To analyze the role of core needle biopsy of axillary lymph nodes with suspected metastases from breast cancer and to correlate the imaging and histologic findings.
Material and methodsWe retrospectively studied 74 patients diagnosed with breast cancer who underwent ultrasound-guided core needle biopsy of axillary lymph nodes with characteristics suggestive of metastases on ultrasonography. The following ultrasonographic findings were considered suspicious for metastases: cortical thickening and changes in the hilar fat and/or non-hilar cortical vascular flow. Patients with negative findings after axillary biopsy underwent sentinel node biopsy.
ResultsCore needle biopsy confirmed lymph node metastases in 47 (63.5%) patients. The 27 patients (36.5%) with negative findings after lymph node biopsy underwent sentinel node biopsy; 3 (11%) of these had a positive sentinel lymph node and underwent axillary lymph node resection. Of the 50 lymph nodes with metastases, 44 (88%) had cortical thickening, 20 (40%) had changes in the hilar fat, and 29 (58%) had non-hilar cortical vascular flow. All biopsies of lymph nodes with both cortical thickening and non-hilar cortical vascular flow were positive, yielding a 100% positive predictive value in this series.
ConclusionsUltrasound-guided core needle biopsy of axillary lymph nodes that are suspicious for metastases from breast cancer at ultrasonography is a highly effective procedure that has low morbidity. Findings of cortical thickening and non-hilar cortical vascular flow in the same lymph node yield a positive predictive value of 100% in this series.
Analizar el papel de la biopsia con aguja gruesa de adenopatías axilares con sospecha ecográfica de metástasis en pacientes con cáncer de mama, y la correlación radiopatológica.
Material y métodosEstudio retrospectivo de 74 pacientes diagnosticadas de cáncer de mama, a las que se realizó biopsia con aguja gruesa ecoguiada de adenopatías axilares con características ecográficas indicativas de metástasis. Consideramos hallazgos ecográficos sospechosos de metástasis: engrosamiento cortical, alteración de la grasa hiliar y/o flujo vascular cortical no hiliar. Las pacientes con biopsia axilar negativa se incluyeron en el protocolo de biopsia de ganglio centinela.
ResultadosLa biopsia con aguja gruesa confirmó metástasis ganglionar en 47 (63,5%) pacientes. Las 27 pacientes (36,5%) con biopsia negativa se incluyeron en el protocolo de biopsia de ganglio centinela y 3 (11%) presentaron ganglio centinela positivo, realizándose linfadenectomía axilar. De las 50 adenopatías con metástasis, 44 (88%) presentaron engrosamiento cortical, 20 (40%) alteración de la grasa hiliar y 29 (58%) flujo vascular cortical no hiliar. En todas las adenopatías con engrosamiento cortical y flujo vascular cortical no hiliar la biopsia fue positiva, indicando un valor predictivo positivo del 100% en esta serie.
ConclusionesLa biopsia con aguja gruesa ecoguiada de adenopatías axilares con sospecha ecográfica de metástasis por cáncer de mama es un procedimiento con alta efectividad diagnóstica y baja morbilidad. La presencia de engrosamiento cortical y flujo vascular cortical no hiliar en la misma adenopatía mostró un valor predictivo positivo del 100% en esta serie.
Traditionally, axillary lymphadenectomy (ALD) has been an integral part of the surgical treatment of breast cancer for its staging and prognostic value.1–3 The technological development and increased mammographic screening have allowed a more frequent diagnosis of early-stage breast cancer, with low probability of axillary disease,4 which can be treated in a more conservative manner, and selective sentinel lymph node biopsy (SLNB) has emerged as an alternative for ALD.5,6
SLNB is a valid and widespread procedure for breast cancer with no preoperative evidence of axillary lymph node involvement.7–11 Nonetheless, this technique requires training of multidisciplinary teams, a longer operating room time and is not a perfect procedure,5,6,12,13 being the selection of the patients benefiting from SLNB a top priority in order to prevent unnecessary ALD or non-indicated SLNB.12
Preoperative confirmation of axillary metastases excludes the use of SLNB. Several imaging techniques are aimed to detect metastasis3,14,15 and ultrasound (US) is the most widely used modality,16–18 being useful as a guide for fine needle aspiration (FNA) and/or core needle biopsy (CNB) of lymph nodes suspicious for metastasis.12,19,20 The aim of the present study is to analyze the usefulness of US-guided core needle biopsy in the preoperative diagnosis of metastatic axillary lymph nodes in patients with breast cancer and the US findings that allow for their identification. The reference standard was the histologic result of the SLNB and/or the ALD. We report our experience in 74 patients.
