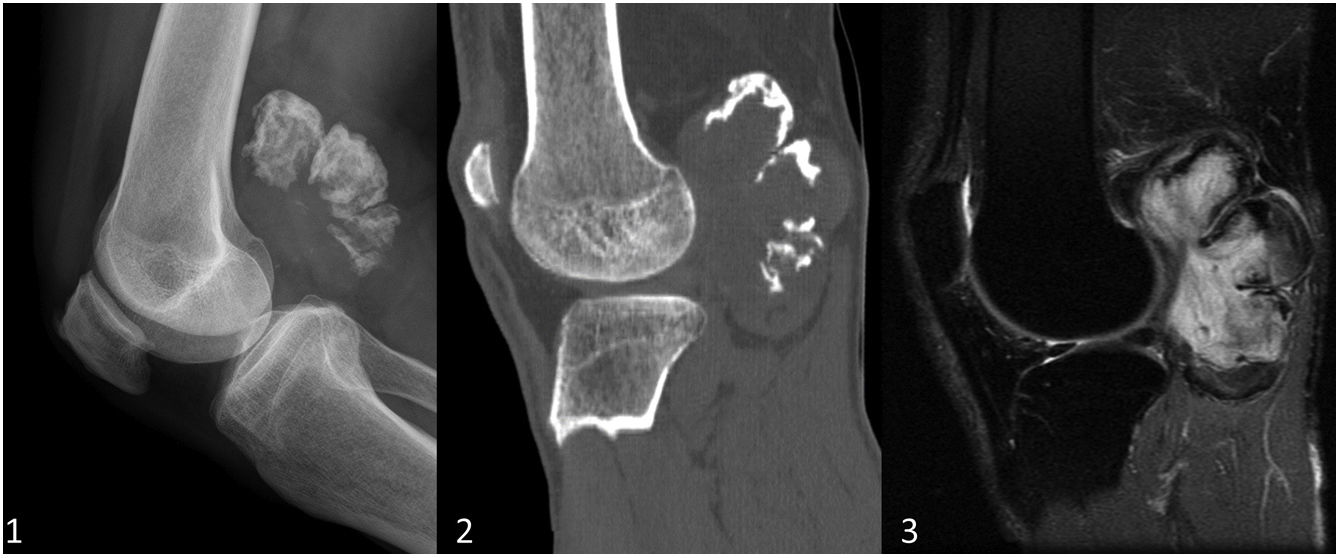
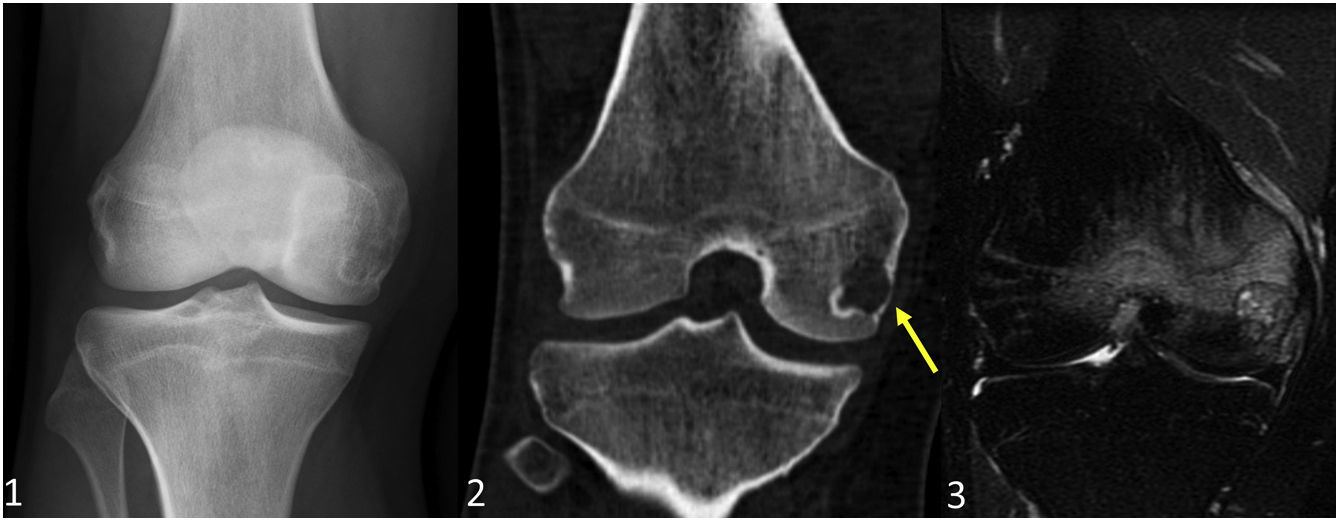
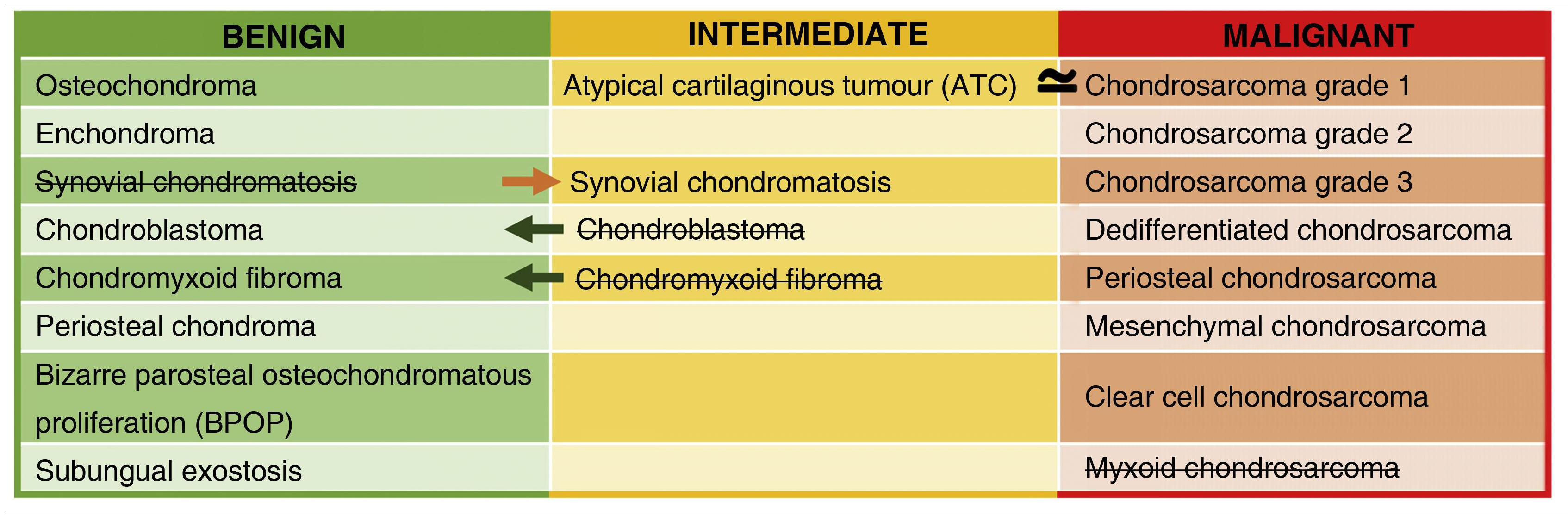
In the WHO classification, cartilaginous bone tumours are described as benign, intermediate (locally aggressive and/or rarely metastasising) or malignant. Table 1 shows the changes introduced in 2020 compared to the 2013 classification. The most notable change is the introduction of a new entity: the atypical cartilaginous tumour (ATC).2–5 Another addition is synovial chondromatosis, included in the intermediate group due to its locally aggressive growth and high local recurrence rate (3–23%). Malignant transformation to chondrosarcoma occurs in 5% of cases and manifests as multiple recurrences and bone marrow invasion (Fig. 1).2,6
Synovial chondromatosis in a 66-year-old man with a persistent popliteal tumour. Both the lateral radiograph (1) and the sagittal bone window CT image (2) of the right knee show a polylobulated mass in the popliteal region with coarse predominantly peripheral and some intralesional cerebriform calcifications. On the sagittal T2-weighted MR image (3) the lobular growth mass shows intense hyperintensity suggestive of cartilaginous origin. No bone erosion or joint effusion is identified.
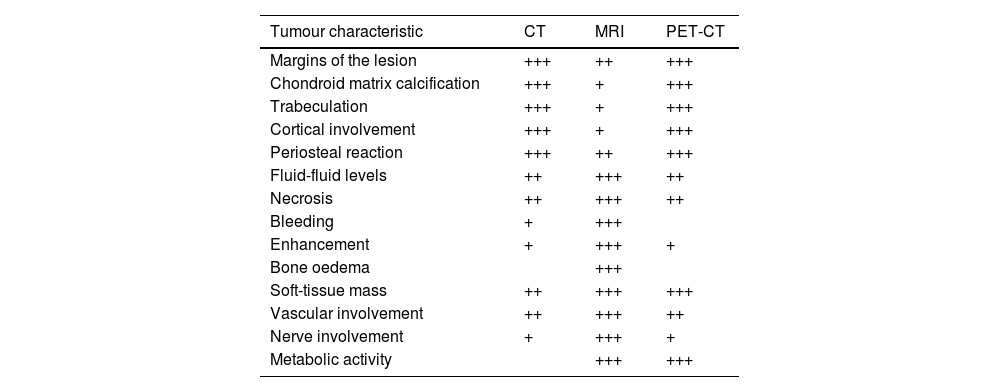
The chondroid matrix is the cardinal sign in the various imaging techniques (Fig. 2).1,7,8 A well-differentiated chondroid matrix is characterised by lobular growth and characteristic ‘rings and arcs’ or popcorn calcification, which are the result of endochondral ossification. On MRI, the non-mineralised chondroid matrix characteristically shows signal hyperintensity in T2-weighted sequences due to its high water content (Fig. 2).4,5 After contrast administration, lobulated or septal enhancement is observed in rings and arcs due to the fact that the cartilage is irrigated by septal capillaries and the perichondrium.9 Once the diagnosis of a cartilage tumour has been established, the next step is to determine its degree of aggressiveness. Table 2 shows the main imaging findings to be evaluated in the study of cartilage tumours and the most appropriate test for this purpose.
