In March 2020, the World Health Organization declared a global pandemic of COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); epidemic conditions continue in nearly all countries today. Although the symptoms and imaging manifestations of COVID-19 predominantly involve the respiratory system, it is fundamental to know the manifestations of the disease and its possible complications in other organs to help in diagnosis and orient the prognosis. To improve the diagnostic process without increasing the risk of contagion unnecessarily, it is crucial to know when extrathoracic imaging tests are indicated and which tests are best in each situation. This paper aims to provide answers to these questions. To this end, we describe and illustrate the extrathoracic imaging manifestations of COVID-19 in adults as well as the entire spectrum of imaging findings in children.
El síndrome de distrés respiratorio grave por el virus coronavirus 2, conocido como SARS-CoV-2, fue declarado pandemia mundial en marzo de 2020 por la Organización Mundial de la Salud y sigue activo actualmente en casi todos los países del mundo. Aunque los síntomas y manifestaciones en pruebas de imagen predominan en el aparato respiratorio, conocer las manifestaciones y posibles complicaciones en otros órganos será fundamental para ayudar al diagnóstico y orientar hacia el pronóstico de la enfermedad. Saber cuándo están indicadas las pruebas de imagen extratorácicas y cuáles son más rentables en cada circunstancia será crucial para mejorar el proceso diagnóstico sin aumentar innecesariamente el riesgo de contagio. En este trabajo hemos tratado de proporcionar estas respuestas, y hemos descrito iconográficamente las manifestaciones radiológicas de la enfermedad COVID-19 en regiones extratorácicas en adultos, así como en su conjunto en el paciente pediátrico.
Lung impairment is the primary manifestation of COVID-19. However, SARS-CoV-2 infection is not limited to the respiratory tract. It can affect other organs, and sometimes its impact on other organs dominates the clinical picture. The first part of this article consists of a review of existing knowledge to date on extrathoracic damage caused by COVID-19. The second part is a review of the disease in children.
Imaging of extrathoracic manifestations of COVID-19 in adultsAlthough the scientific literature is growing, the evidence around extrathoracic manifestations is based in part on clinical conjecture, case reports and post-mortem data. Moreover, in many organs, it is difficult to distinguish abnormalities due to the virus itself from thrombotic phenomena and from a clinical picture of immune activation and secondary shock leading to death.1–3 Thus it is not possible to draw definitive conclusions as to the true prevalence of extrapulmonary impairment due to the virus and, therefore, of the radiological manifestations thereof.
Various pathophysiological mechanisms have been put forwards to account for extrathoracic impairment due to COVID-19. Valdés et al.4 presented an outline of these mechanisms. The presence of ACE2 receptors (for which the SARS-CoV-2 virus has a strong affinity) on the cells of different organs would suggest direct damage by the virus, although as yet there are few reports substantiating this from a pathology perspective.1–3 For this reason, and because some of these symptoms develop late and in more seriously ill patients, the pathophysiology of these manifestations has also been linked to an exaggerated systemic immune response, hypoxia, coagulopathy, multifactorial vascular impairment and even drug toxicity.
The main extrapulmonary manifestations that have been reported are haematological, neurological, cardiac, gastrointestinal, hepatic, renal, cutaneous and ocular.5,6
Haematological and laboratory manifestationsMany laboratory abnormalities in COVID-19 are linked to the prognosis of the disease5,7 and signify in many cases this state of immune system activation and shock. Notable among these for their significance are clotting abnormalities, which clinically manifest as an increased incidence of thrombotic phenomena on multiple levels, both in the form of venous thromboembolic disease and in the form of arterial or venous thrombosis in any location. Both thrombi themselves and secondary visceral ischaemic lesions are seen on imaging (Fig. 1) and constitute a common finding on autopsy.1,2 In COVID-19, there is a higher incidence of this type of event than in other viruses,8 both in seriously ill patients9 and in non-hospitalised patients.10 This is among the main reasons why these patients must be assessed with imaging tests.
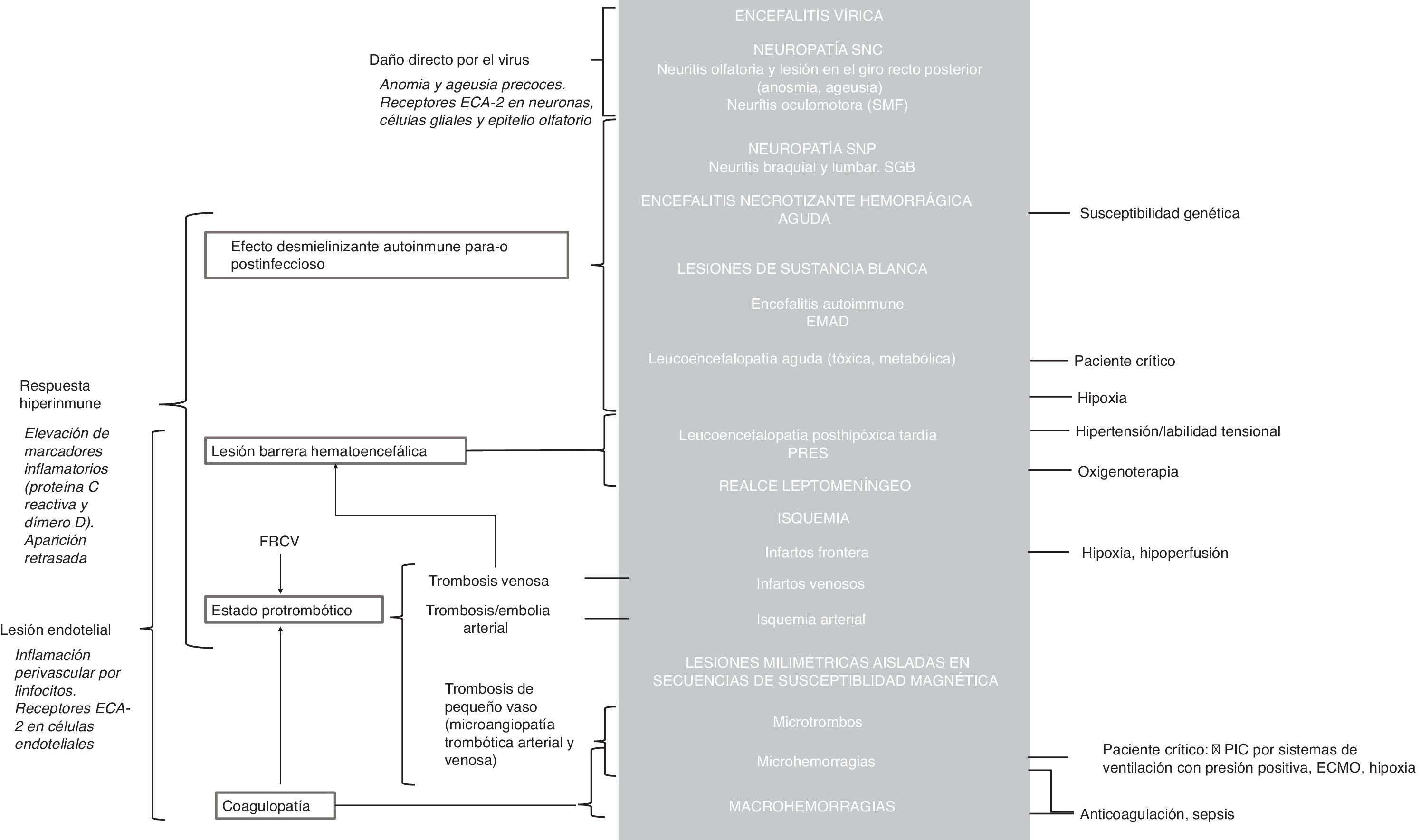
Neurological manifestationsNeurological manifestations resulting from acute COVID-19 are particularly important given their frequency, relative specificity and seriousness, and because they translate to abnormalities in neuroradiology studies. Their pathophysiology is not well known. Three main mechanisms with likely synergistic effects have been postulated6,11: a) direct neuron damage by the virus, b) demyelinating effect of autoimmune origin in the para-infection or post-infection period secondary to inflammatory hyper-response, and c) vascular endothelial injury. However, other concurrent circumstances, regularly seen in critically ill patients, may also play a role12 (Fig. 2).
Possible pathophysiological mechanisms involved in neurological lesions caused by the SARS-CoV-2 virus with their respective manifestations on imaging tests (grey background). The arguments in favour of each of the main mechanisms appear in italics. See the supplementary data for detailed pathophysiological explanations.
