To evaluate the safety and patency of self-expanding stents to treat hepatic venous outflow obstruction after orthotopic liver transplantation. To evaluate differences in the response between patients with early obstruction and patients with late obstruction.
Material and methodsThis is a retrospective analysis of 16 patients with hepatic venous outflow obstruction after liver transplantation treated with stents (1996–2011). Follow-up included venography/manometry, ultrasonography, CT, and laboratory tests. We did a descriptive statistical analysis of the survival of patients and stents, technical and clinical success of the procedure, recurrence of obstruction, and complications of the procedure. We also did an inferential statistical analysis of the differences between patients with early and late obstruction.
ResultsThe mean follow-up period was 3.34 years (21–5331 days). The technical success rate was 93.7%, and the clinical success rate was 81.2%. The rate of complications was 25%. The survival rates were 87.5% for patients and 92.5% for stents. The rate of recurrence was 12.5%. The rate of primary patency was 0.96 (95% CI 0.91–1) at 3 months, 0.96 (95% CI 0.91–1) at 6 months, 0.87 (95% CI 0.73–1) at 12 months, and 0.87 (95% CI 0.73–1) at 60 months. There were no significant differences between patients with early and late obstruction, although there was a trend toward higher rates of primary patency in patients with early obstruction (P=0.091).
ConclusionsTreating hepatic venous outflow obstruction after orthotopic transplantation with self-expanding stents is effective, durable, and effective. There are no significant differences between patients with early obstruction and those with late obstruction.
Evaluar la seguridad y permeabilidad del tratamiento de la obstrucción del drenaje venoso hepático tras trasplante ortotópico con endoprótesis autoexpandibles. Valorar las diferencias en la respuesta en pacientes con obstrucción precoz y tardía.
Material y métodosAnálisis retrospectivo de 16 pacientes trasplantados con obstrucción del drenaje venoso hepático tratados con endoprótesis (1996–2011). El seguimiento se realizó mediante venografía/manometría, ecografía, TC y pruebas de laboratorio. Se realizó análisis estadístico descriptivo de supervivencia de pacientes e injertos, éxito técnico y clínico, recurrencia y complicaciones del total de la muestra, así como inferencial para comparar las diferencias entre pacientes con obstrucción precoz y tardía.
ResultadosLa media de seguimiento fue de 3,34 años (21–5.331 días). La tasa de éxito técnico fue del 93,7%, y la de éxito clínico, del 81,2%. La tasa de complicaciones fue del 25%. La tasa de supervivencia para pacientes fue de 87,5%, y para injertos, de 92,5%. La tasa de recurrencia fue del 12,5%. La tasa de permeabilidad primaria a los 3, 6, 12 y 60 meses fue de 0,96 (IC 95% 0,91–1), 0,96 (IC 95% 0,91–1), 0,87 (IC 95% 0,73–1) y 0,87 (IC 95% 0,73–1), respectivamente. No hubo diferencias significativas entre los pacientes con obstrucción precoz o tardía, aunque las tasas de permeabilidad primaria mostraron tendencia a ser significativamente superiores en el grupo precoz (p=0,091).
ConclusionesEl tratamiento con endoprótesis autoexpandibles en obstrucciones del drenaje venoso hepático tras trasplante ortotópico es efectivo, duradero y seguro. No hay diferencias significativas entre pacientes con obstrucción precoz y tardía.
The piggyback technique for liver hepatectomy and orthotopic liver transplant (OLT) described by Calne and Williams in 1968 and Tzakis et al. in 19891,2 is one of the reference techniques. It is characterized by the preservation of the retrohepatic inferior cava vein (ICV) of the receiver to be connected to the donor's ICV by terminolateral anastomosis through a vascular cuff created from the receptor's suprahepatic veins. Potential pros of this technique are: (a) the absence of the receptor's venovenous bypass and associated complications; (b) lesser time of warm ischemia and of the total duration of the procedure, and (c) a reduction of the complications associated with the dissection of the receiver's retrohepatic ICV. Yet despite all its pros the piggyback technique does not restore completely the pre-transplant physiological situation; this is why the risk of obstruction of the liver venous drainage increases. As a consequence the OLT performed through the piggyback technique eliminates the risk of potentially serious complications.