Materials and methodsA total of 197 new breast cancers were diagnosed in our unit between 2008 and 2009. We retrospectively studied 74 patients diagnosed with breast cancer who underwent US-guided CNB of axillary lymph nodes because of US findings indicative of metastasis. We reviewed the US images and the histologic results of CNB of the axillary lymph nodes, of SLNB and/or of ALD.
All patients were studied with digital mammography (two projections) and bilateral breast and axillary US. Breast lesions were histologically characterized as malignant with CNB or directional vacuum-assisted biopsy, with US or digital stereotactic guidance on prone table. All tumors were T1 or T2, N0 and M0. The patients were informed about the biopsy procedure and SLNB, signed the informed consent and none was pregnant or breastfeeding. SLNB was performed in accordance with the criteria recommended by the “meeting of experts” hold in Murcia in 2006.
US studies were performed with a high-resolution US system Antares (Siemens®, Erlangen, Germany) and a 10–5MHz multifrequency linear transducer, placing the patient supine or on the side contralateral to the breast being examined, with the arms raised above the head. The axilla was imaged in the caudocraneal direction evaluating Berg levels I, II and III.
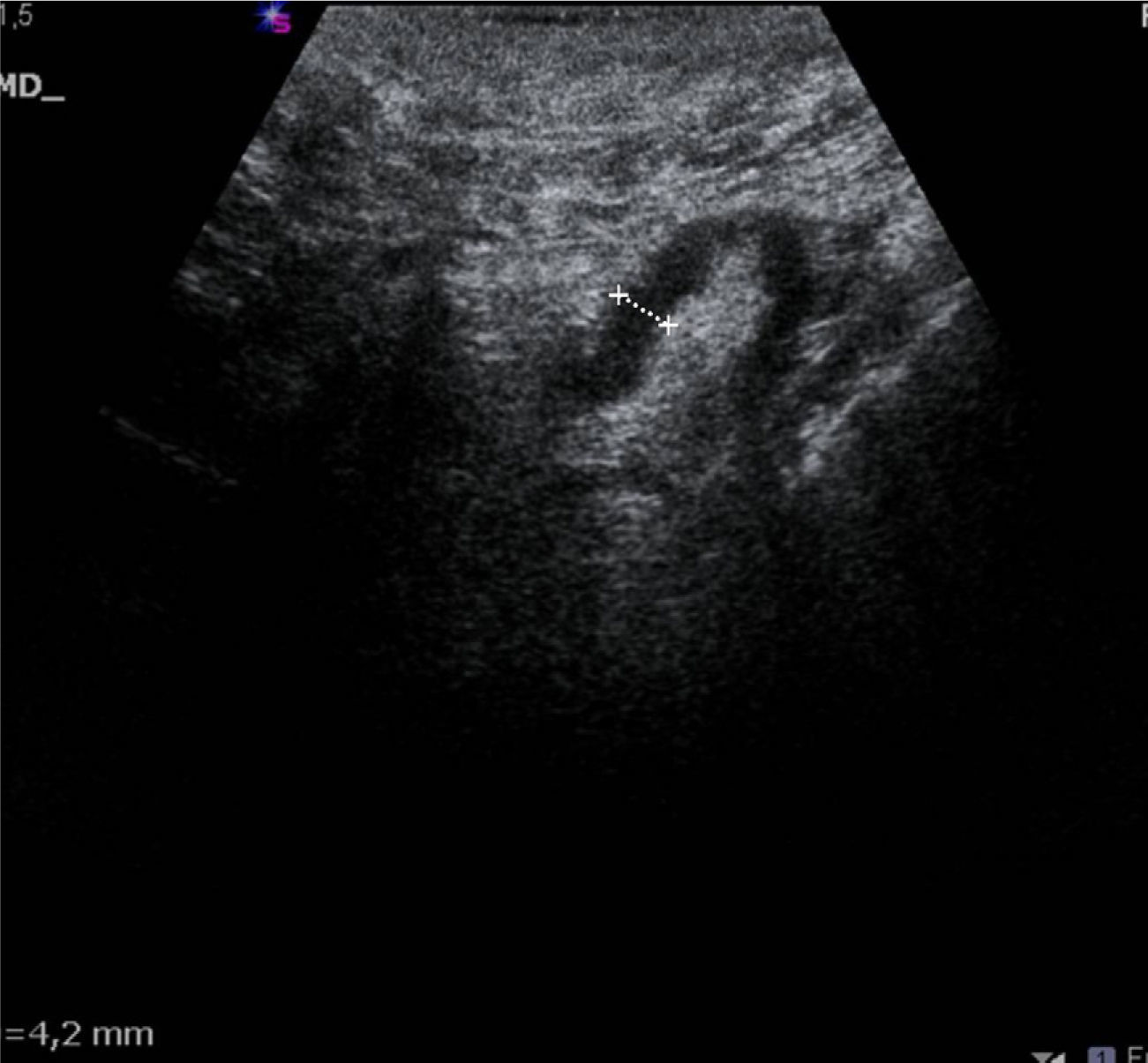
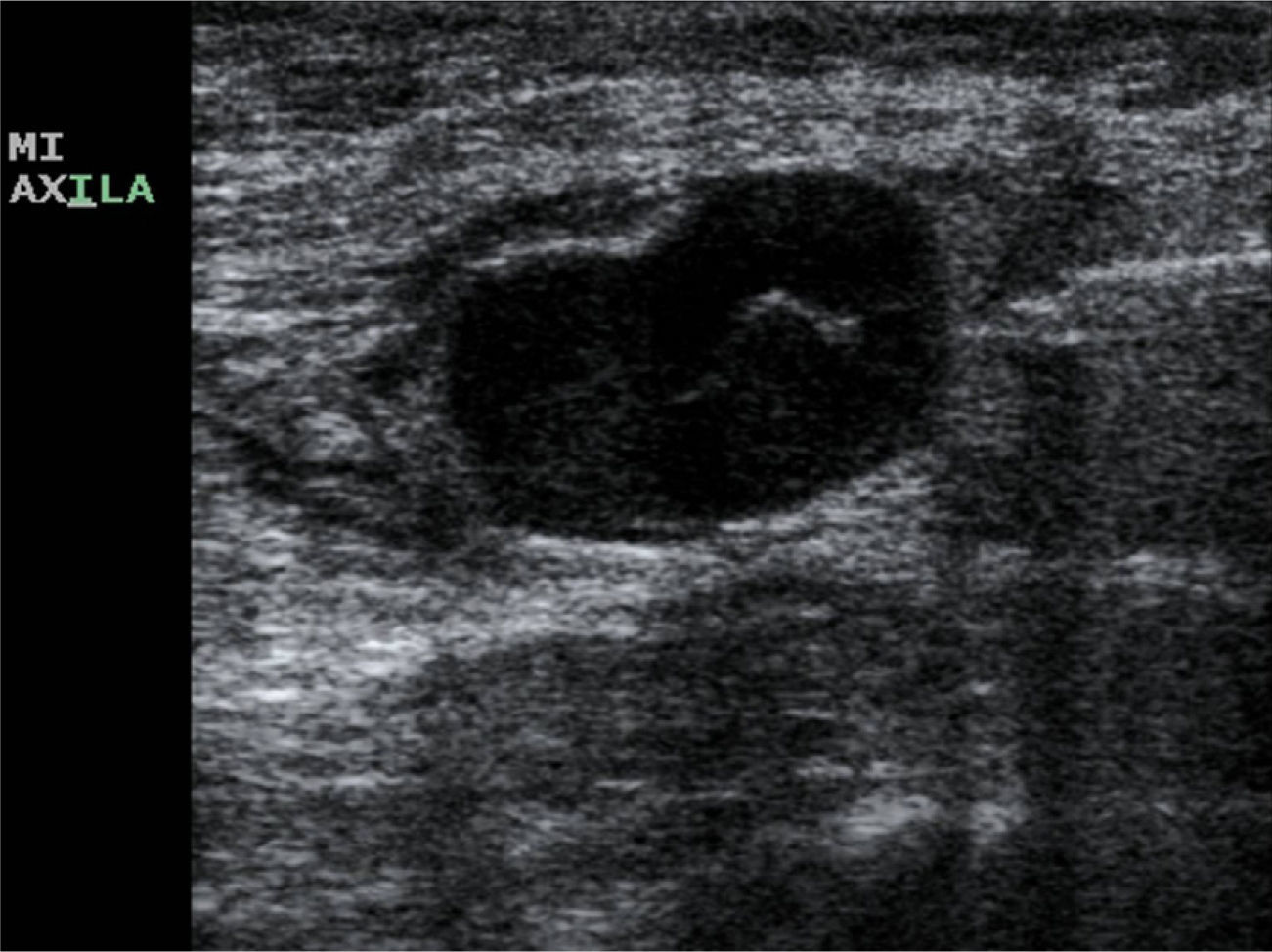