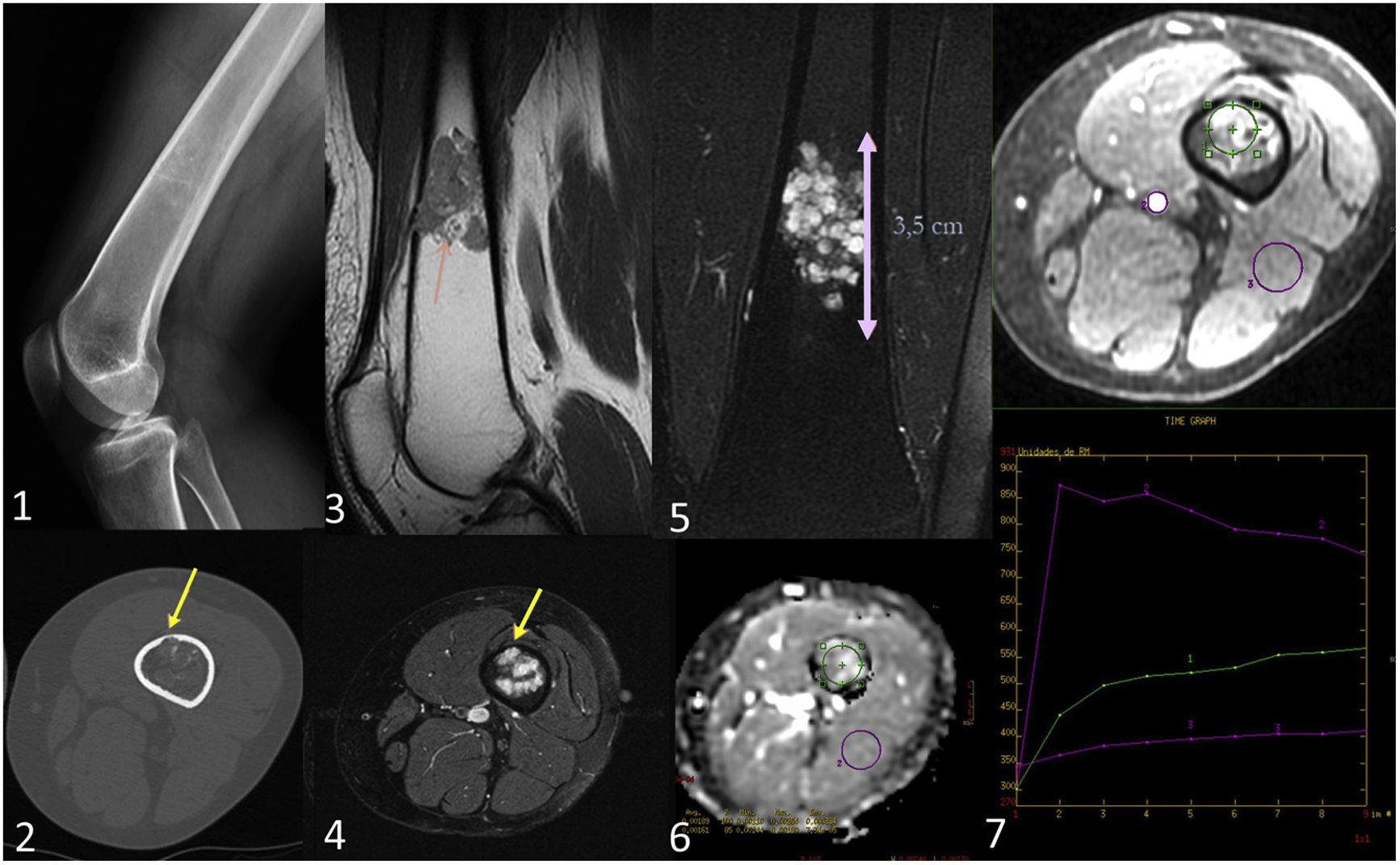
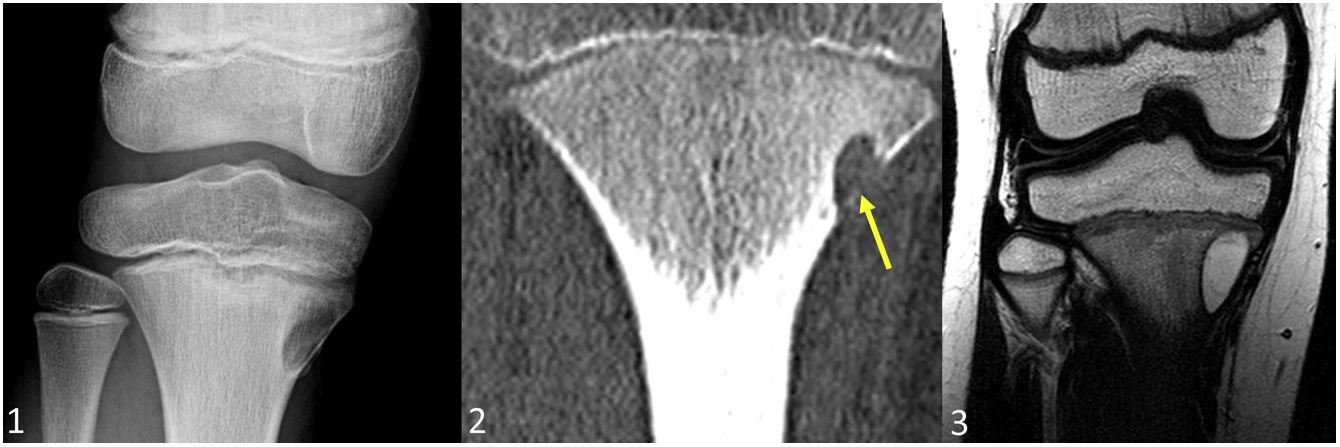
Enchondroma in a 46-year-old woman. Lateral radiograph of the knee (1) shows a lytic 1B lesion with scarce mineralisation in rings and arcs. In the axial bone window CT image (2) the lesion reveals endosteal scalloping of less than two thirds of cortical thickness (yellow arrows). In the PD MRI sequence (3) the lesion displays a typical lobular architecture of hyaline cartilage, while in the T2FS (4) and STIR (5) sequences, the non-mineralised components of the chondroid matrix exhibit high signal intensity. In the DWI/ADC sequence (6) no restriction is visible in the images (ADC = 1.89 × 10–3). After contrast administration, a homogeneous, slow and mild contrast enhancement is observed. The perfusion curve shows enhancement with a type II curve (ROI reference in the popliteal artery).
Comparison of tomographic techniques for the assessment of radiological features of cartilaginous tumours.
| Tumour characteristic | CT | MRI | PET-CT |
|---|---|---|---|
| Margins of the lesion | +++ | ++ | +++ |
| Chondroid matrix calcification | +++ | + | +++ |
| Trabeculation | +++ | + | +++ |
| Cortical involvement | +++ | + | +++ |
| Periosteal reaction | +++ | ++ | +++ |
| Fluid-fluid levels | ++ | +++ | ++ |
| Necrosis | ++ | +++ | ++ |
| Bleeding | + | +++ | |
| Enhancement | + | +++ | + |
| Bone oedema | +++ | ||
| Soft-tissue mass | ++ | +++ | +++ |
| Vascular involvement | ++ | +++ | ++ |
| Nerve involvement | + | +++ | + |
| Metabolic activity | +++ | +++ |
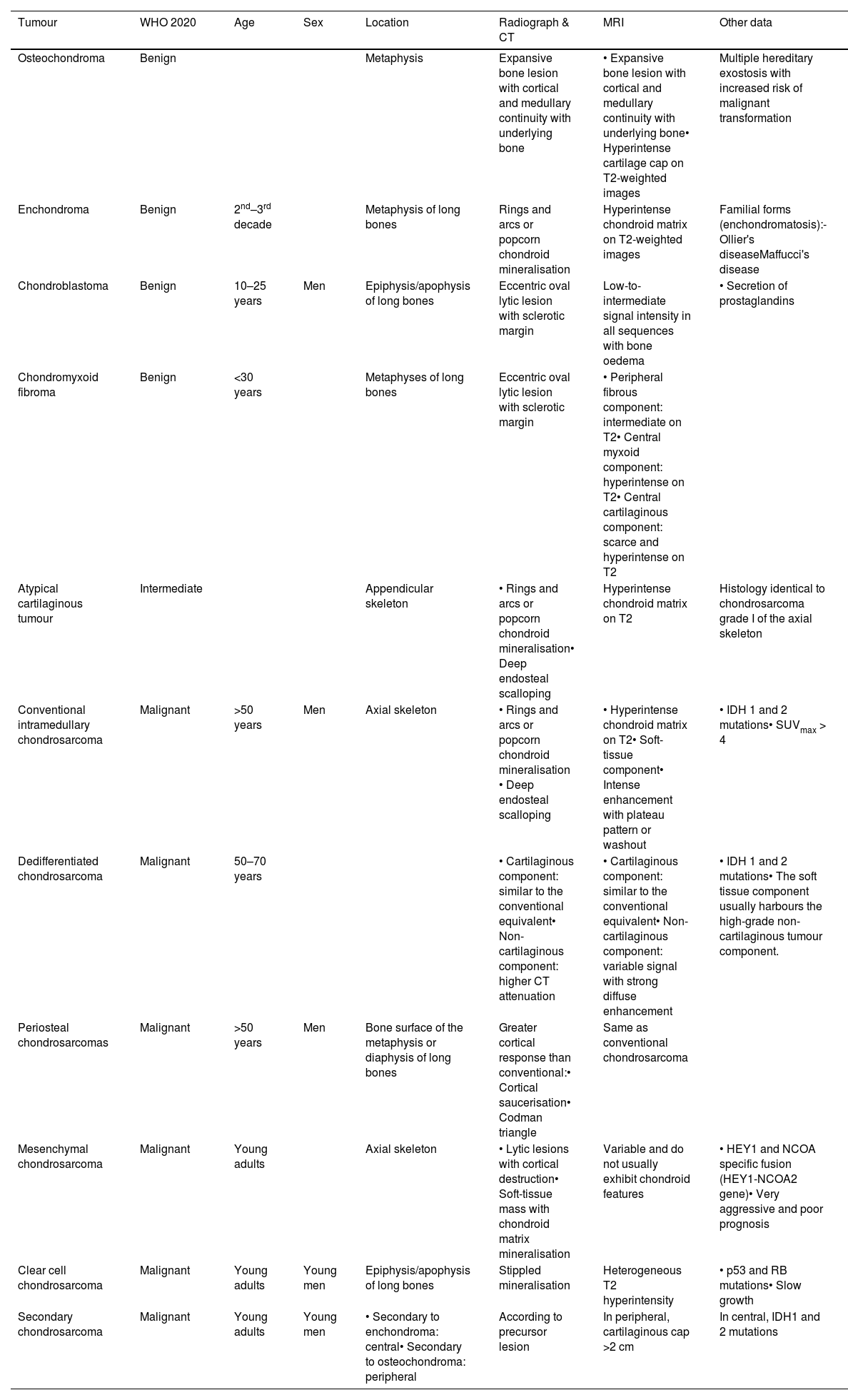
Despite extensive research efforts, the differentiation of enchondromas from ATC/chondrosarcomas remains a challenge, not only for radiologists but also for pathologists. Therefore, the aim of this paper is to review the most common cartilaginous tumours (Table 3), and emphasise the differences between enchondromas and the different types of chondrosarcoma.