The incidence of the obstruction of the liver venous drainage post-OLT ranges between 1% and 7% when taking all transplant variants into consideration–cadaver donor, living donor, reduced graft, pediatric transplant. With the piggyback technique the incidence in the cadaver donor is 1–2.5%.3–6 The clinical manifestations include ascites, hepatomegaly and liver failure. Endovascular therapy through balloon angioplasty and/or endoprosthesis has been widely covered for the post-OLT7–9 management of the ICV and portal vein stenoses. However there are not too many studies that have analyzed the results of endovascular therapy of suprahepatic vein-stenoses post-OLT.10–12 One first issue that needs to be assessed is choosing the access route that will be used since there are several options available: transjugular, transfemoral, and transhepatic. The most common access route described in the reference is transjugular access. This access makes the use of catheters in the suprahepatic veins easier on a technical level–in particular in piggyback type of anastomoses.13 Transfemoral access can be used to release the endoprosthesis in suprahepatic veins, yet in piggyback type-anastomoses the angle between the ICV and suprahepatic veins is steeper making at times the catheterization difficult or even impossible. Ultrasound controlled-transhepatic access allows the simultaneous catheterization of multiple suprahepatic veins to better view venous orifices and the relation of these orifices with the donor's ICV; however, there is a higher risk of immediate complications due to the hepatic capsule perforation.14,15 When choosing one or another access route the number and localization of stenoses is a very important factor. In patients with single stenoses the preferred access route is transjugular access. In patients with multiple stenoses transhepatic access allows the endoprosthesis simultaneous release, so it can be a good alternative to transjugular access–the type of endoprosthesis to use is another kettle of fish. When it comes to stenoses of suprahepatic veins self-expandable and balloon-expandable endoprostheses can be used.16 Several studies have showed that the radial resistance of balloon-expandable endoprostheses is greater,17 yet we still need studies to confirm if this implies a better response. To these issues we need to add the fact that there is little information on long-term safety and patency outcomes.18,19
The goal of this study is to assess retrospectively both the long-term safety and patency of endovascular therapy with self-expandable endoprostheses in patients with OLT and obstruction of the liver venous drainage. As a secondary goal we will study the differences in the therapeutic response of patients with early and late obstructions.
Material and methodsPatientsFrom January 1996 and February 2011, 16 out of the 295 patients (5.4%) who underwent OLT in our institution showed obstruction of the liver venous drainage and were managed with self-expandable endoprostheses. We studied them retrospectively with the endorsement of the hospital ethical committee. All proceedings were performed under prior written informed consent.
The study group included 12 men and 4 women (mean age 53.2 years; 95% CI 31–65 years). The most common cause of liver transplant was alcoholic cirrhosis with or without concomitant diseases (n=8) followed by cirrhosis due to the virus of hepatitis C (n=3) and other causes (n=5). In 15 cases the complication was diagnosed during the first transplant and in only one case in one re-transplant. In all cases it was one OLT with complete graft of the cadaver donor. The piggyback technique was used in 15 patients (3 vessel-cuff [n=12]; 3 vessel-cuff + cavocavostomy [n=2]; 2 vessel-cuff [n=1]), and in one patient one terminoterminal cavocavostomy was used. In 8 cases (50%), the obstruction was found in the early postoperative period (during the first 30 days after the surgery). In the other 8 (50%), the obstruction occurred during the late postoperative period–between 1 month and 4 years after the transplant.
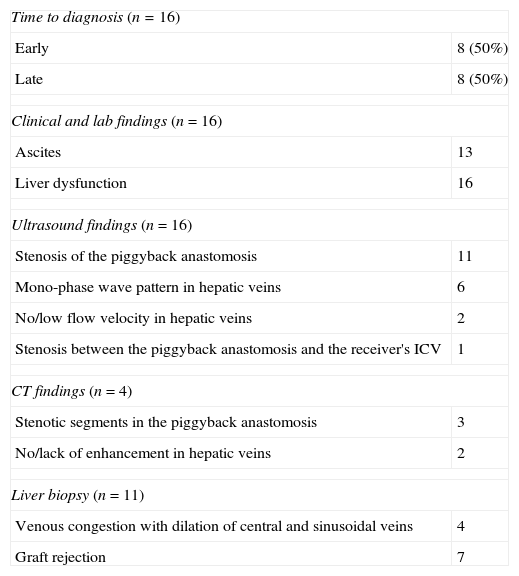
Diagnosis was initially based on clinical and lab findings and then completed through ultrasound and/or computed tomography (CT) (Table 1). In 11 patients one liver biopsy was performed to discard graft-rejection or other processes that might cause clinical and lab alterations. The evaluated parameters included abdominal pain, weight gain, ascites, lower limb swelling, pleural effusion, hepatosplenomegaly and clinical alterations of the liver function. The most common manifestation was the analytical alteration of the liver function expressed as an increase in bilirubin levels and coagulation parameters and reduction of serum albumina. This finding was present in all patients (n=16).
Diagnostic summary.
| Time to diagnosis (n=16) | |
| Early | 8 (50%) |
| Late | 8 (50%) |
| Clinical and lab findings (n=16) | |
| Ascites | 13 |
| Liver dysfunction | 16 |
| Ultrasound findings (n=16) | |
| Stenosis of the piggyback anastomosis | 11 |
| Mono-phase wave pattern in hepatic veins | 6 |
| No/low flow velocity in hepatic veins | 2 |
| Stenosis between the piggyback anastomosis and the receiver's ICV | 1 |
| CT findings (n=4) | |
| Stenotic segments in the piggyback anastomosis | 3 |
| No/lack of enhancement in hepatic veins | 2 |
| Liver biopsy (n=11) | |
| Venous congestion with dilation of central and sinusoidal veins | 4 |
| Graft rejection | 7 |
CT: computed tomography; ICV: inferior cava vein.