ACE2: angiotensin converting enzyme 2; ADEM: acute disseminated encephalomyelitis; CNS: central nervous system; CVRFs: cardiovascular risk factors; ECMO: extracorporeal membrane oxygenation; GBS: Guillain-Barré syndrome; ICP: intracranial pressure; MFS: Miller Fisher syndrome; PNS: peripheral nervous system; PRES: posterior reversible encephalopathy syndrome; RT-PCR: reverse transcription polymerase chain reaction.
Clinically, they manifest with anosmia, ageusia, myalgia, headache, dizziness, dysautonomia, focality due to cerebrovascular episodes, seizures and encephalopathy,13,14 which are present in between 36%15 and 50% of patients with acute COVID-19 admitted to hospitals. Of these, between 21%16 and 60%11,17 show abnormalities on computed tomography (CT) and/or magnetic resonance imaging (MRI), with this rate reaching 84% in patients admitted to intensive care units (ICUs).18 The incidence could be even higher, given the challenges involved in examinations and imaging tests (isolation, agitation and transfer difficulties) in these patients.19 Therefore, neuroimaging tests are indicated in patients with new neurological or psychiatric symptoms, except in cases of mild symptoms characteristic of the clinical picture of the disease (ageusia and anosmia). CT is the neuroimaging test of choice in patients with suspected cerebrovascular lesions, the majority of which will be ischaemic strokes. If the CT scan is negative, but clinical suspicion persists, an abbreviated MRI protocol can be done with T1-weighted, T2-FLAIR and diffusion-weighted sequences, as well as T1-weighted sequences with contrast if meningitis or encephalitis is suspected.6
There is a broad spectrum of neuroradiological manifestations, with patterns that are sometimes difficult to classify:
- A
Central nervous system (CNS):
- a
Acute ischaemic lesions. These account for 27%–65% of all acute neurological lesions.11,16,19 They are seen in between 1.1%19 and 11%16 of hospitalised patients and constitute a marker of a poor prognosis, with a high rate of mortality.19,20 They are predominantly seen in men, elderly patients and patients with vascular risk factors.11 They usually develop from a week after the onset of signs and symptoms. They are more serious in patients with serious lung impairment due to COVID-19.15 Compared to ischaemic strokes in patients without SARS-CoV-2 infection, they are more serious and affect younger patients.20
- a
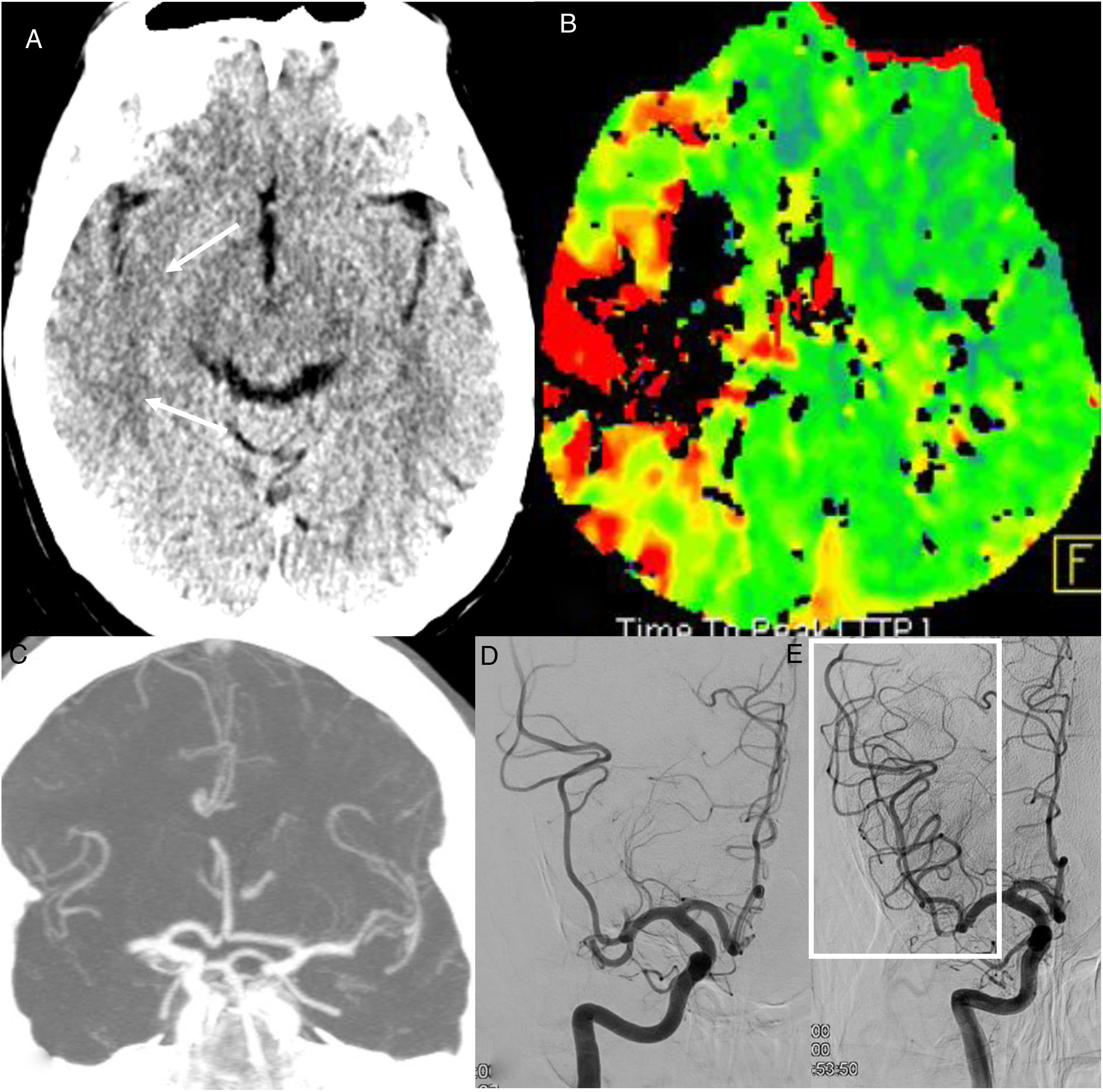
On neuroradiology studies, they manifest as acute/subacute, multiregional, supratentorial or subtentorial infarctions (Fig. 3),16 due to large-vessel injury (45% of the total) (Fig. 4) or small-vessel injury (21% of the total6), or due to hypoperfusion, with the development of infarctions in the frontal region.18 The most common origin is cryptogenic (52%), followed by cardioembolic (25%).16
- b
Non-ischaemic lesions:
- i
White-matter (WM) lesions. These are usually supratentorial. In 25% of cases, two or more patterns are combined21:
- 1
Non-confluent WM lesions. The frequency of these is unknown (uncommon11 versus 30%21). These non-confluent lesions are hyperintense on T2-FLAIR sequences and hypodense on CT, with a variable degree of enhancement with contrast. They may be associated with microhaemorrhagic lesions. It has been suggested that they mimic the radiological presentation of acute disseminated encephalomyelitis (ADEM), with predominant impairment of the centrum semiovales, as well as acute haemorrhagic leukoencephalitis, although these diagnoses require specific cerebrospinal fluid (CSF) abnormalities that have not been found in samples of patients with confirmed SARS-CoV-2 infection. The subcortical WM is affected more commonly in seriously ill patients.22 These lesions are most likely of para-infectious inflammatory demyelinating origin, given the signs of hyperacute ADEM in histology samples,23 the lag in their presentation of at least one week following infection and the radiological findings.