Clinical-radiological characteristics of the cartilaginous tumours described in the article.
| Tumour | WHO 2020 | Age | Sex | Location | Radiograph & CT | MRI | Other data |
|---|---|---|---|---|---|---|---|
| Osteochondroma | Benign | Metaphysis | Expansive bone lesion with cortical and medullary continuity with underlying bone | • Expansive bone lesion with cortical and medullary continuity with underlying bone• Hyperintense cartilage cap on T2-weighted images | Multiple hereditary exostosis with increased risk of malignant transformation | ||
| Enchondroma | Benign | 2nd–3rd decade | Metaphysis of long bones | Rings and arcs or popcorn chondroid mineralisation | Hyperintense chondroid matrix on T2-weighted images | Familial forms (enchondromatosis):- Ollier's diseaseMaffucci's disease | |
| Chondroblastoma | Benign | 10–25 years | Men | Epiphysis/apophysis of long bones | Eccentric oval lytic lesion with sclerotic margin | Low-to-intermediate signal intensity in all sequences with bone oedema | • Secretion of prostaglandins |
| Chondromyxoid fibroma | Benign | <30 years | Metaphyses of long bones | Eccentric oval lytic lesion with sclerotic margin | • Peripheral fibrous component: intermediate on T2• Central myxoid component: hyperintense on T2• Central cartilaginous component: scarce and hyperintense on T2 | ||
| Atypical cartilaginous tumour | Intermediate | Appendicular skeleton | • Rings and arcs or popcorn chondroid mineralisation• Deep endosteal scalloping | Hyperintense chondroid matrix on T2 | Histology identical to chondrosarcoma grade I of the axial skeleton | ||
| Conventional intramedullary chondrosarcoma | Malignant | >50 years | Men | Axial skeleton | • Rings and arcs or popcorn chondroid mineralisation • Deep endosteal scalloping | • Hyperintense chondroid matrix on T2• Soft-tissue component• Intense enhancement with plateau pattern or washout | • IDH 1 and 2 mutations• SUVmax > 4 |
| Dedifferentiated chondrosarcoma | Malignant | 50–70 years | • Cartilaginous component: similar to the conventional equivalent• Non-cartilaginous component: higher CT attenuation | • Cartilaginous component: similar to the conventional equivalent• Non-cartilaginous component: variable signal with strong diffuse enhancement | • IDH 1 and 2 mutations• The soft tissue component usually harbours the high-grade non-cartilaginous tumour component. | ||
| Periosteal chondrosarcomas | Malignant | >50 years | Men | Bone surface of the metaphysis or diaphysis of long bones | Greater cortical response than conventional:• Cortical saucerisation• Codman triangle | Same as conventional chondrosarcoma | |
| Mesenchymal chondrosarcoma | Malignant | Young adults | Axial skeleton | • Lytic lesions with cortical destruction• Soft-tissue mass with chondroid matrix mineralisation | Variable and do not usually exhibit chondroid features | • HEY1 and NCOA specific fusion (HEY1-NCOA2 gene)• Very aggressive and poor prognosis | |
| Clear cell chondrosarcoma | Malignant | Young adults | Young men | Epiphysis/apophysis of long bones | Stippled mineralisation | Heterogeneous T2 hyperintensity | • p53 and RB mutations• Slow growth |
| Secondary chondrosarcoma | Malignant | Young adults | Young men | • Secondary to enchondroma: central• Secondary to osteochondroma: peripheral | According to precursor lesion | In peripheral, cartilaginous cap >2 cm | In central, IDH1 and 2 mutations |
Osteochondromas are the most common benign tumours of the bones (20–50%) and result from the separation of a fragment of epiphyseal cartilage from the growth plate, which, after it has grown and enchondral ossification has occurred, leads to a subperiosteal bony outgrowth that protrudes from the cartilage-capped bone surface. This cartilaginous cap is what defines osteochondromas as nosological entities and differentiates them from other types of bone exostosis.1,10,11 Osteochondromas enlarge from growth at the cartilaginous cap, and typically show no further growth after adolescence and skeletal maturity. Growth of the lesion or cartilaginous cap beyond this point suggests malignant transformation.10–12
Osteochondromas are usually found in the metaphysis or metaphysis-diaphysis and have a predilection for the knee (especially the distal femur) and the proximal humerus. They may have a sessile or pedunculated morphology and tend to grow towards the diaphysis.
Imaging shows an expansive bone lesion with cortical and medullary continuity with the underlying bone. When the cartilaginous cap has a mineralised matrix, it can be detected on radiographs (Fig. 3).3 A cartilaginous cap thickness >3 cm in children and >2 cm in adults is considered suspicious for malignant transformation, with sensitivity, specificity, positive and negative predictive values of more than 90%.10,11 MRI is the technique of choice to measure cartilaginous cap thickness on T2-weighted images and to assess soft tissues. Ultrasound is useful for superficial lesions, especially in children and adolescents.10,11
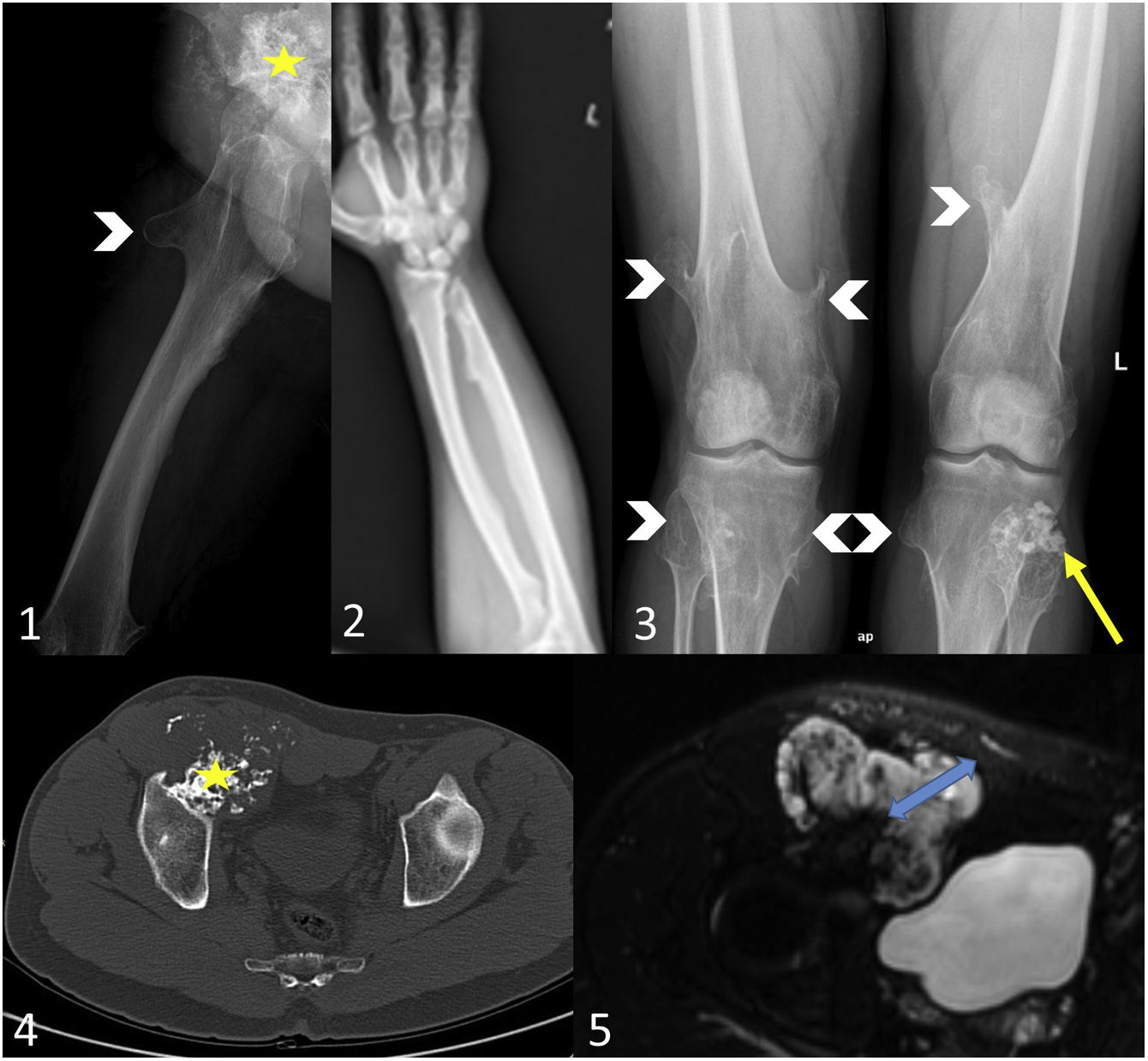
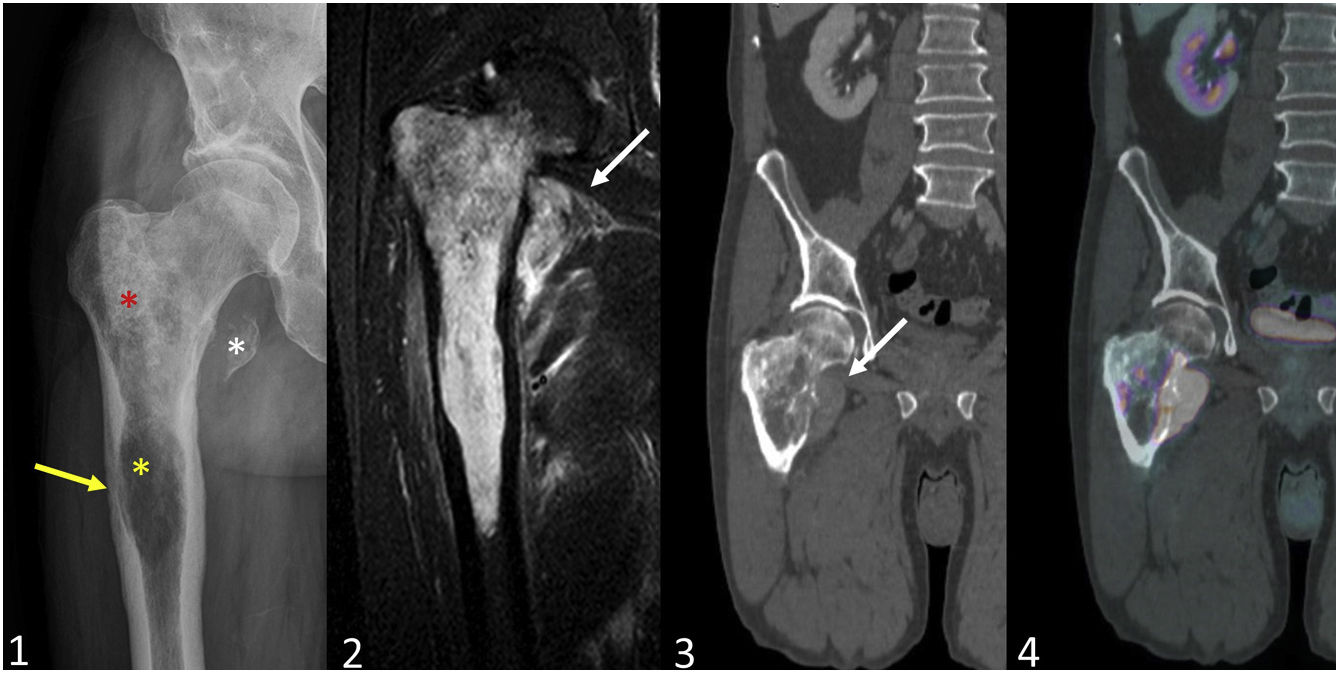
Secondary peripheral chondrosarcoma in a patient with hereditary multiple exostosis. A 31-year-old man presented with a right inguinal tumour with paraesthesia and venous ectasia of the right lower limb. The axial radiograph of the right hip/femur (1) shows an exophytic mass of 10 cm dependent on the anterior wall of the right acetabulum (yellow star), as well as an exostosis in the proximal femur (white arrowhead). On the bone window CT scan (4) the lesion exhibits cortical and medullary continuity (yellow star) and a soft-tissue mass with rings and arcs mineralisation. In the axial STIR MR image (5) the cartilaginous cap is 4 cm thick (blue arrow). Findings indicate osteochondroma with malignant degeneration to secondary peripheral chondrosarcoma. In view of the suspicion of hereditary multiple exostosis, a bone series was performed (2,3), in which the right ulna appears shortened with a deformity of the diaphysis of the radius (pseudo-Madelung) and metaphyseal flaring of both distal femurs (Erlenmeyer flask deformity), as well as exostoses in the right radius and ulna, on the anterior surface of the right proximal femur, on both distal femurs, both proximal tibiae and right proximal fibula (white arrowheads) and an osteochondroma on the head of the left fibula with rings and arcs mineralisation of the cartilaginous cap (yellow arrow).