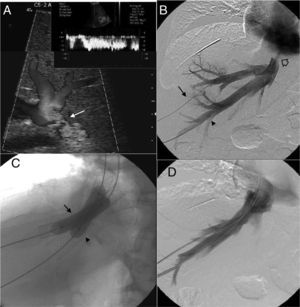
Ultrasounds were performed in all patients (n=16), including one mode B study and pulsed and color Doppler-studies (Antares/Acuson S2000™; Siemens Healthcare, Erlangen, Germany). Findings suggestive of obstruction of the liver venous drainage20 were the stenosis of anastomosis (Fig. 1A), the absence of flow or the absence of low velocity, or one mono-phase wave pattern in suprahepatic veins. CT scan (TCMD; Somatom Sensation and Definition/Axiom, Siemens Healthcare, Erlangen, Germany) was performed in 4 cases in an attempt to morphologically characterize the stenosis and locate it precisely. In all cases there was a prior ultrasound study of the obstruction of the liver venous drainage. CT findings compatible with one obstruction of the liver venous drainage included the stenosis of venous anastomosis and absent or little enhancement of suprahepatic veins with alterations associated with parenchyma perfusion.
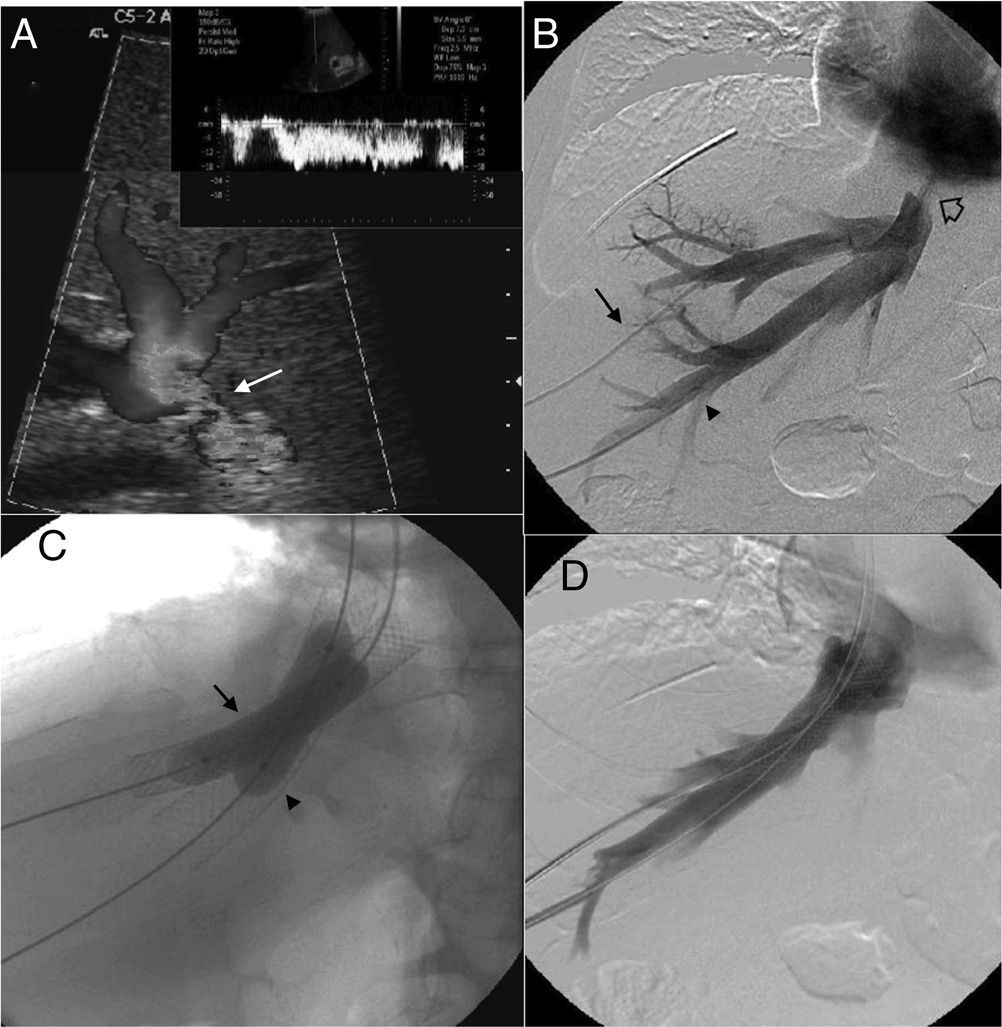
Patient with orthotopic hepatic transplant with the piggyback technique showing ascites and alteration in the liver function tests. (A) Ultrasound. Color Doppler images showing stenosis of the piggyback anastomosis (arrow). The wave shape of the hepatic vein in the pulsed Doppler study shows flattening with no breathing alterations. (B) Venographic study. Selective catheterism of the right and middle hepatic veins through transhepatic access with ultrasound control (arrow and arrowhead). The contrast agent confirms stenosis of the piggyback anastomosis (hollow arrow). (C) Venographic study. Dilation with 2 self-expandable endoprostheses balloon implanted consecutively in the right and middle hepatic veins (arrow and arrowhead). (D) Venographic study. Two self-expandable endoprostheses identified in the right and middle hepatic veins running through the anastomosis stenosis. The injection of the contrast agent does not show stenosis or contrast reflux toward the hepatic veins.
Liver biopsy was performed in 11 patients to discard other processes that might justify the clinical and analytical situation. Access route was transhepatic or transjugular. Several degrees of graft-rejection were found in 7 patients (63.3%).