- 2
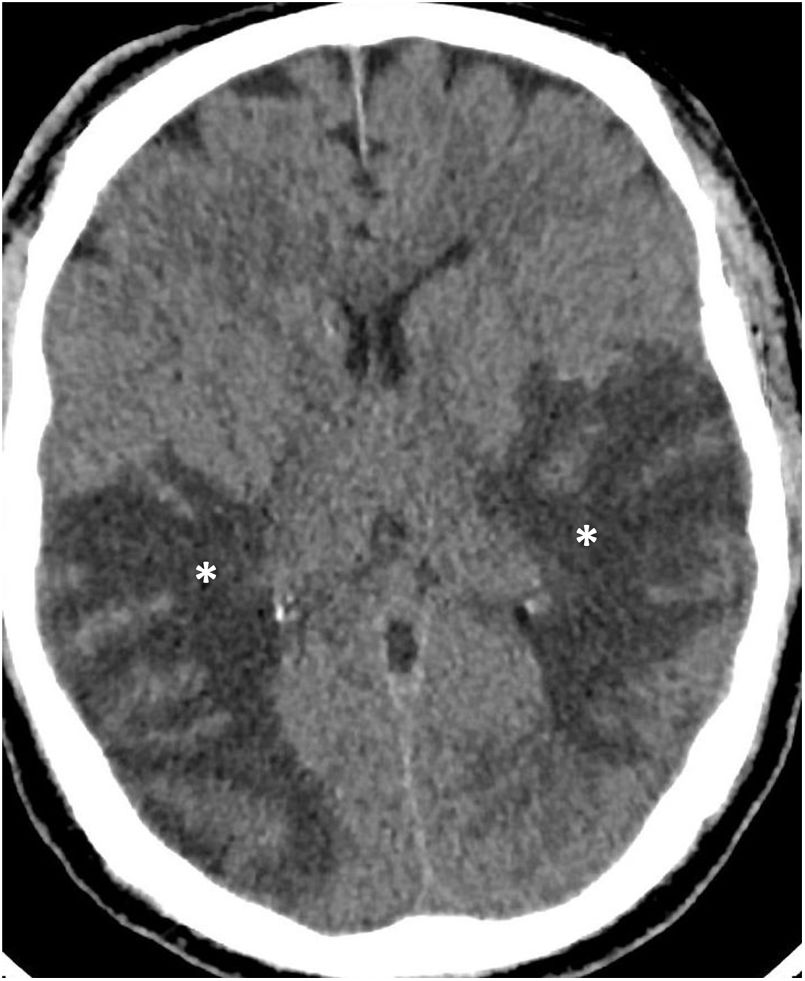
Confluent WM lesions or diffuse leukoencephalopathy (Fig. 5). These lesions are hyperintense on T2-FLAIR sequences, more extensive, confluent12,21,24 and symmetrical25 with moderate diffusion restriction. They affect the periventricular and subcortical deep WM, leaving the juxtacortical WM intact to a certain degree, rarely affecting the subtentorial WM and sparing the deep grey matter.24 They have been reported in critically ill patients, with acute respiratory distress syndrome and persistently altered mental state, suggesting a hypoxic lesion mechanism and, given the diffusion restriction,24 demyelinating behaviour. They have been likened to: late posthypoxic leukoencephalopathy,24,26 with frontal region injury; sepsis-associated encephalopathy of metabolic or toxic origin; and posterior reversible encephalopathy syndrome (PRES)27 with confluent lesions hyperintense on T2-FLAIR sequences in typically (but not exclusively) occipital subcortical WM,28 related to increased or labile blood pressure,11 and kidney failure (Fig. 6).
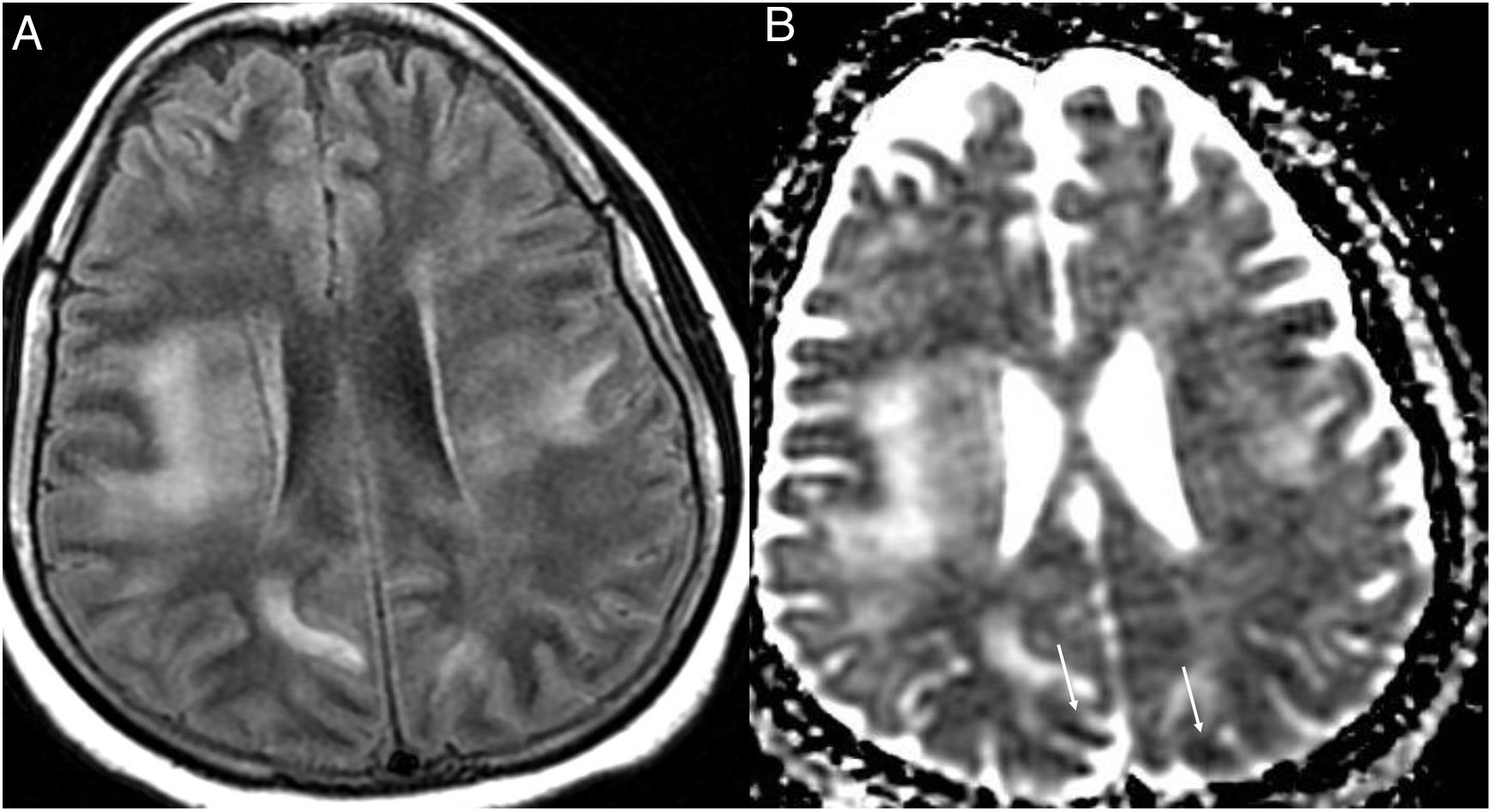
Figure 5.A 60-year-old man admitted to ICU due to COVID-19 pneumonia requiring intubation. During admission he presented left hemiparesis. A single computed tomography scan showed hypodense lesions in the periventricular and subcortical white matter (asterisks) in both temporal and parietal lobes. Acute diffuse leukoencephalopathy in a critically ill patient was suspected. (Figure courtesy of Dr Cristina Utrilla [Hospital La Paz, Madrid]).
Figure 6.A 35-year-old woman with nosocomial SARS-CoV-2 infection, with headache and low-grade fever. Magnetic resonance imaging showed confluent lesions in the periventricular and subcortical supratentorial white matter hyperintense on the T2-FLAIR sequence (A) and with diffusion restriction in the apparent diffusion coefficient (ADC) (arrows) (B). Posterior reversible encephalopathy syndrome was suspected. (Figure courtesy of Dr Cristina Utrilla [Hospital La Paz, Madrid]).