Hereditary multiple exostosis (HME) is also referred to as osteochondromatosis, diaphyseal aclasis, hereditary multiple osteochondromas or multiple osteochondromas. HME is an autosomal inherited syndrome characterised by the coexistence of osteochondromas, bone exostoses and bone deformities, such as epiphyseal-metaphyseal flaring of the long bones (Erlenmeyer flask deformity) or shortening of the ulna (pseudo-Madelung deformity) (Fig. 3). In our experience, a shortened ulna was observed in 3 of the 12 patients with HME in our institution, which is a lower prevalence than that found in the literature, namely 30–74%.13,14 In HME, osteochondromas have a higher risk of malignant transformation (2–5%).10,12
EnchondromasEnchondromas are common cartilaginous tumours (3–17% of all bone tumours), being the second most common benign bone tumour (12–14%) after osteochondromas. While they can be diagnosed at all ages, there is a predilection for the second and third decade of life.1,15–18
Enchondromas are hypocellular tumours made up of chondrocytes surrounded by a mature hyaline matrix. They are not cytologically atypical.15
They are most commonly found in the central medullary region in the metaphysis of long bones (very rarely in the epiphyseal region), especially in the proximal humerus and the distal and proximal femur, and in the diaphysis of short tubular bones of the hands and feet.1,16 The central location in the metaphysis of long bones suggests that the enchondromas are the result of the continued growth of benign cartilaginous debris displaced from the growth plate.18 Enchondromas in flat bones are the exception (6%), and are most commonly found in the pelvis and ribs.19
Conventional radiography and CT identify geographic lytic lesions using the Lodwick classification, in which type 1A represents sclerotic margin and 1B a well-marginated non-sclerotic margin. The characteristic ‘rings and arcs’ or popcorn mineralisation of the chondroid matrix is best assessed using CT.20 Due to their slow expansive lobular growth, enchondromas can cause cortical thinning known as endosteal scalloping, affecting up to a third of the cortical thickness; any more would indicate a more aggressive lesion.7,10 Endosteal scalloping can be assessed with conventional radiography, CT and MRI, but CT is the test of choice. On MRI they appear as markedly hyperintense lobular lesions on T2-weighted sequences with more or less hypointense calcification on all sequences. In the diffusion study of enchondromas, it should be remembered that well-differentiated cartilaginous tissue does not present significant restriction of water diffusion and that mineralisation of the lesion can constitute a significant artefact. Therefore, diffusion imaging has not been shown to be useful in differentiating between enchondromas, ATCs and chondrosarcomas, but may be helpful in identifying a dedifferentiated tumour component where applicable, and in demonstrating that the lesion is not an enchondroma.21–23 After the administration of contrast media, enhancement is mild peripheral or transseptal, while in the dynamic study a progressive increase in enhancement (type II curve) is more frequently observed, suggestive of benignity, which differs from the plateau pattern (type III) or early enhancement (types IV and V), considered malignant. However, the ability to differentiate between enchondromas and low-grade chondrosarcomas is limited1,7,24 (Fig. 2). In 18F-FDG-PET/CT there is a significant overlap between the values2–4,5 of enchondromas and ATCs/chondrosarcomas grade 1.25,26
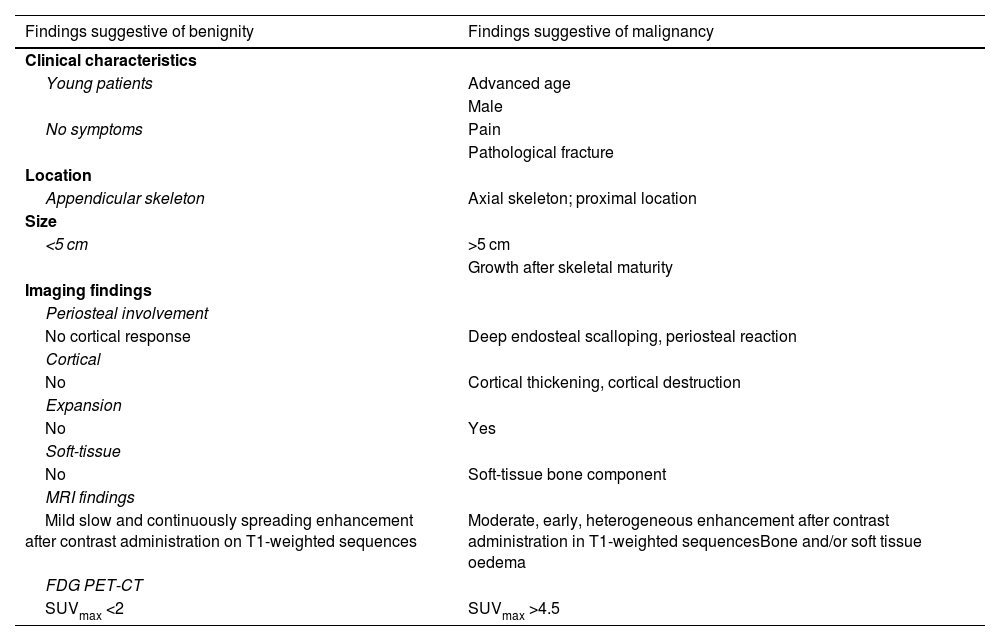
Malignant progression occurs in 1–4% of solitary enchondromas and 20–60% of multiple enchondromas.1,12,15 Malignant transformation should be suspected in enchondromas that become painful or grow continuously after skeletal maturity. Other factors that raise suspicion of malignancy are: an epiphyseal-metaphyseal or axial skeletal location, large tumour size (>5 cm), presence of deep endosteal scalloping, bone oedema or soft tissue enlargement (Table 4).1,7,8,18,21,22,27,28
Clinical and radiological differences between benign and malignant cartilaginous tumours.
| Findings suggestive of benignity | Findings suggestive of malignancy |
|---|---|
| Clinical characteristics | |
| Young patients | Advanced age |
| Male | |
| No symptoms | Pain |
| Pathological fracture | |
| Location | |
| Appendicular skeleton | Axial skeleton; proximal location |
| Size | |
| <5 cm | >5 cm |
| Growth after skeletal maturity | |
| Imaging findings | |
| Periosteal involvement | |
| No cortical response | Deep endosteal scalloping, periosteal reaction |
| Cortical | |
| No | Cortical thickening, cortical destruction |
| Expansion | |
| No | Yes |
| Soft-tissue | |
| No | Soft-tissue bone component |
| MRI findings | |
| Mild slow and continuously spreading enhancement after contrast administration on T1-weighted sequences | Moderate, early, heterogeneous enhancement after contrast administration in T1-weighted sequencesBone and/or soft tissue oedema |
| FDG PET-CT | |
| SUVmax <2 | SUVmax >4.5 |
Enchondromas are the most common type of bone tumours in the short tubular bones of the hands and feet, while chondrosarcomas are very rare. In this location, matrix mineralisation is uncommon and deep endosteal scalloping may be present, so this feature should not be used to differentiate enchondromas from chondrosarcomas.7,18 They often present in hands and feet through pathological fractures.