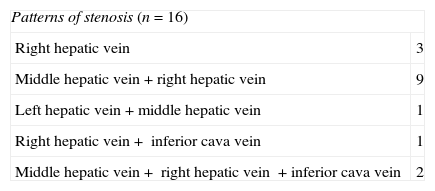
After establishing the initial diagnosis of the obstruction of the liver venous drainage initiating one anticoagulant therapy the 16 patients underwent venography and manometry. The goal of these tests was to confirm and precisely locate the obstruction (Fig. 1B) and quantify the portal pressure gradient and between the suprahepatic veins and the right atrium considered significant when >3mmHg.21 The direct view of the obstruction was achieved in 11 patients. In the other 5 it was spotted through indirect signs (collateral, inverse flow). The pressure gradient was significant in the 16 patients. When it comes to the obstruction pattern 3 patients showed single stenosis of suprahepatic veins, 10 patients, multiple stenoses of suprahepatic veins and 3 patients stenosis in suprahepatic veins and ICV (Table 2).
ProceedingEndovascular therapy was performed immediately after venographic or differed confirmation based on the need for additional tests to schedule therapy. The proceeding was done under general anesthesia in 15 patients. The proceeding was done under general anesthesia in an attempt to guarantee the immobility of patient during such proceeding to maximize the quality of venography and increase pressure during selective catheterization and deployment of endoprosthesis.22 In one patient we used deep sedation recommended by the anesthetist. Access to suprahepatic veins was performed though ultrasound guided-transhepatic access in 15 patients and transjugular access in one patient. Transjugular access was the preferred one in the presence of stenosis of one single suprahepatic vein while the transhepatic access was the preferred one when stenosis affected more than just one vein or when the catheterization of single stenoses through transjugular access was difficult on the technical level. In the 3 cases in which there was coexisting stenosis of the receiver's ICV this stenosis was managed with one endoprosthesis through transfemoral access. In all cases safety guidewire we placed in the ICV through transfemoral or transjugular access. In all those cases in which the transhepatic access was used the suprahepatic veins were initially punctured with one 21 gauge needle for the introduction of one 0.035″ guidewire (Terumo, Tokyo, Japan) through the AccuStick™ II kit (Boston Scientific, Spencer, IN, USA). The one vascular introducer (4–11F) (Cordis Co., Miami, FL, USA) inside the lumen of each suprahepatic vein was inserted and ran though each stenosis through rigid Amplatz™ guidewire (Boston Scientific) leaving the guideguide distal edge in the superior cava vein. The deployment of self-expandable endoprostheses (Wallstent®, Boston Scientific, Natick, MA, USA) through the stenosis from supraheptic veins and/or the receiver's ICV was always followed by the dilation of endoprostheses with angioplasty balloons with the appropriate size (Wanda™, Boston Scientific, Galway, Ireland) (Fig. 1C). The diameter of endoprosthesis ranged between 10 and 20mm and length between 60 and 100mm. All cases after the proceeding through venography (Fig. 1D) and pressure gradient were monitored to determine the technical response to the proceeding. Transhepatic tracts were embolized through 0.035″-coils. The immediate follow-up after the proceeding consisted of clinical assessment, lab tests and Doppler ultrasound 24–72h after implanting the endoprosthesis. Long-term follow-up consisted of one clinical assessment, lab tests every 1–3 months and one Doppler ultrasound after 1, 3, 6, 9 and 12 months and then every three months. CT studies and/or venography were saved for situations of clinical suspicion of complications or obstruction relapse.
Technical success was tagged as the angiographic resolution of stenosis with an improved blood flow after contrast injection and a significant reduction of pressure gradient. Clinical success was tagged as the immediate and significant improvement of the patient's clinical status assessed through signs and symptoms like abdominal pain, ascites, lower limb swelling, and pleural effusion.
We considered as major complications associated with the proceeding those implying a greater level of patient care, surgical intervention, extension of hospital stay, permanent sequelae or death including not only those associated with the implantation of endoprosthesis (bleeding, pneumothorax, hemothorax or visceral lesion) but also complications that might occur much time after the intervention – malposition, migration or occlusion.
Relapse was defined as the occurrence of clinical, ultrasound, tomographic or angiographic findings indicative of new obstruction of the liver venous drainage. Among some of these findings ascites (after showing significant improvement after the intervention), the relapse of significant pressure gradients in suprahepatic veins or ICV and the appearance of new stenoses. For us the survival of patients and grafts was the rate of patients and living grafts when follow-up was over.
Primary patency was defined as the time interval elapsed between the implantation of endoprosthesis and the first time the signs of obstruction of the liver venous drainage appeared requiring one skin angiographic study. Assisted primary patency was defined as the time frame elapsed between the first endovascular proceeding to insure flow in one compromised endoprosthesis and the realization of a new endovascular proceeding to maintain patency was necessary.
Statistical analysisTo compare survival of patients and grafts, the technical success, the clinical success, relapse and complications among patients with early and late obstructions the Pearson Chi-square test was used. To estimate patency rate of endoprostheses the Kaplan–Meier test was used. Log-rank test was used to compare patency rates between patients with early and late obstructions of venous flow. All statistical analyses were performed with the SPSS® statistical package (version 15.0, SPSS, Chicago, IL, USA). Statistical significance was established in a P value <0.05.