- 1
- ii
Lesions in specific locations:
- 1
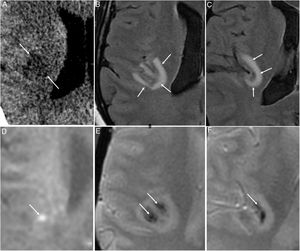
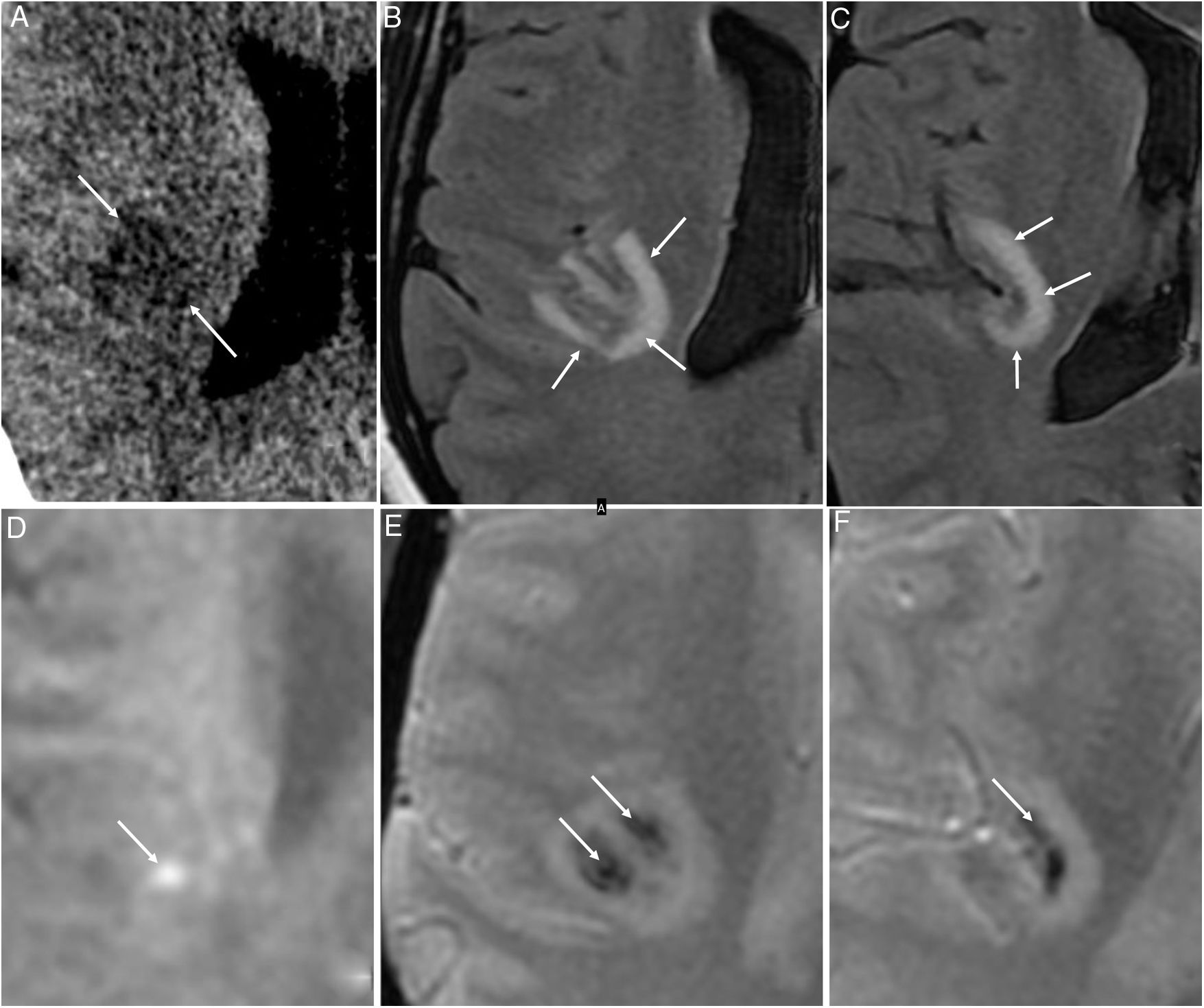
Medial thalamus, medial temporal lobe and subinsular region, as acute haemorrhagic necrotising encephalitis. These lesions are hypodense on CT, with a haemorrhagic component and ring enhancement on MRI, and are generally bilateral and symmetrical (Fig. 7). They have been linked to inflammatory hyper-response29 in genetically susceptible patients.
Figure 7.A 48-year-old woman with acute COVID-19 who had developed sensory abnormalities in the left half of her body four to five days earlier. An initial computed tomography scan showed a right insular hypodense lesion (arrows in A). Subsequent magnetic resonance imaging determined the lesion to be juxtacortical and hyperintense on the T2-FLAIR sequence (arrows in B and C), with some bright foci on the diffusion-weighted sequence (arrow in D) and haemorrhagic foci on T2-weighted imaging* (arrows in E and F). These findings raised suspicion of acute haemorrhagic necrotising encephalitis.
- 2
Medial temporal lobe. Unilateral lesions hyperintense on T2-FLAIR and diffusion sequences, similar to viral encephalitis (caused by herpes simplex virus, human herpesvirus 6 or Epstein-Barr virus) and to autoimmune limbic encephalitis.21 According to some series, these are the most common neurological findings (43%).21 However, no data were found for encephalitis with the SARS-CoV-2 virus in histology studies.30
- 3
Splenium of the corpus callosum, affected by lesions hyperintense on T2-FLAIR sequences and microhaemorrhages. This has been likened to Susac syndrome, which is linked to immune-mediated arteriolar occlusion. It is uncommon.31
- 1
- i
- c
Haemorrhages:11
- i
Parenchymal. These are reported in 3.6% of patients with neuroimaging tests.16 They may be hemispheric, lobar, cerebellar and/or in the basal ganglia, and have an opening to a ventricle or subarachnoid space.11 In a long series,16 60% exceeded 5 cm, were open to a ventricle and showed displacement from the midline and cerebral hernia. They may be associated with ischaemic lesions, with the non-ischaemic lesions described,6,21,23 or spontaneous. Some 24% of all infarctions in patients with COVID-19 are haemorrhagic.6 This worsens their prognosis19 and the intrahospital mortality rate exceeds 50%.
- ii
Subdural.16
- iii
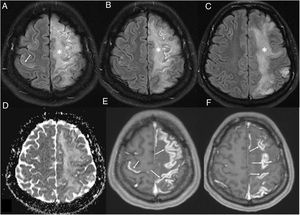
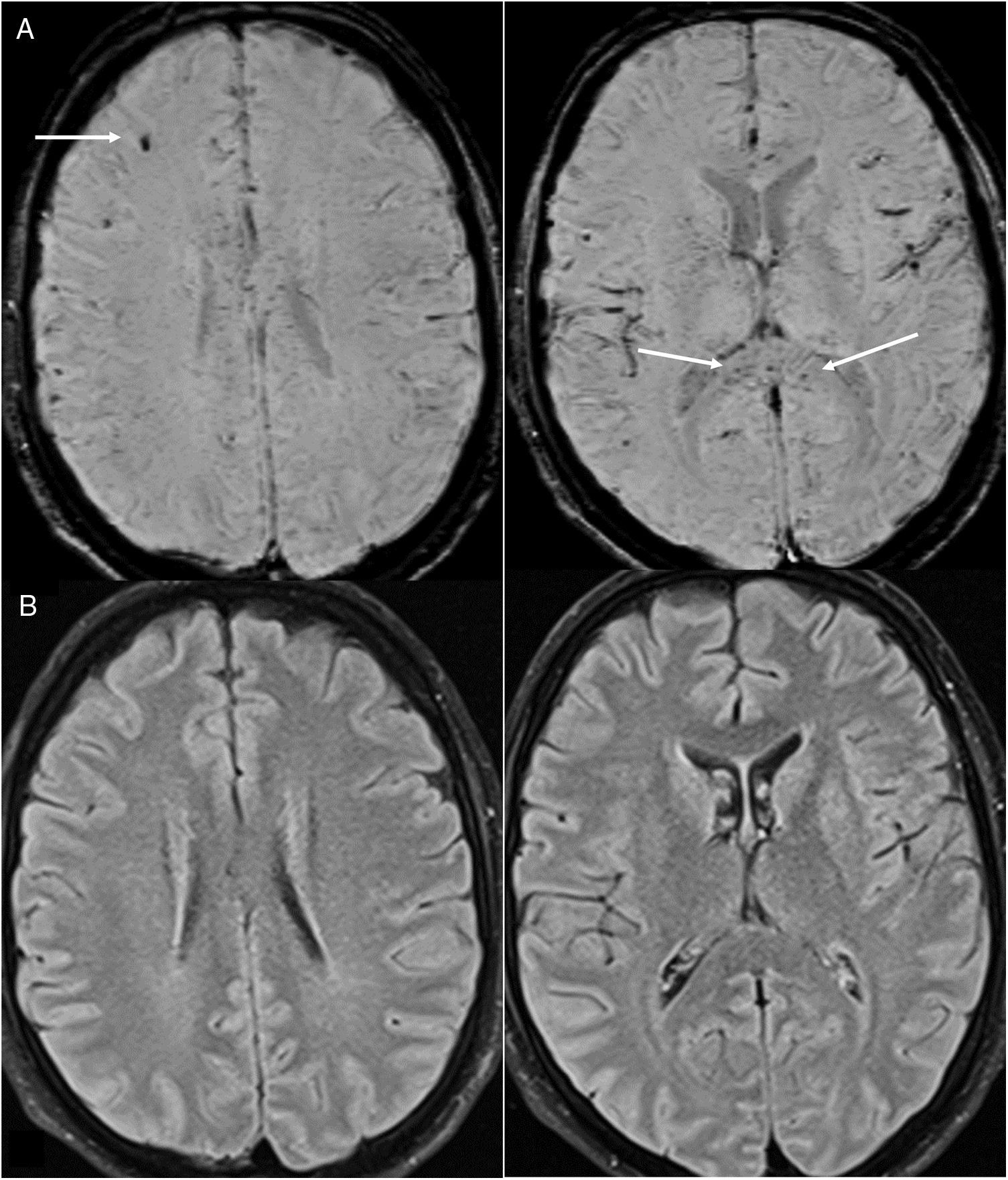
Lesions measuring millimetres on susceptibility-weighted sequences. They are reported in up to 24% of patients who undergo MRI,21 most of whom are critically ill.24 They consist of multiple foci measuring millimetres (more than 15) that are hypointense on susceptibility-weighted sequences and located in the juxtacortical WM and/or corpus callosum (Fig. 8). They may be accompanied by confluent lesions in the WM. They have been attributed to microhaemorrhages23 originating from endothelial dysfunction, microbleeding in critically ill patients16,23,24 and, among those in the corpus callosum, abnormal cerebral venous return due to increased intrathoracic pressure in patients with positive-pressure ventilation.32 Others believe them to be due to thrombotic microangiopathy.33 Thrombi could be located both in arteries, which would account for foci of cortical infarction visible on MRI, and in small cortical vessels, given their hyperdensity, possibly due to a slow flow rate.