EnchondromatosisEnchondromatosis, in which patients develop multiple enchondromas, is a very rare disease (1:100,000). It affects the metaphysis and diaphysis of long and short tubular bones and usually occurs before the second decade of life. The most common subtypes are Ollier disease, with multiple enchondromas and an asymmetrical distribution, and Maffucci syndrome, which also features soft-tissue vascular malformations such as haemangiomas. The vast majority of cases are not hereditary, but up to 90% of cases involve mutations in the isocitrate dehydrogenase 1 or 2 (IDH 1/2) gene, although these mutations are also observed in up to 50% of solitary enchondromas.29
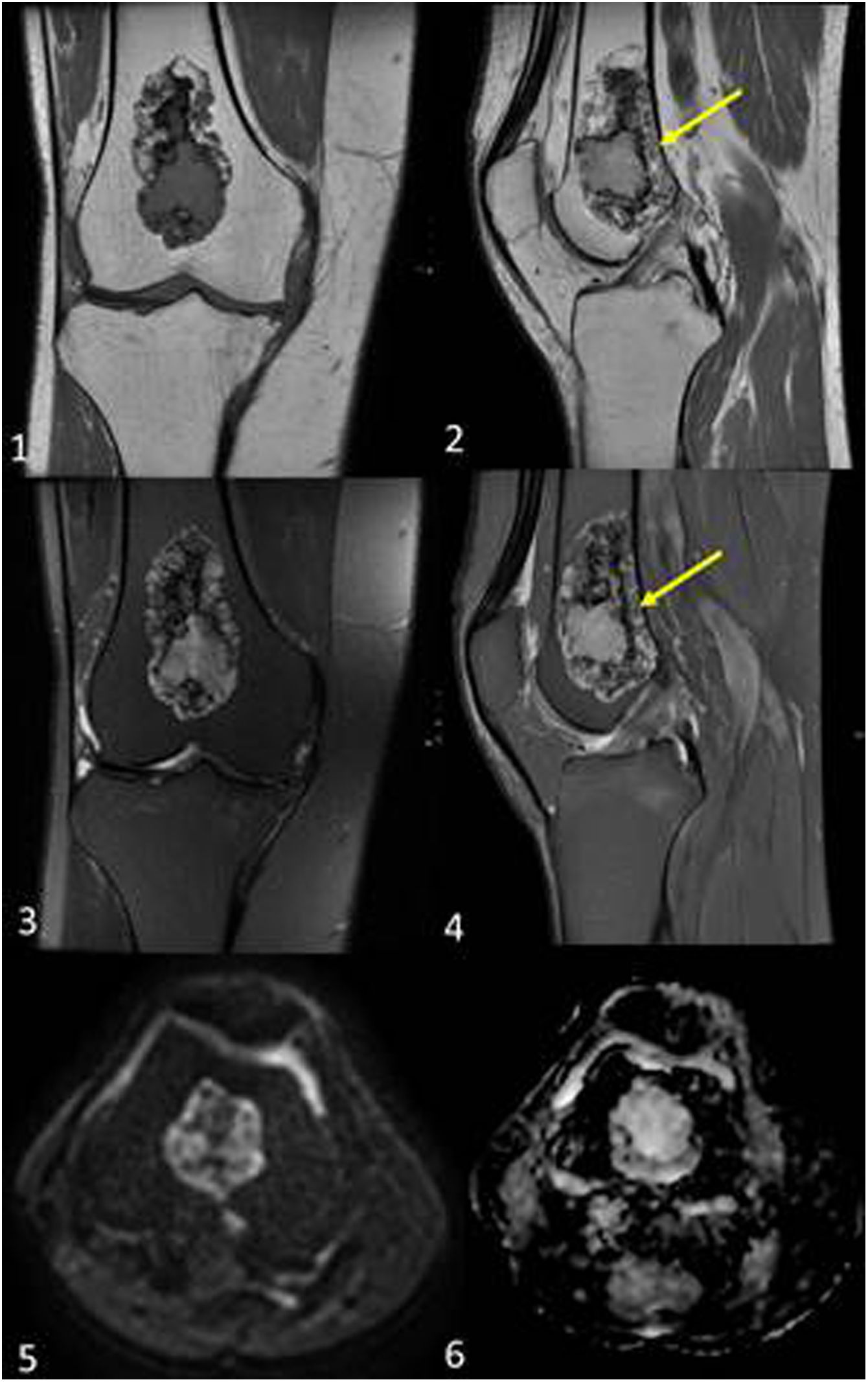
It is not uncommon for these tumours to develop malignant degeneration in the long term. The rate of degeneration in Maffucci syndrome is up to 60% higher than in Ollier's disease, for which the rate is 20–50% (Fig. 4).12,15
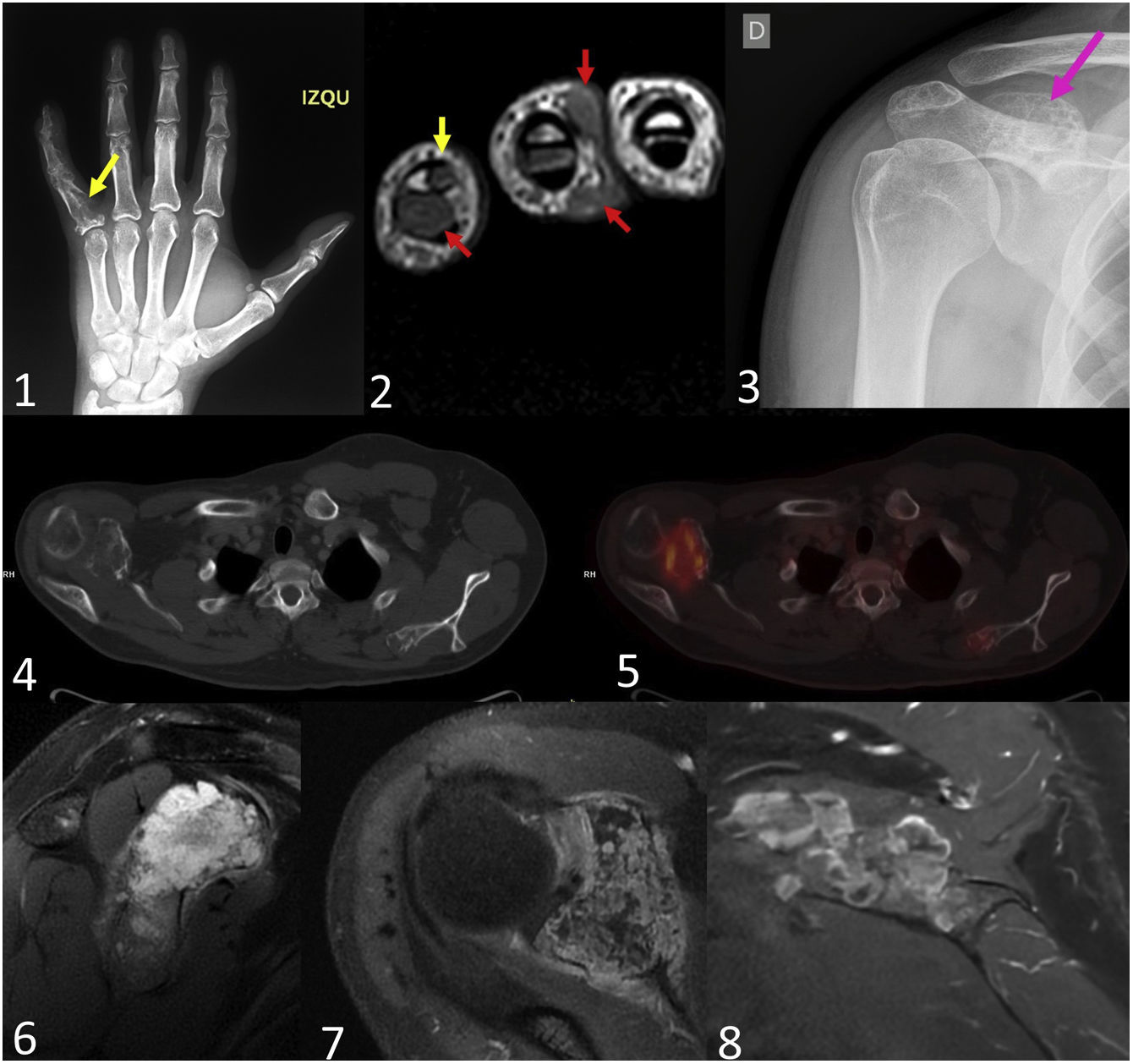
Secondary central chondrosarcomas in a 28-year-old male with Maffucci syndrome. The patient presented with a tumour on the proximal phalanx of the 5th finger of the left hand. Posteroanterior radiograph (1) shows an enchondroma (yellow arrow). In the MRI of the hand (2), in addition to multiple enchondromas (yellow arrow), multiple small subcutaneous lesions were identified, which extensively involved the dermal plane, compatible with haemangiomas (red arrows). Multiple diffusely distributed enchondromas were identified in the bone series. In the right scapula (3) there is a glenoid-dependent outgrowth bone lesion with rings and arcs calcification (pink arrow), which shows significant endosteal scalloping in the CT scan with bone window (4). T2-weighted MRI sequences (6) identify the chondroid matrix of the lesion and, after contrast administration (7), intense septal enhancement is identified. On PET-CT (5) the lesion had an SUVmax of 6.2, while another chondroid-like lesion was identified in the left scapula with an SUVmax of 2.7. The patient underwent surgery on the right lesion, which was diagnosed as a secondary central chondrosarcoma grade 2. Two years later, the lesion grew on the left scapula and the SUVmax increased to 4.7, suggestive of malignant transformation. Post-contrast MR images of the left scapula (8) show postseptal enhancement. The final histological diagnosis was secondary central metachronous chondrosarcoma grade 1.