ResultsThe number of suprahepatic veins characterized per patient ranged between 1 and 3 (median 2). The right suprahepatic vein was characterized in 14 patients, the median suprahepatic vein in 12 patients, and the left suprahepatic vein in 2 patients. The total number of self-expandable endoprostheses implanted was 34. The number of prostheses implanted per patient was between 1 and 4. Endoprostheses were implanted in the right suprahepatic vein, the median suprahepatic vein, the left suprahepatic vein or the receiver's ICV based on the location and degree of stenosis in the vascular anastomosis. Thirteen out of 16 (81.25%) patients required more than one endoprosthesis. In all cases the endoprostheses were implanted in the area of stenosis without immediate complications like shortening or malpositioning. The information on the implantation of endoprostheses is summed up in Table 3.
Insights on endoprosthesis implantation.
| Type of treatment (n=16) | |
| Primary therapy (1st endovascular therapy) | 14 |
| Secondary therapy (after angioplasty) | 2 |
| Time to endoprosthesis implantation (n=16) | |
| Immediately after venography/manometry | 7 |
| Days after venography/manometry | 9 |
| Catheter route of suprahepatic veins (n=16) | |
| Transhepatic (with ultrasound control) | 15 |
| Transjugular | 1 |
| Catheterized suprahepatic veins (n=28) | |
| Right hepatic vein | 14 |
| Middle hepatic vein | 12 |
| Left hepatic vein | 2 |
| Number of implanted endoprostheses | 34 |
| Diameter of endoprostheses (average) | 16mm (10–20) |
| Length of endoprostheses (average) | 60mm (60–100) |
| Number of endoprostheses per patient (n=16) | |
| 1 | 3 |
| 2 | 10 |
| 3 | 1 |
| 4 | 2 |
| Site of endoprosthesis implantation | |
| Right hepatic vein | 15 |
| Middle hepatic vein | 11 |
| Left hepatic vein | 3 |
| Receiver's inferior cava vein | 6 |
| Immediate complications (shortening or displacement) | 0 |
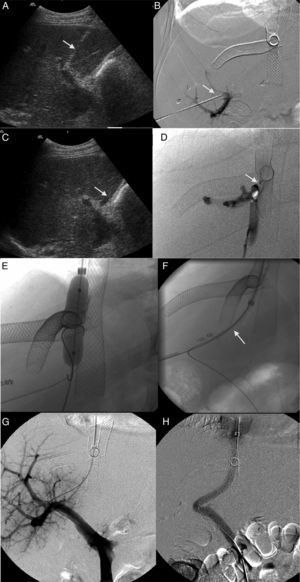
During follow-up 2 patients required one percutaneous portosystemic shunt (TIPS) post-endovascular therapy (223 and 256 days post-proceeding) due to persistence of significant portal hypertension (Fig. 2). In the first case the patient showed significant clinical improvement but died 20 days later due to disseminated intravascular coagulation. In the second case the patient showed significant clinical improvement, was still alive and without relapses 2 years 4 months after implanting the TIPS.
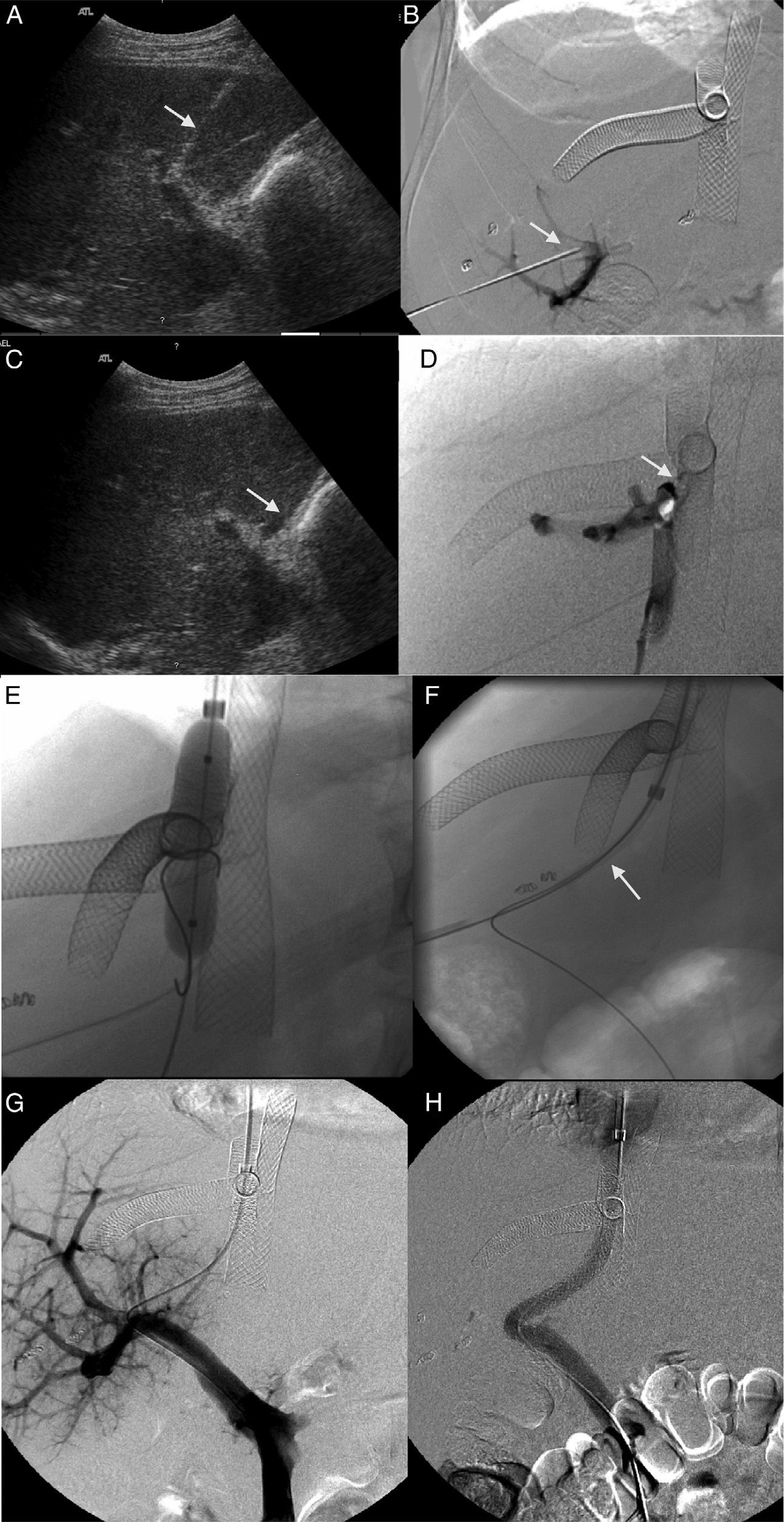
Patient with obstruction of the liver venous drainage treated with endoprosthesis in the middle hepatic vein (MHV), left hepatic vein (LHV), and the inferior cava vein (ICV). The patient showed sudden onset-serious portal hypertension due to the occlusion of the endoprosthesis implanted in the MHV. (A) Ultrasound. Selective catheterism of the right portal vein (RPV) through transhepatic access with ultrasound control (arrow). (B) Venographic study. The injection of the contrast agent through transhepatic access confirms the catheterism of the RPV (arrow). The endoprosthesis deployed in the MHV, LHV, and ICV can be identified here. (C) Ultrasound. Selective catheterism of the donor's