Figure 8.A 35-year-old man admitted for acute COVID-19 and serious lung impairment which required treatment with an extracorporeal membrane oxygenation system. He presented altered consciousness. Brain magnetic resonance imaging showed multiple hypointense foci that were juxtacortical and in the corpus callosum on MRI susceptibility-weighted imaging (arrows in A); these could be attributed to microhaemorrhages or microthrombosis and were not associated with parenchymal lesions on T2-FLAIR imaging (B).
- i
- d
Other lesions:
- i
Abnormal cortical signals on T2-FLAIR sequences in ICU-admitted patients.12
- ii
Lesions hyperintense on T2-FLAIR sequences in the medial cerebellar peduncles and the medial portions of the cerebellar hemispheres.23
- iii
Pseudotumor cerebri.
- iv
Increase in the subarachnoid space around the optic nerves, possibly due to an increase in intracranial pressure caused by ventilation mechanisms.25
- i
- e
Extra-axial lesions:
- i
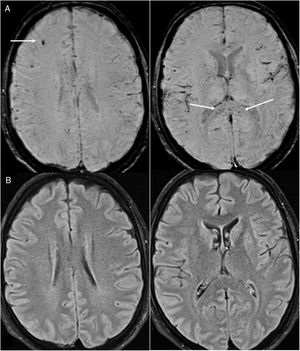
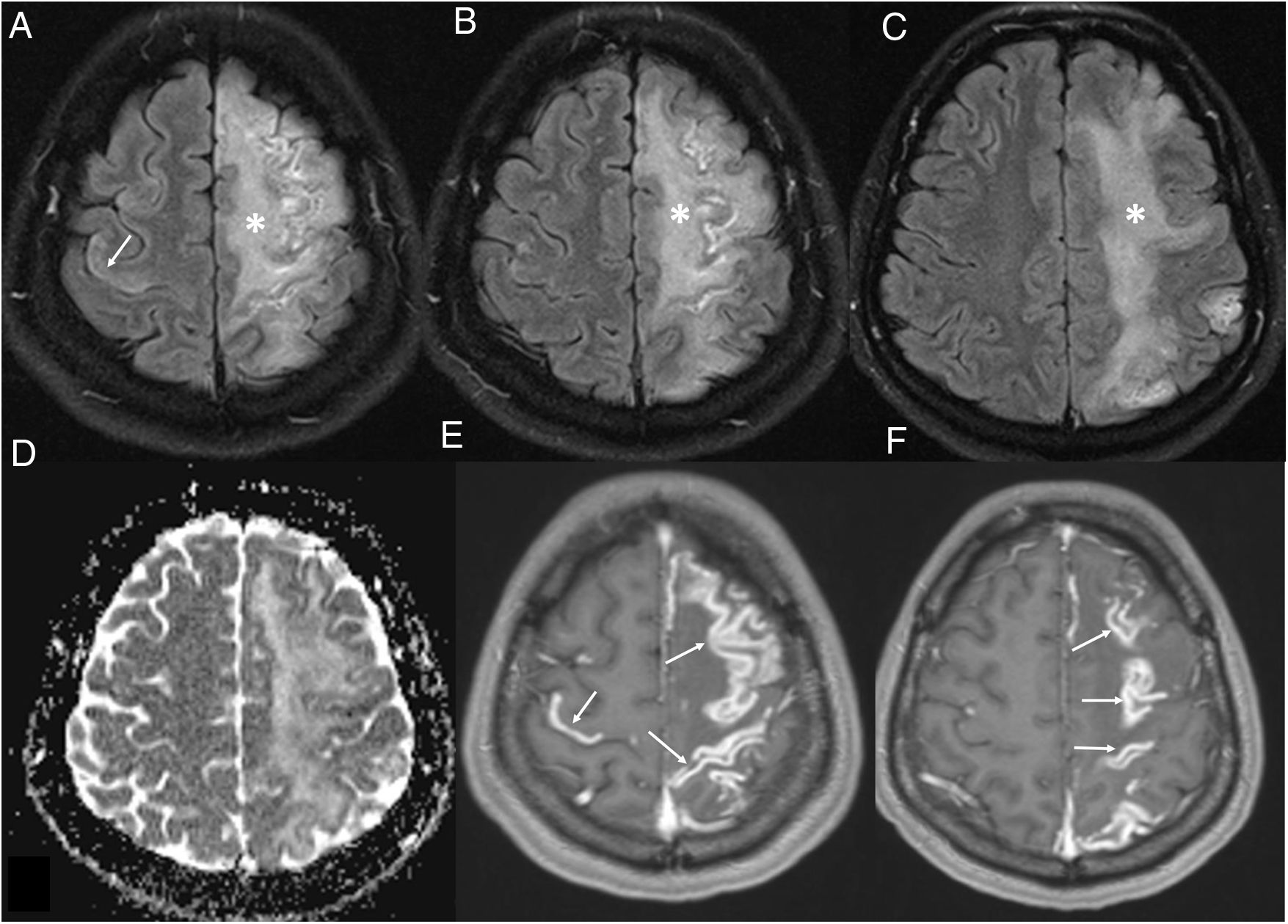
Meninges. Leptomeningeal enhancement has been reported in 17% of patients with neurological symptoms.19,34,35 It reflects leptomeningeal inflammation, also reported in pathology samples, although it could also result from oxygen therapy.18 It may be associated with leukoencephalopathy (Fig. 9).
Figure 9.A 36-year-old woman admitted for COVID-19 pneumonia who presented an acute neurological deficit in the right half of her body. Brain magnetic resonance imaging showed diffuse left frontoparietal (asterisks in A–C) and right prefrontal (arrow in A) corticosubcortical abnormalities on T2-FLAIR imaging, with no restriction on the apparent diffusion coefficient map (D), which was associated with intense leptomeningeal enhancement on T1-weighted imaging with contrast (arrows in E-F). Meningoencephalitis was suspected.
- ii
Vessels. Free-floating thrombi in the carotid arteries,11 carotid dissection and venous sinus thrombosis have been documented. Venous infarctions should be suspected in lesions in areas not corresponding to arterial regions. These lesions are often bilateral and haemorrhagic.
- i
- b
- B
Neuropathy:
- a
Intracranial nerves. Some 2.2% of patients are affected16:
- i
Olfactory neuritis. Anosmia and ageusia of sudden, early (during the first week) onset have been reported in more than 50% of cases, and are considered a possible biomarker of the disease,16 primarily when they are not associated with nasal congestion. This is more common in young women and patients with mild signs and symptoms. Patients with anosmia have been reported to show hyperintense lesions in the olfactory bulbs16 and in the cortical region of the posterior gyrus rectus (related to olfaction 5,15,36,37). Most cases are reversible within weeks. They are not associated with paranasal sinus abnormalities.38
- ii
Oculomotor nerve neuritis. This has been linked to Miller Fisher syndrome (MFS),16 an intracranial variant of Guillain-Barré syndrome (GBS), which presents with diplopia, ataxia and areflexia. Enlargement and hyperintensity of oculomotor nerves have been found on T2-FLAIR sequences in MRI, with marked contrast enhancement.
- i
- b
Peripheral nerves. This is reported exclusively in men, with a mean age of 60 years, one to two weeks after the onset of viral signs and symptoms, with a variable prognosis23:
- i
GBS.23,34,39 This appears earlier than in other viral infections. There are no specific neuroradiological manifestations.
- ii
Plexopathy. This presents with weakness but no pain. Increased signal and enhancement in brachial and lumbar plexus nerves have been found on MRI.
- i
- a
A 71-year-old man with a history of chronic kidney disease, liver failure, hypertension and acute COVID-19 infection. Chest computed tomography (CT) (A) showed patchy ground-glass opacities in both lungs that could be attributed to COVID-19. The patient presented right hemiparesis and dysarthria during admission. Brain magnetic resonance imaging (B, diffusion-weighted sequence) identified multiple small multiregional infarctions, and CT angiography (C and D) identified calcified atheromatous plaques in both carotid bifurcations.