Chondroblastomas, now included in the benign tumour group, are cartilaginous tumours located in the epiphysis or apophysis of long tubular bones, which distinguishes them from most other bone tumours. They are rare (<1%) and seldom metastasise (<2%), especially in cases of recurrence. They predominantly affect young males (10–25 years) in the distal femur or proximal humerus.1,30
Chondroblastomas are characterised by the proliferation of immature cartilage cells, which secrete prostaglandins that produce local pain, and a focal formation of cartilage matrix.1,30
Conventional radiography and CT show type 1A or type 1B geographic lytic lesions in eccentric locations in the epiphyses of the long bones.20,30 In 30–50% of chondroblastomas, the internal structure of the lesion exhibits mottled calcification.1,30 On MRI, chondroblastomas are heterogeneously hypointense on T1-weighted sequences and heterogeneously hypointense and partially hyperintense on T2-weighted sequences. Due to the production of prostaglandins, they are almost always associated with bone and soft tissue oedema, and joint effusion where adjacent to joints1,7,30,31 (Fig. 5). Areas of haemorrhagic-cystic degeneration may appear as T2 signal hyperintensity, which corresponds to cystic degeneration that is more or less heterogeneous depending on the haemorrhagic content. In the acute phase of bleeding, it is possible to detect foci of signal hyperintensity in T1-weighted sequences, while in the chronic phase, the gradient echo sequence is very sensitive to the paramagnetic effect of haemosiderin.30
Chondroblastoma in a 19-year-old male with right-sided knee pain. The anteroposterior radiograph (1) shows an eccentric focal bone lesion in the distal epiphysis of the femur, with an incomplete margin and mottled mineralisation inside. The coronal CT image with bone window (2) shows slight cortical thinning (yellow arrow). In the coronal T2FS sequence (3) the lesion is markedly hyperintense with a linear hypointense component corresponding to the chondroid matrix mineralisation, and is associated with significant oedema in the adjacent trabecular bone.
Chondromyxoid fibromas (CMFs) are also now classified as benign cartilaginous tumours. They are very rare (<1%) but with locally aggressive growth.1,30,32 They are most commonly diagnosed before the age of 30, principally during the second decade of life.30
They consist of three tissue components: a peripheral fibrous matrix, a central myxoid matrix and a central chondroid matrix.1,32 They are usually located in the medulla of the metaphyses, and less frequently in the medulla of the diaphyses of the long tubular bones. Approximately 50% occur in the knee joint.1,30
The images show an eccentrically located, oval, geographic lytic lesion with a sclerotic margin and its longitudinal axis parallel to the axis of the bone (Fig. 6). Its expansive nature is manifested by cortical thinning and insufflation.30,32 Its appearance on MRI varies depending on the percentage of each of the components. The peripheral fibrous component exhibits intermediate signal on T2-weighted images and nodular enhancement on post-contrast images. The central myxoid component presents as marked signal hyperintensity on T2-weighted images and does not usually show up as enhancement on images.32 The amount of cartilage is usually minimal and mineralisation is rare (<10%).30,32 Haemorrhagic-cystic degeneration is possible.30
Chondromyxoid fibroma in a 10-year-old male with right knee pain. The anteroposterior radiograph shows an eccentric focal lesion type 1B in the proximal metaphysis of the right tibia with scarce mineralisation. The coronal CT image with bone window (2) highlights the expansive nature of the lesion with cortical thinning (yellow arrow). The coronal T2-weighted MR image (3) shows marked homogeneous central signal hyperintensity corresponding to the myxoid component.
Enchondromas and ACTs/chondrosarcomas grade 1 are difficult to differentiate anatomopathologically, because they have overlapping histological features. Lobules of hyaline cartilage surrounded by bone tissue and low cellularity are observed in both entities, the most significant difference being that ACTs/chondrosarcomas grade 1 invade the medullary spaces of the spinal cord.4,7 ACTs and chondrosarcomas grade 1 show no histological differences, but are instead differentiated by their location:
- •
In the appendicular skeleton, they are classified as ACTs (intermediate group) (Fig. 7).
Figure 7.Atypical cartilaginous tumour in a 79 year-old woman with right knee pain. The lateral radiograph shows a focal bony lesion in the right distal femoral metaphysis. Coronal T1 (1) and sagittal DP (2) MRI sequences show a lobular lesion with a maximum diameter >5 cm and cortical endosteal scalloping greater than two thirds of the cortical thickness (yellow arrows). Coronal T2FS (3) and sagittal PDFS (4) sequences confirmed the cartilaginous aetiology of the lesion by identifying a characteristically hyperintense lobular chondroid matrix with hypointense foci corresponding to chondroid matrix mineralisation. Diffusion imaging showed no restriction (5,6).
- •
In the axial skeleton (including the pelvis and skull base), they are designated as chondrosarcomas grade 1 (malignant group) (Figs. 8 and 9).
Figure 8.Chondrosarcoma grade 1 of the rib cage in a 38-year-old male. Posteroanterior radiograph (1) shows a lytic lesion dependent on the anterior costal arch of the left eighth rib (red asterisk). The CT scan (2) shows a solid nodular and expansive lesion dependent on the anterior costal arch of the left eighth rib, with small ring and arch calcifications, suggestive of a chondroid tumour (red asterisk). On MRI T2-weighted sequences (3) the lesion shows hyperintense chondroid matrix with sharp lobulated contours and hypointense linear foci. Mild transseptal enhancement is seen after contrast administration (4).
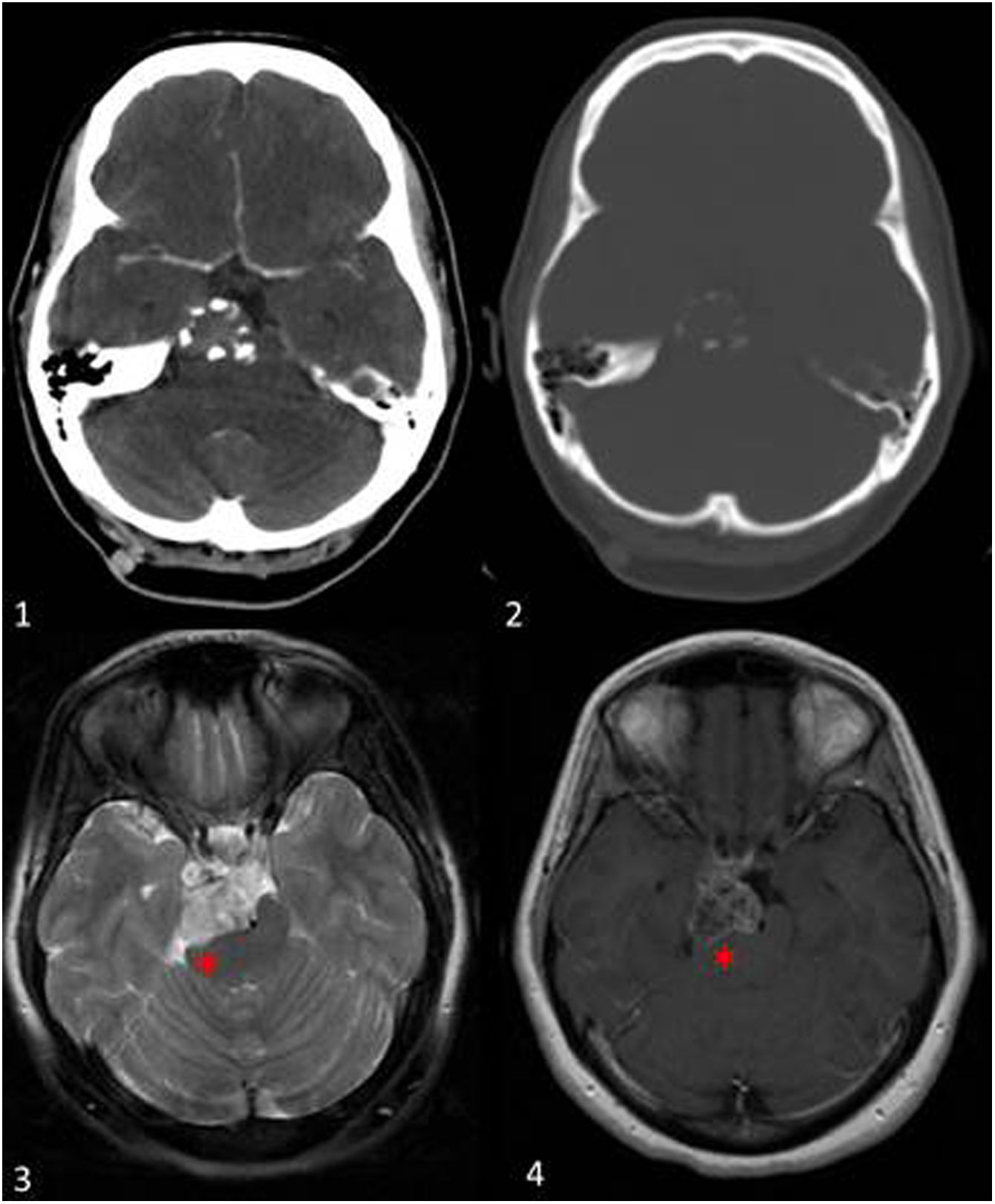
Figure 9.Chondrosarcoma grade 1 at the base of the skull. A 40-year-old woman presented with a drug-resistant headache and a CT scan was performed, identifying a lesion in the right petroclival region, which exerted a significant mass effect on the right cerebral peduncle (red asterisk). Axial soft tissue CT scan after contrast administration (1) shows slight enhancement of the lesion, and in the bone window (2) small peripheral punctate calcifications. On MRI the lesion shows heterogeneous hyperintense signal in T2-weighted sequences (3). After administration of intravenous contrast (4), slight transeptal enhancement is observed.
The purpose of this distinction is to highlight the different behaviours and prognoses despite their having the same histology: ACTs are locally aggressive and rarely metastasise, so they are classified as intermediate tumours.
ChondrosarcomasChondrosarcomas are the third most common primary malignant bone tumour (20–27%) after multiple myelomas and osteosarcomas.7 Chondrosarcomas that arise de novo are referred to as primary, while those that develop from benign cartilage neoplasms, such as enchondromas or osteochondromas, are secondary. Chondrosarcomas are also classified as central or peripheral depending on their location.
The new WHO classification does not recognise myxoid chondrosarcomas of bone as a specific entity. It does, however, refer to prominent myxoid changes of conventional high-grade chondrosarcomas. Extraskeletal myxoid chondrosarcomas are not currently classified as chondrosarcomas, but rather as very rare (<1/1,000,000) mesenchymal neoplasms as there is no evidence of cartilaginous differentiation. They arise principally in the deep soft tissue of the proximal extremities, and the name has only been retained for historical reasons.2,3,33,34
A. Primary chondrosarcomasPrimary chondrosarcomas constitute a heterogeneous group of tumours with numerous categories, including conventional intramedullary (80–85%) and less frequent subtypes (dedifferentiated, juxtacortical, mesenchymal and clear cell). Chondrosarcomas are almost always symptomatic. They can metastasise, often late, mainly to the lungs.