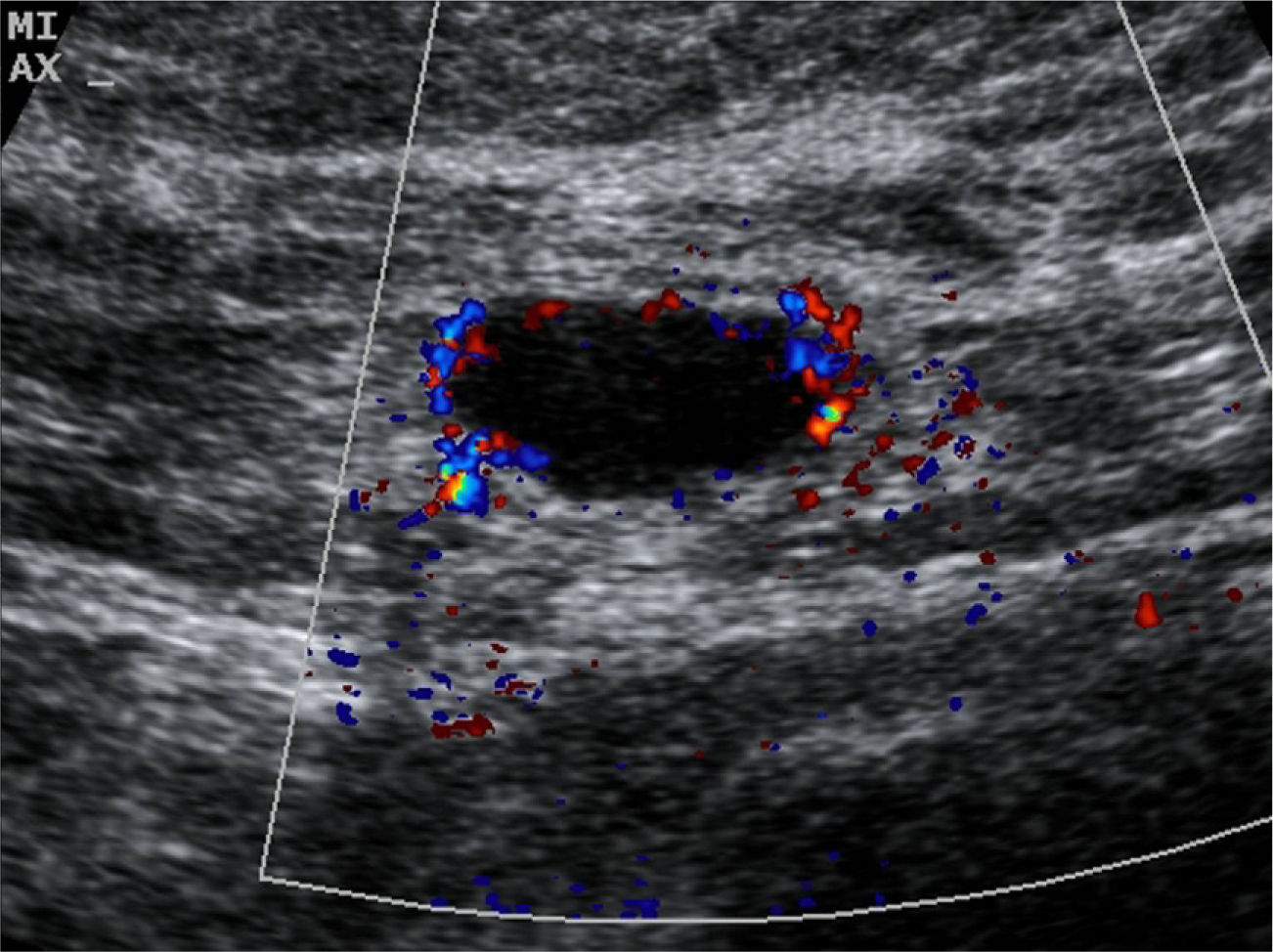
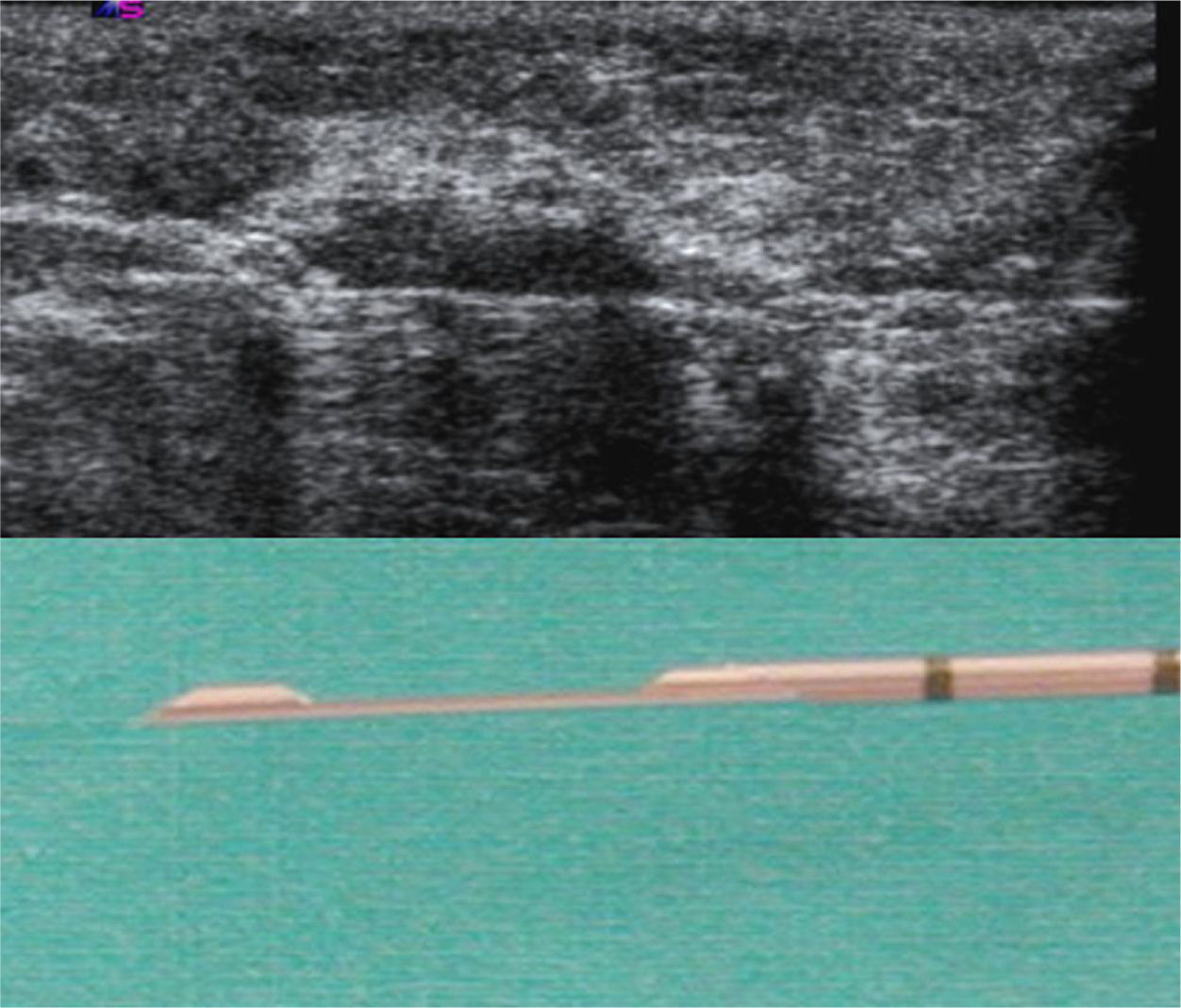
The US parameters evaluated were: cortical thickness, morphological changes in the hilar fat and characteristics of the cortical vascular flow. The following were considered findings suspicious for metastasis: local or diffuse cortical thickness ≥3mm (Fig. 1), absence, obliteration or eccentric location of the hilar fat (Fig. 2), and/or non-hilar cortical vascular flow (NHCVF) (Fig. 3) on color Doppler-duplex US with parameters set for low velocity flow detection (pulse-repetition frequency at 488, velocity scale at 3cm/s), and individualized setting for the rest of parameters. CNB was performed in those lymph nodes showing one or more of these parameters. When several lymph nodes showed US abnormalities, the most suspicious node was sampled, considered as the one with the largest number of parameters indicative of metastasis. At an equal number of parameters, the absence of hilar fat was the prevailing criterion in the selection of the lymph node, since this is the parameter with the highest positive predictive value (PPV) described in the literature.12,21,22 None of the patients in our series had several suspicious lymph nodes with one single US finding different in each lymph node. Access to the lesions was not a limitation for CNB in any of the patients.