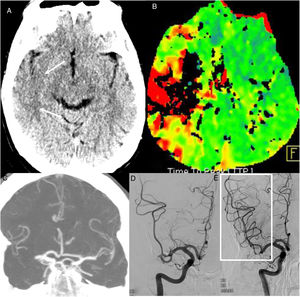
Acute arterial ischaemic lesions. A 63-year-old man attended at the hospital after having a stroke in the region of the right medial cerebral artery four hours earlier. PCR was positive for COVID-19. A single computed tomography (CT) scan (A) showed a right temporal cortical hypodensity (arrows) and perfusion CT (time-to-peak map) (B) showed a circulatory delay in the region of the right medial cerebral artery. CT angiography showed an occlusion of the right medial cerebral artery (C). Arteriography showed an occlusion of two divisions of the M2 segment of the medial cerebral artery (D) which were recanalised by means of mechanical thrombectomy (box in E).
Patients with SARS-CoV-2 infection have a reported incidence of cardiac impairment of 7%–30%; this incidence is higher (55%) in ICU patients and patients with cardiovascular risk factors (CVRFs).6,40 However, it may present with mild respiratory symptoms.6 Elevated levels of D-dimer and troponin and the presence of CVRFs have been associated with a higher mortality rate. Although the lesion mechanism is unknown, it is believed to be multifactorial, including direct effects by the virus, an imbalance between myocardial oxygen supply and demand, coronary artery thrombosis, immune system reaction and effects of administered treatments.40 Acute myocardial infarction is among the most common manifestations; others are stress myocardiopathy, myocarditis, pericarditis, arrhythmias and cardiogenic shock,40 in addition to multiregional thromboembolic phenomena. The best technique for evaluating cardiac effects is MRI.6
Hepatic and gastrointestinal manifestationsAbdominal symptoms are not rare in COVID-19: diarrhoea, vomiting and abdominal pain have been reported,41 in some cases as sole symptoms.42 For this reason, a finding of lesions at the bases of the lungs suggestive of COVID-19 on abdominal CT scans in patients with abdominal signs and symptoms but without suspected COVID-19 is of enormous clinical interest.43
Liver clinical chemistry abnormalities are common in patients with COVID-197 and are linked to more serious forms of the disease and administered treatments.44 Their radiological manifestations may be non-specific and include periportal oedema, diffuse liver abnormalities and steatosis.6,45 One study46 reported the presence of distension and biliary sludge due to cholestasis in 54% of the patients studied, more commonly in intensive care patients.
The radiological manifestations of the intestinal impairment reported were thickening and enhancement of the walls of both the small intestine and the large intestine42,45,46 and the presence of pneumatosis and portal gas (in 20% of ICU-admitted patients who underwent imaging studies46) which was linked to ischaemia in laparotomy.
An association has also been found between COVID-19 and acute pancreatitis,47 as well as splenomegaly and splenic infarctions (Fig. 1B) in a small percentage of patients.45
Renal and genitourinary manifestationsKidney abnormalities have been commonly reported in COVID-19, manifesting primarily with kidney failure, with a typically multifactorial aetiology that includes impairment by the virus itself, systemic inflammatory signs and symptoms and clotting abnormalities, in some cases secondary to rhabdomyolysis,3,6,48 and associated with more serious infection and a higher infection mortality rate.49
No extensive series of ultrasound evaluation of these patients have been published, although increased echogenicity and abnormal corticomedullary differentiation are to be expected.6,50
On CT without contrast, decreased attenuation of the kidney parenchyma and perirenal fat abnormalities can be identified,51 which are correlated with abnormal creatinine levels. Studies with contrast have also reported kidney infarctions46 (in up to 20% in one series45) and may demonstrate decreased kidney perfusion. This finding may even precede abnormal kidney function.52
The bladder wall may be thickened or show signs of cystitis.45
Finally, in the spectrum of genitourinary lesions, a link between COVID-19 and testicular pain has been observed, and there have been cases of orchitis in relation to SARS-CoV-2 infection.53
Musculoskeletal abnormalitiesMusculoskeletal impairment requiring evaluation on imaging is uncommon, even though myalgia and arthralgia are very common symptoms.54 There have been reports of myositis and rhabdomyolysis that present as oedema in the different imaging techniques. In advanced cases, these may lead to necrosis.6 Arthritis caused by the virus is uncommon, although rheumatological disease triggered by COVID-19 has also been reported.54 Finally, lesions of vascular origin may present in the soft tissues, due to either thrombosis with gangrene or haemorrhagic complications. Notable among these are haematomas of the rectus abdominis and haematomas of the iliopsoas muscles, which is an unusual location but may be characteristic of these patients, reported in one series in up to 7.8 per 1000 hospitalised patients.55
Cutaneous and ocular manifestationsThese include chilblains, erythematous rash, viral rash and generalised urticaria, which are usually self-limiting. They constitute useful markers of infection, and sometimes are the only sign thereof.6 The most common ocular manifestations are pain, secretion, redness and follicular conjunctivitis.6
Imaging of COVID-19 impairment in childrenSARS-CoV-2 infection can affect children of all ages, with less serious clinical manifestations than in adults.56–58 Fever, cough and dyspnoea are the most common symptoms; gastrointestinal symptoms, rhinorrhoea, chest pain and headache are less common.59,60 Maternal perinatal transmission has been reported in cases of asymptomatic or mild disease in newborns.61
Paediatric lung impairmentTo date, there are few studies on diagnostic imaging of COVID-19 in paediatric patients. In one study with 91 confirmed cases, the most common finding on chest X-ray was thickening of the peribronchial/perihilar wall.60 Children who follow a more serious clinical course and require ICU admission show ground-glass opacities and bilateral multifocal consolidations on chest X-ray.62
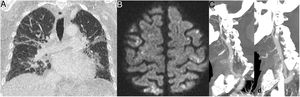
The most common radiological finding on CT consists of ground-glass opacities, visible in 62%–81% of cases, predominantly affecting the lower lobes. Consolidations, interstitial lesions and the tree-in-bud pattern have also been reported.59,60 Thickening of the peribronchial/perihilar wall63 and the halo sign64 (Fig. 10), defined as an area of consolidation surrounded by a ring of ground-glass opacity, are more common in children with COVID-19 than in adults. Lymphadenopathy, cavitation and pleural effusion are rare in this disease.59 Most cases in which follow-up studies were obtained showed radiological improvement.
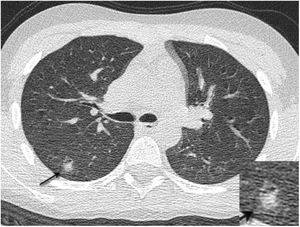
A 12-year-old boy with Wilms tumour with fever, cough and positive PCR for SARS-CoV-2. Preoperative computed tomography revealed multiple small pseudonodular consolidations (arrow) with a ground-glass halo (halo sign) on the periphery of both lower lobes, which had disappeared on the CT scan that was done two weeks later.