Conventional intermedullary chondrosarcomasAlthough conventional chondrosarcomas can occur at any age, they tend to occur in older people (>50 years).
In some locations, Chondrosarcoma grade 1 or ACTs present predominantly chondroid stromas with greater cellularity than enchondromas and which infiltrate the medullary cord. Chondrosarcomas grade 2 present less chondroid and more myxoid stroma and there may be small foci of necrosis. Therefore, they present greater cellularity, predominantly in the periphery, and greater atypia, but mitoses are only occasionally identified. Chondrosarcomas grade 3 have higher cellularity and nuclear pleomorphism than the other grades, the chondroid matrix is scarce or absent, the small amount of intercellular material is usually myxoid and there are extensive foci of necrosis.7,15 Histological grading is relevant for prognosis and treatment management, as local recurrence is more frequent in chondrosarcomas grades 2 and 3. In some cases, highly malignant degeneration may also occur.
Histologically, conventional chondrosarcomas contain chondroblastic differentiations, for which reason specifying the IDH1 or 2 mutation is crucial when diagnosing chondroid tumours as this differentiates them from chondroblastic osteosarcomas. It does not however provide information on the level of malignancy of the lesions.15,29
Conventional radiographyConventional chondrosarcomas show a mixed appearance, with the lytic component showing a multilobulated geographic pattern and 60–80% of the sclerotic areas exhibit the characteristic ‘rings and arcs’ or popcorn mineralisation of the chondroid matrix. More aggressive patterns, such as permeative or moth-eaten appearances, can be seen in conventional high-grade chondrosarcomas, but are more common in mesenchymal or dedifferentiated subtypes. High-grade chondrosarcomas usually involve less extensive areas of mineralisation.
Cartilaginous tumours usually grow with a lobular architecture, leading to endosteal scalloping, which, if it measures more than two thirds of the cortical thickness, is considered deep and is highly indicative of chondrosarcoma.7,18 According to Murphey et al., the depth of endosteal scalloping is the best distinguishing feature between enchondromas and chondrosarcomas.7,18 Longitudinal extension of endosteal scalloping in long bones (>2/3 of the lesion length) is also suggestive of chondrosarcoma. Should lobular growth continue, cortical destruction and soft-tissue mass would occur18 alongside endosteal scalloping. The presence or absence of periosteal reaction depends on the rate of tumour growth.7,18
Computed tomographyCT is a superior tomographic imaging technique to conventional radiography for detecting periosteal reaction and chondroid matrix mineralisation, as well as for assessing endosteal scalloping, because it enables axial assessment of the lesional bone.
Intraosseous and extraosseous non-mineralised components usually have low attenuation, due to the high water content of hyaline cartilage. Mild enhancement is seen peripherally or across the septum after the administration of contrast media. However, higher-grade lesions with higher cellularity and lower water content may show greater attenuation and more prominent diffuse or nodular enhancement.
Magnetic resonanceMRI is the best technique for studying the extent of spinal cord involvement, the presence and extent of peritumoural oedema and soft-tissue mass. The depth and extent of endosteal scalloping is well assessed and while cortical response and chondroid matrix mineralisation can be determined with MRI, it is best evaluated with conventional radiography or CT. The non-mineralised components of the hyaline cartilage show marked signal hyperintensity in T2-weighted sequences, reflecting its high water content, and the mineralised chondroid matrix exhibits signal hypointensity in all sequences.
The presence of a soft-tissue mass (20–30%) is indicative of the level of aggressivity and practically excludes a diagnosis of enchondroma.7,9,18,28 Post-contrast enhancement is typically faint, peripheral and septal, consistent with the hypoxic and hypovascular nature of cartilaginous tumours.9 Post-contrast MRI studies increase specificity for the diagnosis of chondrosarcoma and facilitate biopsy planning.1,9,10,18,23 Lesions with progressively enhancing curves (type II) are usually considered benign in aetiology, type III curves with a plateau pattern are considered malignant, while type IV curves (wash-out pattern, with early and intense enhancement and late washout) and type V curves (maintained early enhancement) are strongly suggestive of malignancy1,7,18,24 (Fig. 2 and Fig. 10). A diffusion study can be useful to distinguish between different tumour components within the lesion, as well-differentiated cartilaginous tissue does not present significant restriction of water diffusion, while areas of histological dedifferentiation do restrict diffusion, as long as there is no artefact in this sequence caused by the presence of a mineralised chondroid matrix (Fig. 7).1,21
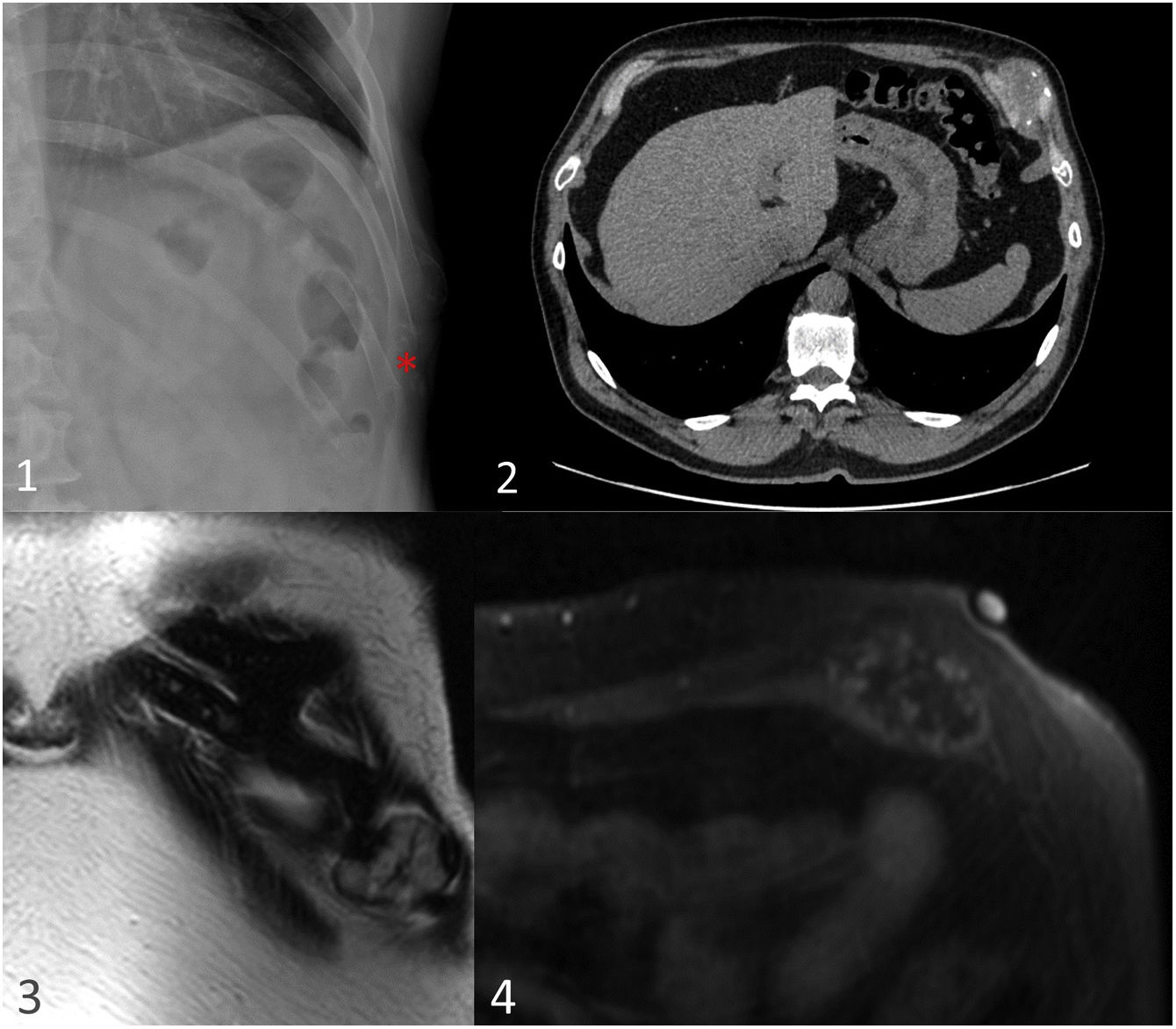
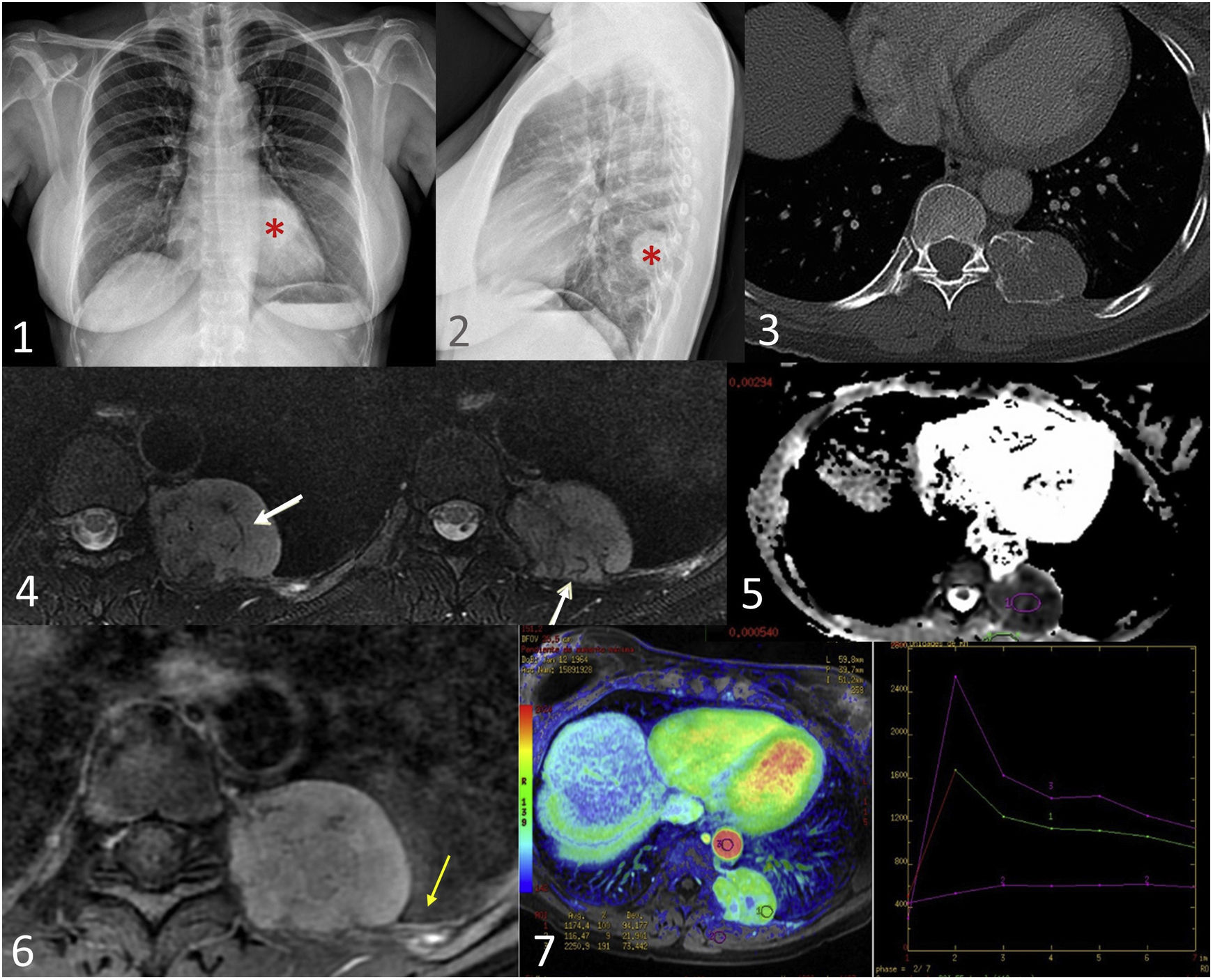
Mesenchymal chondrosarcoma in a 73-year-old woman with respiratory pathology. Posteroanterior and lateral chest radiographs (1,2) show a left paravertebral extrapulmonary mass (red asterisk). The CT scan with bone window (3) shows a mass with an insufflating appearance, located in the left sixth chondrocostal joint, with a rings and arcs calcification pattern and a significant soft-tissue component. MRI shows an expansive hyperintense lesion in T2 sequences compatible with chondroid matrix and hypointense linear foci compatible with matrix mineralisation (white arrows) (4). Diffusion sequences (5) show restriction and, after contrast administration (6), homogeneous and diffuse enhancement of the mass and adjacent pleural tissue (yellow arrow). The perfusion curve (7) shows rapid enhancement with progressive washout, highly suggestive of malignancy (type IV curve).