Patients with positive axillary CNB underwent ALD, and those with negative CNB underwent SLNB, completed with ALD in case of positive sentinel lymph node.
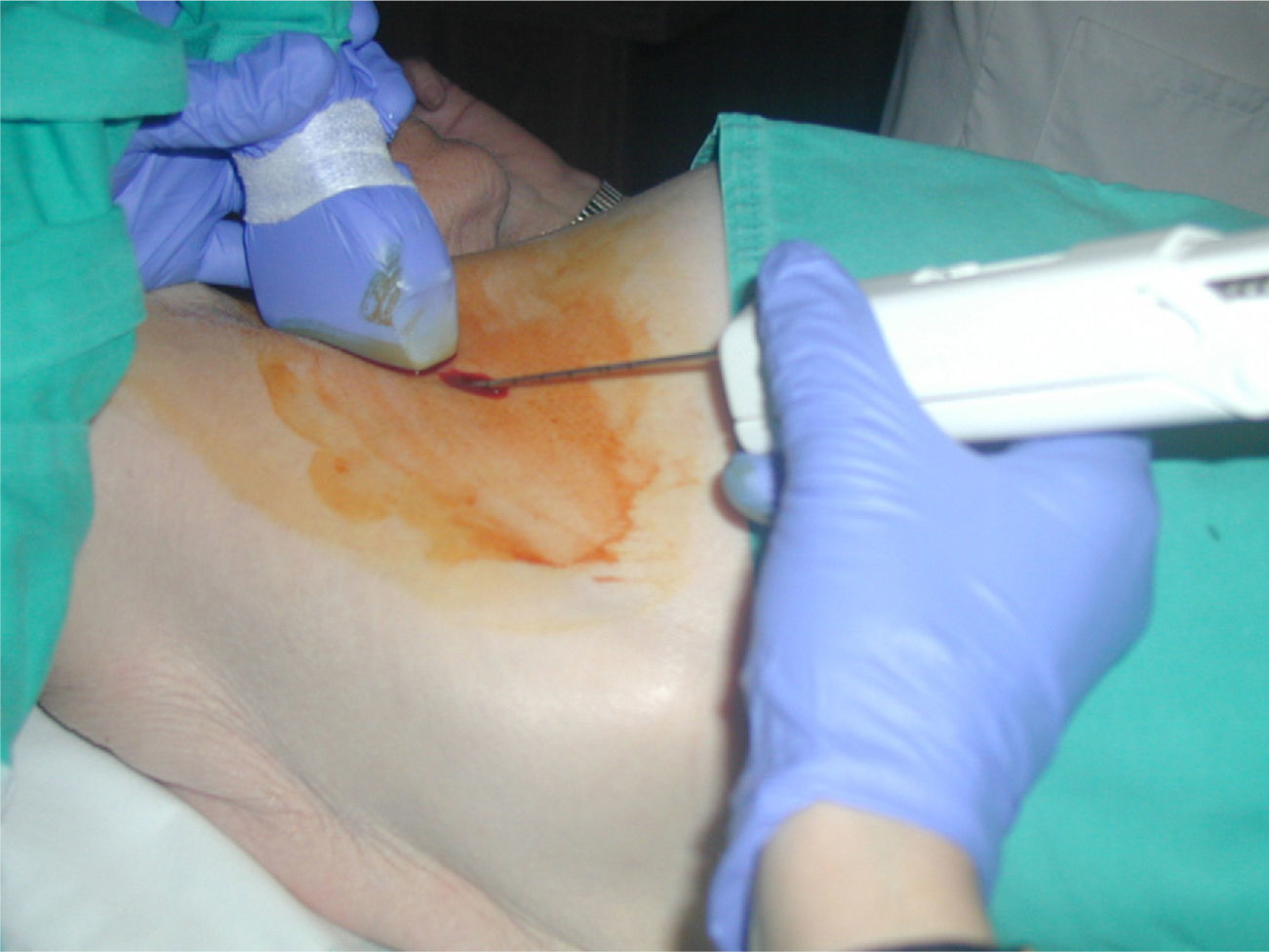
All biopsies of axillary nodes were performed by the same radiologist, with the freehand technique (Fig. 4). We used a disposable biopsy system (Acecut, Leleman®, Hirayanagicho, Japan) following a two-stage sampling process, with a 75mm, 14-gauge cutting needle with a specimen collection trough measuring 11 or 22mm in length (Fig. 5). The first firing was performed placing the tip of the needle at the periphery of the lymph node, at a safe distance from vascular and nervous structures; the needle was then advanced (with the needle trough open) and placed through the lymph node manually. Use of this procedure ensures that only nodal tissue that has already been traversed by the needle is cut and sampled. At least 3 samples were collected from each node, including tissue from the cortex and hilum (Fig. 6). The cores were sent to the anatomic pathology department fixed in formaldehyde in separate labeled containers. Different containers and biopsy systems were used for breast lesions and lymph nodes, avoiding possible false positive results caused by contamination of the samples.
The histologic diagnosis was performed on processed specimens stained with hematoxylin and eosin (H&E), and negative nodes on H&E were further evaluated with immunohistochemical (IHC) tests.
The statistical analysis compared the US findings and the histologic results of the CNB, using the results of the SLNB and/or of ALD as the reference standard. We created a database that included the following variables: patient age, US criteria used to evaluate the lymph nodes (cortical thickness, changes in the hilar fat and presence of NHCVF), histologic results of the CNB, sentinel biopsy and/or ALD, number of lymph nodes removed, histologic type and tumor size (in the surgical specimen). Positive CNB were considered true positives, negative CNB with negative SLNB were considered true negatives, and negative CNB with positive SLNB were considered false negatives. We estimated the sensitivity, the negative predictive value (NPV) of the axillary biopsy and the PPV of every US parameter analyzed.