The studies conducted to date have shown that imaging findings of SARS-CoV-2 infection in children are non-specific and that it is not possible to distinguish this infection from other viral and bacterial diseases of the airways.60
Given that findings on X-ray and CT of the chest are absent or non-specific, imaging is unlikely to support or rule out the suspected diagnosis or change the clinical management. Therefore, it is not recommended that imaging studies be done to screen for SARS-CoV-2 infection in paediatric patients.65 X-ray, if deemed clinically indicated, should be the first-line imaging test.65 CT should be reserved for cases in which complications are suspected, in particular in children with other associated diseases (Fig. 10). Ultrasound may play a role in COVID-19 follow-up in paediatric ICUs,66 but it is not routinely recommended due to the risk of exposure for healthcare personnel.65 The use of standardised reports that suggest or rule out the presence of this infection is not recommended in paediatric patients.65
Paediatric neurological impairmentThe neuroimaging findings most commonly found in children with neurological impairment associated with SARS-CoV-2 infection were patterns similar to acute disseminated encephalomyelitis, foci of myelitis and neural enhancement. Neurovascular complications are less common than in adults and the majority of these patients follow a favourable clinical course.67
Paediatric abdominal impairmentThere is little literature concerning abdominal disease associated with SARS-CoV-2 infection,68 which is most commonly reported as being in association with paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2. Abdominal pain, vomiting and diarrhoea may be symptoms of presentation of this infection in paediatric patients.60 The most common findings in children who required imaging tests were inflammatory changes in the terminal ileum, mild ascites and mesenteric lymphadenopathy.68
Multisystem inflammatory syndrome in children temporally associated with SARS-CoV-2In April 2020, paediatricians from England and the United States detected an increase in cases with characteristics similar to septic shock, with signs of myocarditis and symptoms mimicking Kawasaki disease, and suggested a possible link to SARS-CoV-2 infection.69,70 Since then, more than a thousand cases have been reported at hospitals around the world.71,72
The diagnosis of multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection is complex and multidisciplinary. It requires elevation of clinical chemistry markers of inflammation, the absence of another infectious cause, signs of organ dysfunction (e.g. hypotension, shock or myocardial dysfunction) and occurrence in regions with a high prevalence of SARS-CoV-2 infection.73,74 The most common forms of presentation are fever, gastrointestinal symptoms, skin rash and conjunctivitis. Many of the children show imaging and laboratory findings suggestive of cardiac impairment, with clinical deterioration requiring ICU admission. Respiratory symptoms at the onset are uncommon and suggest acute SARS-CoV-2 lung infection.75
The pathophysiology of this disease seems to be related to a cytokine-mediated late postviral immunological reaction, rather than a direct attack on organs on by the virus.76 Therefore, serology studies should show an increase in immunoglobulin G with negative RT-PCR.75
The most common findings on chest X-ray and CT are signs of pulmonary vein congestion and of an acute lung inflammation process, ranging from mild impairment to signs of acute respiratory distress syndrome (Fig. 11A). Cardiomegaly, bilateral peribronchial/perihilar thickening, perihilar ground-glass opacities and consolidations are some of the most common radiological findings.75
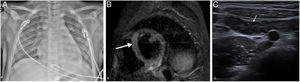
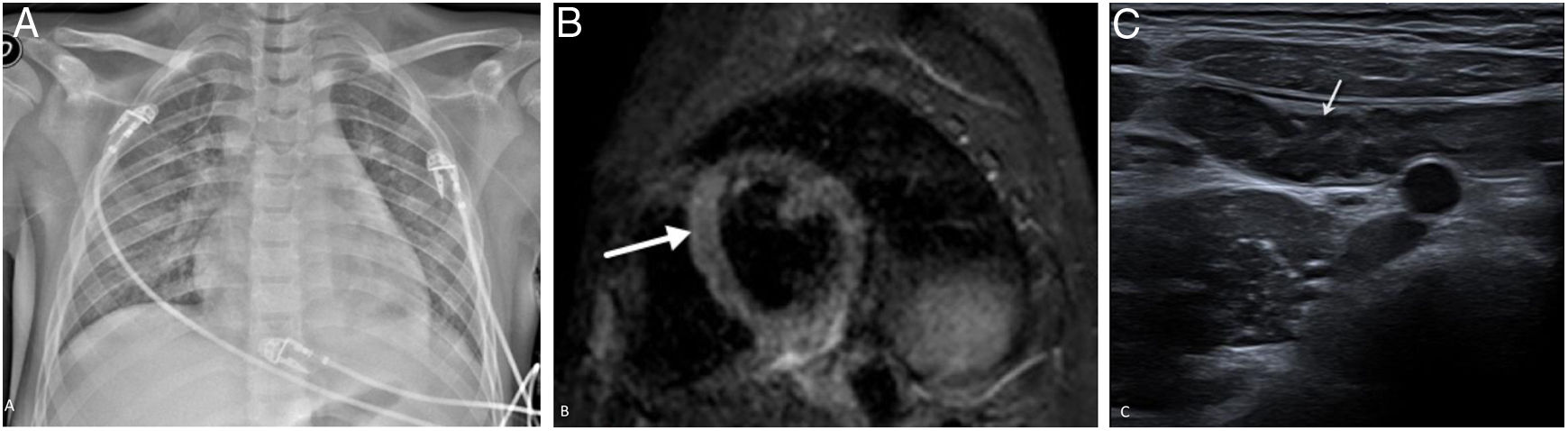
A 10-year-old boy with fever, rash, abdominal pain, chest pain and diarrhoea. He showed clinical, laboratory and imaging signs suggestive of myocarditis, requiring admission to the ICU, where he was diagnosed with MIS-C associated with SARS-CoV-2. PCR for SARS-CoV-2 performed six days after the onset of symptoms was negative, and inmunoglobulin G was positive after eight days. The X-ray (A) taken on admission to the ICU showed signs of pulmonary oedema, cardiomegaly, bilateral ground-glass opacities, basal laminar atelectasis and mild left pleural effusion. Cardiac magnetic resonance imaging identified a focus of myocarditis in the left ventricular septum (arrow) and slight pericardial effusion on the short-axis STIR sequence (B). Abdominal ultrasound showed ileitis (C), as well as hepatosplenomegaly ileitis, ascites and non-specific hyperechogenic matter in the bladder (not shown).
On echocardiography and MRI, signs of left ventricular dysfunction were detected, with a decrease in ejection fraction and in some cases pleural effusion.77,78 Cardiac MRI is useful in this situation to detect oedema, myocardial fibrosis/ischaemia and in some cases coronary artery dilation.72 The imaging pattern of this disease more closely resembles myocarditis (oedema and pericardial effusion) (Fig. 11B) than Kawasaki disease (aneurysms).
This disease can also affect the gastrointestinal tract, and the most common findings are inflammation of the terminal ileum and mild ascites (Fig. 11C). Hepatomegaly, splenomegaly, infarctions of abdominal solid organs, perivesicular/periportal oedema, an increase in the echogenicity of the renal cortex and lymphadenopathy are also common findings.75,79 Central nervous system impairment is possible in this disease, showing foci of diffusion restriction in the corpus callosum and leptomeningeal enhancement on MRI imaging.80
ConclusionBeing familiar with the manifestations on imaging tests of neurological, vascular, gastrointestinal, genitourinary and cutaneous abnormalities in SARS-CoV-2 infection in adults, as well as the expression of the infection in children, is essential for improving management. Multiorgan damage in COVID-19 suggests a complex, multifactorial pathophysiology that is not well known, and carries high rates of morbidity and mortality. Neurological and cutaneous manifestations can increase diagnostic specificity. Thrombotic complications, due to their seriousness and frequency, have a particular impact on prognosis.
Authorship- 1
Responsible for study integrity: JMPM.
- 2
Study concept: JJAJ.
- 3
Study design: JJAJ. JMPM.
- 4
Data collection: N/A.
- 5
Data analysis and interpretation: N/A.
- 6
Statistical processing: N/A.
- 7
Literature search: JMPM, AR, PCD, IB, EGG, JJAJ.
- 8
Drafting of the manuscript: JMPM, AR, PCD, IB, EGG, JJAJ.
- 9
Critical review of the manuscript with intellectually significant contributions: JMPM, AR, PCD, IB, EGG, JJAJ.
- 10
Approval of the final version: JMPM, AR, PCD, IB, EGG, JJAJ.
The authors declare that they have no conflicts of interest.
We would like to thank Dr Luis Gorospe (Thoracic Radiology Unit, Hospital Ramón y Cajal, Madrid) and Dr Cristina Utrilla (Neuroradiology Unit, Hospital La Paz, Madrid) for providing some of the images used to prepare this article.
Please cite this article as: Plasencia-Martínez JM, Rovira À, Caro Domínguez P, Barber I, García-Garrigós E, Arenas-Jiménez JJ. Manifestaciones extratorácicas de la COVID-19 en adultos y presentación de la enfermedad en niños. Radiología. 2021;63:370–383.