18F-FDG-PET/CT provides useful information on tumour biology by depicting the metabolic potential of tumours and identifying hypermetabolic foci that may suggest more aggressive behaviours. 18F-FDG-PET/CT plays an important role in the assessment of responses to neoadjuvant chemotherapy and tumour recurrence.25,26 The SUVmax value correlates with the histological grading of cartilaginous tumours, and values greater than four are indicative of malignant pathology (Figs. 4 and 11).26
Dedifferentiated chondrosarcoma in a 62 year-old male with right trochanteric bursitis. Plain radiograph (1) shows a lytic lesion of poorly defined borders on the right proximal femur with areas of chondroid matrix mineralisation in the more cranial portion (red asterisk), very scarce in the more caudal portion (yellow asterisk), and pathological fracture of the lesser trochanter (white asterisk). The lesion shows endosteal scalloping greater than two thirds of cortical thickness (yellow arrow) with extensive longitudinal involvement. The coronal STIR image (2) delineates the bony and soft-tissue extension (white arrow) of the lesion. The coronal CT image (3) shows lytic areas, chondroid matrix mineralisation and a soft-tissue mass (white arrow), and on PET-CT (4) the SUVmax was 34.7.
Dedifferentiated chondrosarcomas involve a highly malignant degeneration that occurs in 6–10% of low-grade chondrosarcomas.15 This variant consists of two phases: a low-grade/well-differentiated cartilaginous tumour component (enchondroma or ACT) with an abrupt transition to a high-grade sarcoma (most commonly osteosarcoma, undifferentiated sarcoma or fibrosarcoma).1,7 The detection of the IDH1 or IDH2 mutation, which is highly specific for chondroid tumours, is essential for the diagnosis of dedifferentiated chondrosarcomas.29
Patients with dedifferentiated chondrosarcomas tend to be older than those with conventional chondrosarcomas (50–70 years), and there are no differences between genders for prevalence.7
Imaging findings vary depending on the ratio of the low-grade cartilaginous tumour component to the high-grade sarcoma component. The low-grade cartilaginous tumour regions show the typical features of conventional chondrosarcomas. The high-grade non-cartilaginous tumour component usually manifests as increased attenuation on CT, variable signal intensity on T2-weighted sequences and significant diffuse enhancement after contrast administration (Fig. 11). A soft-tissue mass, often large, is almost always seen in areas of high-grade tumour.7
Periosteal chondrosarcomasPeriosteal chondrosarcomas, previously referred to as juxtacortical chondrosarcomas, arise from the bony surface. It is a rare subtype (2–4%), with a male predilection, but histologically identical to conventional chondrosarcoma with a variable degree of enchondral bone formation.7,15,35 They are covered by a fibrous pseudo capsule that is continuous with the underlying periosteum and is often associated with cortical erosion, while medullary involvement is unusual.7,35 They are typically located in the posterior distal metaphysis or diaphysis of the femur.
On imaging, they behave in the same way as conventional chondrosarcomas, although they exhibit a greater cortical response. The underlying cortex is usually thickened and may have associated cortical saucerisation and Codman triangles at the margins of the lesion.35
Mesenchymal chondrosarcomasMesenchymal chondrosarcomas are characterised by a biphasic histology with a malignant cartilaginous tumour component and an undifferentiated stromal component with small round, Ewing sarcoma-like cells and/or spindled cells around haemangiopericytoma-like vessels.1,7,15,36 Unlike dedifferentiated tumours, the undifferentiated stroma is interspersed with islands of well-differentiated cartilage. They involve a specific fusion of the HEY1 and NCOA genes (HEY1-NCOA2 gene) and do not exhibit IDH mutations, typical of cartilaginous tumours.29,36
Mesenchymal chondrosarcomas are very aggressive, with a strong tendency to metastasise and a poor prognosis, but they account for only 2–3% of chondrosarcomas. They can arise from bone or soft tissue, with extraskeletal chondrosarcomas accounting for 10–60%.3,15,36 They mostly affect young adults, with no gender differences in prevalence.
Unlike conventional chondrosarcomas, they most commonly affect the axial skeleton, especially the craniofacial region, and more specifically the mandible and maxilla. Other typical locations are the vertebral bodies, the ilium and the ribs (Fig. 9).7
Imaging tests can identify biphasic morphology, which helps in biopsy planning. Radiographs and CT show lytic or mixed lesions with cortical destruction, often with large soft-tissue masses and chondroid matrix calcification.36 The undifferentiated stroma with high cellularity explains why this type of chondrosarcoma behaves differently from conventional chondrosarcoma in diffusion and other sequences.1 MRI features are highly variable and do not usually present a typical chondroid appearance. The enhancement pattern may be homogeneous or heterogeneous and varies widely in the literature, but is often diffuse and more evident than in conventional chondrosarcomas.7,36 Some lesions show serpiginous, high-flowing vessels of low signal intensity, with a pattern similar to that of a solitary fibrous tumour, a feature not seen in other chondrosarcomas.7,36
Clear cell chondrosarcomasClear cell chondrosarcomas constitute a very rare low-grade variant (1%). They usually affect young adults, most commonly males (3:1).7,15 As in mesenchymal chondrosarcomas, IDH mutations are not detected, but P53 and RB alterations are.15
The most common locations are the epiphyses of the femoral and humeral heads. These locations require a differential diagnosis with chondroblastomas, which present significant perilesional oedema.1,7,15,30 Polyostotic involvement has been described, although this manifestation may represent metastatic disease.
These chondrosarcomas are slow-growing and less aggressive, with a consequent improvement in prognosis.10,31 On imaging, matrix mineralisation is not as common (30%) and they more typically present stippled calcification. In addition, a sclerotic margin, which simulates a benign lesion, is identified in up to 20% of lesions.10,31 On CT the non-mineralised areas usually show low attenuation, and on MRI the solid components show homogeneous intermediate signal intensity on T1 sequences and heterogeneous hyperintensity on T2.7,31 After contrast administration, uptake is heterogeneous, due to the absence of enhancement in the areas of matrix mineralisation.
B. Secondary chondrosarcomasSecondary chondrosarcomas account for 10–15% of chondrosarcomas and arise from the malignant transformation of pre-existing benign cartilaginous lesions: enchondromas (solitary or multiple, Ollier's disease or Maffucci's syndrome) or osteochondromas (solitary or multiple hereditary).15 Enchondromas tend to be the precursors of central secondary chondrosarcomas and osteochondromas the precursors of peripheral chondrosarcomas. In peripheral chondrosarcomas and osteochondromas, it is not IDH1 and IDH2 mutations that are detected, but rather EXT1 and EXT2 mutations that are more common.29
Malignant transformation is seen in 1% of solitary osteochondromas and in 2–5% of patients with HME, and accounts for 8% of all chondrosarcomas. These chondrosarcomas are usually solitary and of low histological grade. Malignant transformation before the age of 20 years is extremely rare.10,11 The main criterion for suspicion of malignant transformation is a cartilage cap thickness >3 cm in children and >2 cm in adults.10,11 Other criteria are local pain, growth after skeletal maturity or irregularities of the cartilage surface (Figs. 3 and 4).10,12
ConclusionChondrosarcomas are a heterogeneous group of malignant bone tumours that produce a chondroid matrix, and whose behaviour varies according to the histological grade. The 2020 WHO classification categorises cartilaginous tumours into three groups: benign, intermediate or malignant, and ACTs are included in the intermediate group. When studying these tumours, it is essential to use conventional radiology, CT and MRI, and establish the radiological-pathological correlation.
Authorship- 1
Research coordinator: Arrazola, Crespo.
- 2
Study concept: Crespo, Gomez-Pena, Rueda,
- 3
Study design: Crespo.
- 4
Data collection: Gómez-Pena, Romero, Rueda.
- 5
Data analysis and interpretation: N/A
- 6
Statistical analysis: N/A
- 7
Literature search: Gómez-Pena, Romero, Rueda.
- 8
Drafting of article: Crespo, Gómez-Pena, Romero, Rueda.
- 9
Critical review for important intellectual content: Moreno, Crespo
- 10
Final approval of the version for publication: Arrazola, Moreno, Crespo.
None.