ICV through transhepatic access with ultrasound control (arrow). (D) Venographic study. The injection of the contrast agent in the donor's ICV shows the stenosis of the piggyback anastomosis (arrow). There is no contrast passing to the receiver's ICV and there is contrast media reflux toward the right hepatic vein. No reflux toward the MHV. (E) Venographic study. Dilation of the anastomosis stenosis from the access to the donor's ICV. (F) Venographic study. Creation of one shunt between the receiver's ICV and the RPV passing through the donor's ICV (arrow) with a Colapinto needle using the right transjugular access. (G) Venographic study. The injection of the contrast media from the right transjugular access opacifies the portal tree confirming the creation of one portosystemic shunt. (H) Venographic study. Implantation of the Viator® and Wallstent® endoprostheses through the shunt between the receiver's ICV and the RPV. One injection of the contrast media confirms the patency of the shunt.
The rate of technical success was 93.7% (15 out of 16 cases). In one case non-significant reduction of the pressure gradient was reported–yet there was a significant clinical improvement. The rate of clinical success was 81.25% (13 out of 16 patients). Two of the failed cases were patients with acute obstruction of the liver venous drainage shortly after the transplant that did not respond clinically to endovascular therapy. One of the cases required transplant 15 days after the intervention and the other case ended with the patient's death 6 days later. The third patient showed significant portal hypertension that did not improve after a repeated endovascular therapy and eventually needed TIPS implantation.
The overall rate of major complications was 25% (4 out of 16 cases). The rate of major immediate complications was 12.5% (50% of complications). The first case consisted of a pleural effusion that required percutaneous drainage and the second case consisted of one intrahepatic hematoma requiring blood transfusions. The rate of major long-term complications was also 12.5% (50% of complications). The first case corresponded with the thrombosis of one endoprosthesis associated with symptomatic portal hypertension (refractory ascites) requiring TIPS implantation. The second case corresponded with the migration of one endoprosthesis toward the receiver's ICV. The patient then showed dyspnea and new onset systolic murmur. The ultrasound study revealed one right aorto-atrial fistula and one atrial septal defect with right overload due to the migration of endoprosthesis from the ICV toward the right atrium. Surgical therapy to extract the migrated endoprosthesis and close the atrial septal defect and the aorto-atrial fistula was performed without any associated complications. The patient was alive and without sequelae after completion of the study.
Follow-up period ranged between 21 and 5331 days (average 3.34 years). The survival rate was 87.5% for patients (14 out of 16) and 92.5% for grafts (15 out of 16). The relapse rate for the obstruction of the liver venous drainage with clinical manifestations requiring reintervention was 12.5% (2 out of 16).