ResultsThe mean age of patients was 51years (range 32–83). The definitive histologic type (surgical specimen) of the primary breast cancer was: infiltrating ductal carcinoma (IDC) in 53 patients (71.62%), IDC with extensive intraductal component in 12 (16.22%), extensive in situ ductal carcinoma (>4cm) in 3 cases (4.05%), and infiltrating lobular carcinoma in 6 (8.11%). The mean size of the tumor was 16.9mm (range 4.7–43mm).
Lymph node metastasis was found at CNB in 47 (63.5%) of the 74 patients. Twenty-seven (36.5%) patients with negative results were further evaluated with SLNB: the sentinel node was negative in 24 cases (89%) (one with isolated tumor cells) and positive in 3 (11%) (micrometastasis). The latter underwent ALD, revealing negative nodes in all cases (11, 15 and 22 nodes were excised, mean 16). The NPV of the CNB was 88.9% (24/27), with a sensitivity of 94% (47/50).
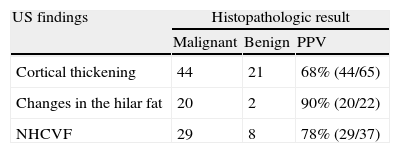
Cortical thickness >3mm was found in 21 (78%) of the 27 patients with negative CNB; morphological changes in the hilar fat were found in 2 patients (7%), and NHCVF in 8 patients (30%), with 2 or more US criteria of metastasis found in several lymph nodes. Of the 50 patients with positive lymph nodes (positive CNB or negative CNB with positive sentinel node), cortical thickness was found in 44/50 patients (88%), changes in the hilar fat in 20/50 patients (40%) and NHCVF in 29/50 patients (58%). Considered individually, the absence or obliteration of the hilar fat was the US parameter with the highest PPV (90%) against NHCVF (78%) and cortical thickening (68%). The combination “cortical thickening and NHCVF” was positive in all cases detected in our series (100% PPV) (Table 1).
Biopsy material was sufficient for histologic analysis in all cases and no major complications such as infection, severe bleeding or neurologic injury occurred.