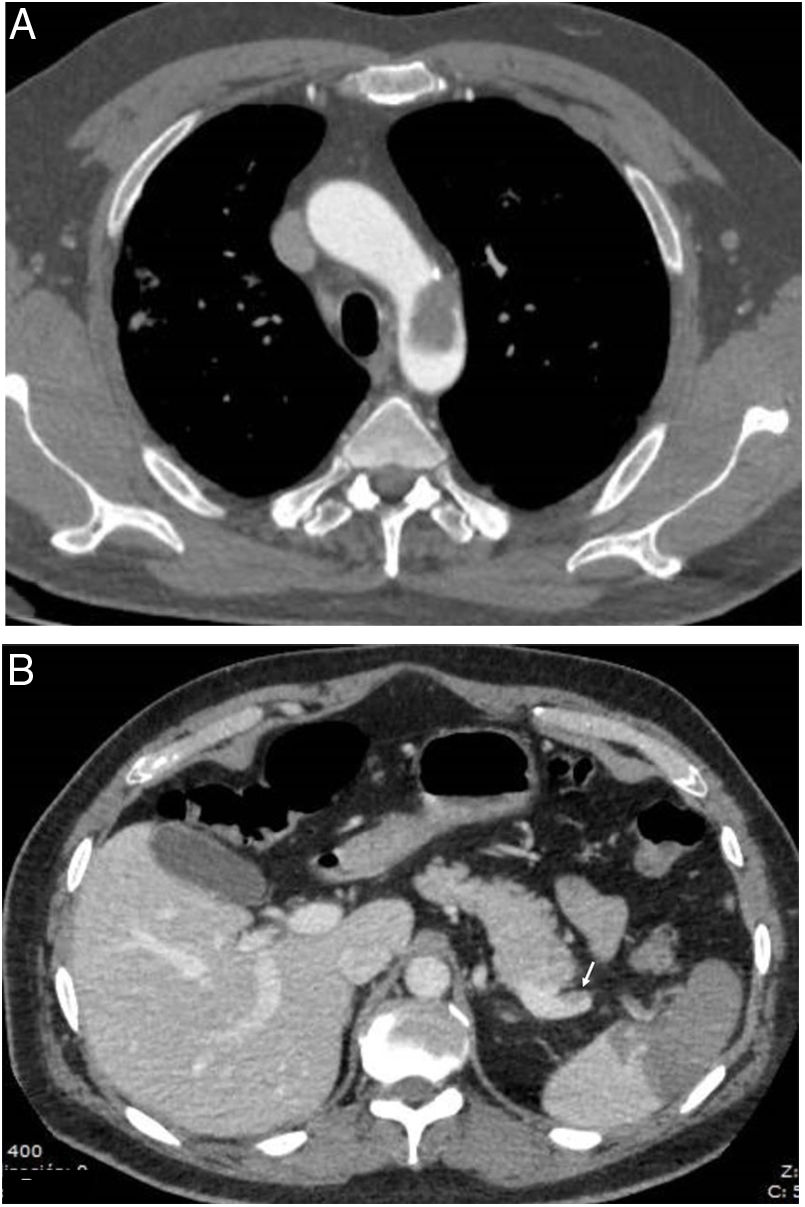
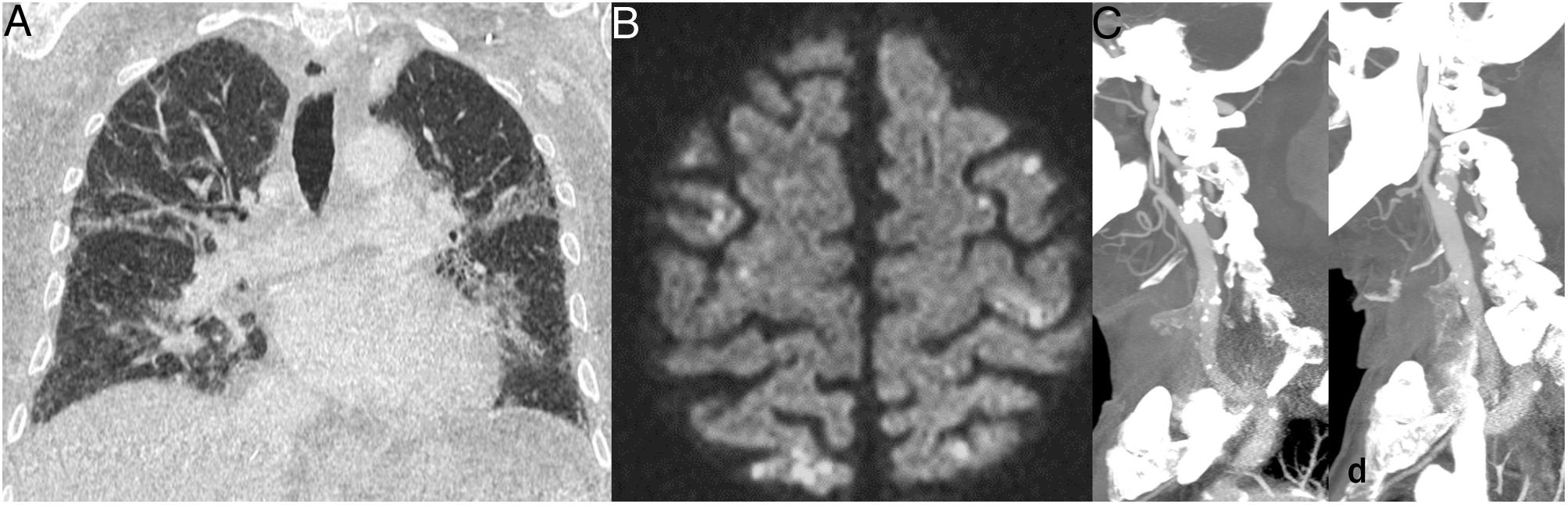
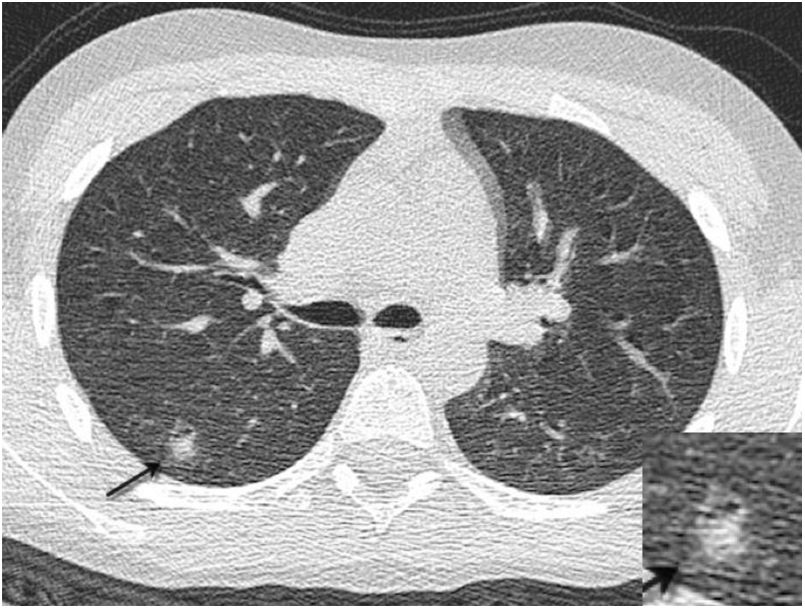
![Thrombotic complications in patients with COVID-19 pneumonia: A) Free-floating thrombus in the aortic arch. B) Splenic infarction and small thrombus visible in the splenic vein (arrow). (Courtesy of Dr Gorospe [Hospital Ramón y Cajal, Madrid]). Thrombotic complications in patients with COVID-19 pneumonia: A) Free-floating thrombus in the aortic arch. B) Splenic infarction and small thrombus visible in the splenic vein (arrow). (Courtesy of Dr Gorospe [Hospital Ramón y Cajal, Madrid]).](https://static.elsevier.es/multimedia/21735107/0000006300000004/v1_202107080630/S2173510721000732/v1_202107080630/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![A 60-year-old man admitted to ICU due to COVID-19 pneumonia requiring intubation. During admission he presented left hemiparesis. A single computed tomography scan showed hypodense lesions in the periventricular and subcortical white matter (asterisks) in both temporal and parietal lobes. Acute diffuse leukoencephalopathy in a critically ill patient was suspected. (Figure courtesy of Dr Cristina Utrilla [Hospital La Paz, Madrid]). A 60-year-old man admitted to ICU due to COVID-19 pneumonia requiring intubation. During admission he presented left hemiparesis. A single computed tomography scan showed hypodense lesions in the periventricular and subcortical white matter (asterisks) in both temporal and parietal lobes. Acute diffuse leukoencephalopathy in a critically ill patient was suspected. (Figure courtesy of Dr Cristina Utrilla [Hospital La Paz, Madrid]).](https://static.elsevier.es/multimedia/21735107/0000006300000004/v1_202107080630/S2173510721000732/v1_202107080630/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![A 35-year-old woman with nosocomial SARS-CoV-2 infection, with headache and low-grade fever. Magnetic resonance imaging showed confluent lesions in the periventricular and subcortical supratentorial white matter hyperintense on the T2-FLAIR sequence (A) and with diffusion restriction in the apparent diffusion coefficient (ADC) (arrows) (B). Posterior reversible encephalopathy syndrome was suspected. (Figure courtesy of Dr Cristina Utrilla [Hospital La Paz, Madrid]). A 35-year-old woman with nosocomial SARS-CoV-2 infection, with headache and low-grade fever. Magnetic resonance imaging showed confluent lesions in the periventricular and subcortical supratentorial white matter hyperintense on the T2-FLAIR sequence (A) and with diffusion restriction in the apparent diffusion coefficient (ADC) (arrows) (B). Posterior reversible encephalopathy syndrome was suspected. (Figure courtesy of Dr Cristina Utrilla [Hospital La Paz, Madrid]).](https://static.elsevier.es/multimedia/21735107/0000006300000004/v1_202107080630/S2173510721000732/v1_202107080630/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)