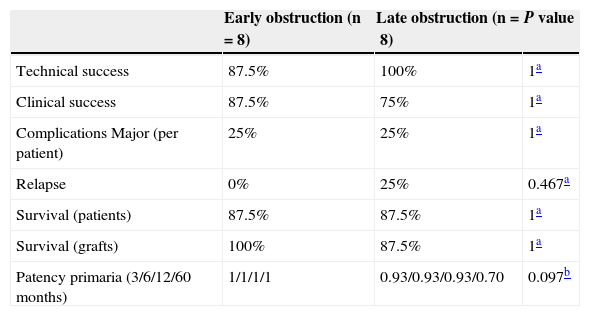
The estimated overall patency based on the number of endoprostheses was 97% (33 out of 34). The overall patency rate was 91.7% (31 out of 34), and the overall global primary patency rate was 5.8% (2 out of 34). The primary patency rate 3, 6, 12 and 60 months after implanting the endoprostheses was 0.96 (95% CI 0.89–1), 0.96 (95% CI 0.91–1), 0.86 (95% CI 0.70–1) and 0.86 (95% CI 0.70–1). The primary assisted patency rate was 1 at 3, 6 and 12 months. After comparing the results among patients with early (n=8) and late obstructions (n=8) no statistically significant differences were found (Table 4). However, there was a trend showing higher primary patency rates in the group of patients with early obstructions.
Result comparison between patients with early and late obstructions of the hepatic venous flow.
| Early obstruction (n=8) | Late obstruction (n=8) | P value | |
|---|---|---|---|
| Technical success | 87.5% | 100% | 1a |
| Clinical success | 87.5% | 75% | 1a |
| Complications Major (per patient) | 25% | 25% | 1a |
| Relapse | 0% | 25% | 0.467a |
| Survival (patients) | 87.5% | 87.5% | 1a |
| Survival (grafts) | 100% | 87.5% | 1a |
| Patency primaria (3/6/12/60 months) | 1/1/1/1 | 0.93/0.93/0.93/0.70 | 0.097b |
Our results support the hypothesis that the therapy of the obstruction of the liver venous drainage post-OLT with self-expandable is safe and efficient. Efficacy parameters show excellent results of both technical and clinical results (>81.25%) with a low relapse rate (12.5%). On the other hand survival rates for patients and grafts are high (>87.5%) showing one acceptable rate of major complications (12.5%) based on the medical reference. The results in patients with early and late obstruction of the liver venous drainage are not significantly different, yet there is a trend to show major primary patencies in cases of early obstructions.
The obstruction of the liver venous drainage is an uncommon and potentially serious complication of OLT with a mortality rate of up to 24%.3 It also affects significantly the survival of the graft. The incidence has been established between 1% and 7%3–5 of patients with OLT, yet some studies show several variations based on the technique used–cadaver donor, living donor, reduced graft, pediatric patients, etc. In our series the incidence is 5.4%, similar to the incidence rates published for the OLT of the cadaver donor.
Over time multiple causes have been proposed as being related with this complication. The early obstruction of venous anastomosis (8 cases in our series) can be related to one direct compression of anastomosis due to one excessively large or congested graft, the torsion of venous anastomosis when the graft is too small or when the stitches are very tight.3,4 Late obstructions–8 cases in our series–have been associated with hyperplasia of the intima and/or fibrous changes in anastomosis.11 One contributing factor to the obstruction of the liver venous drainage in patients with the piggyback technique for OLT is the type of vascular cuff. Parrilla et al.4 retrospectively studied one cohort of 1112 cases, and the rate of complications varied based on the type of vascular cuff used. The incidence of complications (7.3 vs 0.9%) and the risk of early obstruction of flow (1.6 vs 0.28%) were greater in patients with 2 vessel-cuff rather than with 3 vessel-cuff. This greater incidence could be related to a reduction in the diameter of anastomosis–at least 1cm smaller and the greater length of the venous segment between the graft and the receiver's ICV. As a consequence the risk of morbidity can be greater for the graft when a 2 vessel-vascular cuff is used.21–23 In our series 14 out of the 15 cases with the piggyback technique had a 3 vessel-vascular cuff.
The diagnosis of the obstruction of the liver venous drainage post-OLT is based on the finding of clinical, lab and image modality alterations like ultrasound, CT or venography/manometry studies.8,24,25 Pressure gradients have a key role in diagnosis, yet there is no consensus on what gradient values correspond with a significant stenosis. One gradient >5–6mmHg is widely accepted as the threshold to induce symptoms, yet pressure gradients >3mmHg can be hemodynamically significant.21 Ko et al.18 claim that in the presence of symptoms endoprosthesis should be implanted even with low gradients in an attempt to improve the clinical characteristics and prevent obstructions.