DiscussionSLNB was avoided in all patients with positive CNB. In the 3 false negative results obtained with CNB, the sentinel node was the only positive node after ALD and only micrometastases were identified, deposits <2mm that are not usually detected at routine examinations; second-generation echo-enhancers may improve the diagnostic sensitivity.23 A sentinel node with isolated tumor cells was considered non-metastatic (true negative).12
US is the most widely used technique in the preoperative evaluation of axillary lymph nodes, with a wide range of sensitivity (35%-95%) related to the experience of the operator and the variety of parameters considered as possible predictors of metastatic disease.17,18,24–26 The absence of hilar fat is considered an US parameter with high PPV and low sensitivity.12,21,22 This is in line with the results obtained in the present study: this was the least common finding (40%), with a PPV of 90%.
The most common US finding was cortical thickness >3mm, with a PPV of 68% and a sensitivity of 88%, slightly below the results obtained by Deurloo et al.17 and Abe et al.12 in their series; although Deurloo et al. considered a cortical thickness of at least 2.3mm. In their study, Abe et al.12 reported an excessively low sensitivity for this parameter (6%) and propose as possible indicator “the ratio between the cortical thickness and the short axis of the lymph node”, against cortical thickening alone.
Abe et al.12 have reported that the presence of NHCVF may be a good indicator of nodal metastasis, with a high PPV (78%) when is detected in patients with ipsilateral breast cancer. We obtained the same value in our series. This increased NHCVF is probably due to increased peripheral vascularity relative to decreased hilar blood supply that results from infiltration by metastatic disease.26
In the study by Abe et al., the absence of hilar fat was the US finding with the highest predictive value followed by “cortical thickening combined with NHCVF”, with a PPV of 81%. In our series, all the lymph nodes with “increased cortical thickness and NHCVF” were metastatic, indicating a high PPV (100%). This difference may be due to the smaller sample size and to the minimal dependency on the operator of the US study and of axillary lymph node biopsy in our series. Abe et al. included patients from several medical institutions in their study.
US findings suspicious for neoplastic involvement of the axilla should be confirmed by histopathologic analysis. FNA is a common procedure and some authors have reported a high sensitivity (60–85%) and specificity (100%).17,19,23 In comparison with CNB, FNA is a minimally invasive and cost effective procedure, with low morbidity, simple and quick to perform; conversely, this technique is highly operator-dependent, necessitating the cooperation of an experienced cytologist. CNB is less dependent on the operator and yields more material; its main challenge is to avoid damage on the neurovascular bundle.
There were no complications in our series. Small lymph nodes, in a deep location and/or close to a main vascular structure, and in patients with deep axillas (with no fat) were more difficult to biopsy. In order to avoid complications in these settings, a two-stage firing system was used, which also facilitates a more accurate sampling of the selected area. This technique, or a very similar one, has been described by other researchers, with automated and semiautomated biopsy systems and 14-gauge cutting needles, encountering no complications.12,27–31
Our future goal is to increase the sample size including patients who do not undergo CNB of the axillary lymph nodes. This will allow us to establish the sensitivity, specificity, predictive values and odds ratio of the technique and the value of the US parameters analyzed as an indicator of lymph node metastasis.
In conclusion, US-guided biopsy with 14-gauge needles and a two-stage firing technique allows us to obtain positive results without significant clinical complications, avoiding unnecessary SLNB in patients with breast cancer. In our series, the combination of the US parameters “increased cortical thickness and presence of NHCVF” had a PPV of 100%.
AuthorshipResponsible for the integrity of the study: MYTS.
Conception of the study: MYTS, MEBI and JJMG.
Design of the study: MYTS, MEBI and JJMG.
Acquisition of data: MYTS, MEBI, MAO, LMGL and LRO.
Analysis and interpretation of data: MYTS, MLRR, MAO and LMGL.
Statistical analysis: JJMG.
Bibliographic search: M MYTS, MEBI and MAO.
Drafting of the manuscript: MYTS and MLRR.
Critical review with intellectually relevant contributions: MYTS, MEBI, MLRR, MAO, LMGL, JJMG and LRO.
Approval of the final version: MYTS, MEBI, MLRR, MAO, LMGL, JJMG and LRO.
Conflict of interestThe authors declare no conflict of interest.
Please cite this article as: Torres Sousa MY, et al. Estadificación ganglionar axilar prequirúrgica en el cáncer de mama: parámetros ecográficos y biopsia con aguja gruesa ecoguiada. Radiología. 2011;53:544–51.