Balloon-angioplasties and endoprostheses have frequently been reported in patients with OLT for the management of portal vein stenosis and receiver's ICV.7–9 However, they are not as common for the management of suprahepatic veins. Even though some cases10,14 and short series11,12,18,19,26 have been published there is still little information on the long-term results of endovascular therapy. Whether associated or not with OLTs6,26 balloon-angioplasties have proven to be an effective therapy for the obstruction of the liver venous drainage. However, the incidence of relapse for obstructions is high showing primary patencies around 80%, 60% and 60% 3, 12, and 60 months after the proceeding26 suggestive of a high rate of reinterventions. To guarantee long-term primary patency and avoid reinterventions the implantation of endoprosthesis is one strategy that needs to be taken into account in patients with both early and late obstructions.21,24
The vascular accesses more widely reported for the implantation of endoprosthesis in suprahepatic veins are the transjugular and transfemoral accesses.10 Transhepatic access is rarely used though it has some advantages10,27,28 like offering a better view of the orifice of suprahepatic veins and its relation with the receiver's ICV which in our opinion allows us to be way more accurate when implanting the endoprosthesis. This can be especially important when using self-expandable endoprostheses due to the shortening they suffer when deployed. Thus transhepatic access can reduce the risk of endoprosthesis malpositioning or protrusion at the level of the ICV.18 Other advantages are the possibility of catheterizing simultaneously several suprahepatic veins or a lower risk of cardiac perforation or ICV rupture during the proceeding.28 Setbacks have to do with the hepatic capsule perforation and the theoretically higher risk of immediate complications (intraperitoneal hemorrhage and hepatic laceration).15 Most complications can be prevented through ultrasound monitoring of the puncture entry site. When the proceeding is over the transhepatic tract needs to be embolized with coils or other materials.27
When it comes to choosing the type of endoprosthesis to use both self-expandable and balloon-expandable endoprostheses are right and adequate options. Balloon-expandable endoprostheses seem to offer a greater radial resistance which in turn can be better for the management of stenoses of fibrous origin.17 Also they show a smaller intrinsic shortening when deployed. However, self-expandable endoprostheses adapt themselves to the very shape of the vein in which they are implanted which allows us to better maneuver for deployment purposes inside the piggyback anastomosis, yet we still need to be careful with shortening when we implant them. So this election needs to be based on the interventional radiologist's expertise and skills with all types of endoprostheses.
After a follow-up of 5331 days (median 3.34 years) results showed survival rates of 87.5% for patients and 93.7% for grafts. These results are similar to others published ranging between 72% and 100%.11,19 In our series the causes of death corresponded with infectious complications associated with the transplant and immunosuppressant therapy. The rate of technical success (93.7%) was similar to that of other series–ranging from 94% to 100%.11,18,19 Our rate of clinical success (81.25%) was consistent with the results showed by Ko et al.18 (82.2%), but slightly under the rate found by other groups (92.3–100%).11,19 The rate of obstruction relapse was 12.5% lower than the one reported for balloon-angioplasty and similar to the rates published by Ko et al. (12.94%) and Carnevale et al. (11.11%).18,19 The overall patency rate was 97%, and the primary patency rate close to 91.7% (from 6 to 5331 days) while the assisted patency rate was 5.8%. In endoprostheses with a limited primary patency the obstruction reappeared at 62, 301 and 512 days and needed reintervention. While assessing the effectiveness of therapy in patients with early and late obstruction of the hepatic venous flow we did not find significant differences but a trend toward a greater primary patency in patients with early obstructions. When it comes to the security of proceeding, the overall rate of complications was 25%. The incidence of immediate complications (2 cases, 12.5%) is slightly greater than the one found in other series (0–5.5%),7,12 yet in both cases a rapid sequelae-free recovery was reported. The rate of late complications (12.5%) is not greatly different from that obtained by other series ranging from 4.6% to 7.6%.11,18,19
The main limitation of this study is the retrospective study with only one study arm and a small sample. However in most patients the follow-up period was a long one given the analysis is limited to descriptive statistics and the results are not very influenced by limitations. Only the comparisons between early and late groups are limited by the number of patients, so any findings need to be confirmed through directed studies.
In sum the endovascular therapy with self-expandable endoprostheses is a risky but effective technique for the management of the obstruction of the liver venous drainage post-OLT. When results are compared to the management of patients with early and late obstructions post-OLT there are no significant differences, yet there is a trend to believe that primary patency is greater in patients with early obstructions.
Author contributions- 1
Manager of the integrity of the study: JIB.
- 2
Original idea of the study: JIB, FR, JIH.
- 3
Study design: JIB.
- 4
Data mining: GVR, AAB, ISY.
- 5
Data analysis and interpretation: GVR, ISY.
- 6
Statistical analysis: GVR, AAB.
- 7
Reference search: GVR, AAB, ISY.
- 8
Writing: GVR, ISY.
- 9
Manuscript critical review with intellectually relevant contributions: JIB, FR, JIH, AAB.
- 10
Final version approval: GVR, AAB, ISY, FR, JIH, JIB.
Authors confirm that all proceedings and experiments followed relate to the committee of responsible human experimentation ethical rules and regulations in compliance with the World Medical Association and the Declaration of Helsinki.
Data confidentialityAuthors confirm that the protocols of their centers have been followed on matters concerning the publishing of data from patients. They also confirm that all patients included in this study have been given enough information and handed over their written informed consent for their participation in this study.
Right to privacy and informed consentAuthors confirm that in this report there are no personal data of patients.
Conflict of interestsAuthors reported no conflicts of interests.
Please cite this article as: Viteri-Ramírez G, Alonso-Burgos A, Simon-Yarza I, Rotellar F, Herrero JI, Bilbao JI. Obstrucción del drenaje venoso hepático tras trasplante: resultados del tratamiento con endoprótesis autoexpandibles. Radiología. 2015;57:56–65.