Progressive multifocal leukoencephalopathy is a demyelinating disease of the central nervous system caused by the reactivation of the JC virus. This opportunistic encephalopathy mainly affects immunodepressed patients with stage III HIV infection, although in recent years it has also been found in association with treatment with immunosuppressors such as natalizumab. MRI plays an important role in both the early diagnosis and follow-up of this disease. Recently, it has been reported that hypointensities in U-fibers and cortex adjacent to white-matter lesions characteristic of the disease can be identified on T2-weighted gradient-echo and susceptibility-weighted sequences in patients with progressive multifocal leukoencephalopathy.
ObjectiveWe aimed to analyze the presence and usefulness of cortical hypointensity on T2-weighted gradient-echo sequences in relation to the diagnosis of progressive multifocal leukoencephalopathy and to review the literature on the topic.
Material and methodsWe analyze three cases of progressive multifocal leukoencephalopathy seen at our center in three patients with immunosuppression of different origins: one with stage III HIV infection, one with multiple sclerosis being treated with natalizumab, and one with rheumatoid arthritis being treated with rituximab.
ResultsIn all three cases MRI showed the cortical hypointensity adjacent to the white-matter lesion in the T2-weighted gradient-echo sequence. In the patient with multiple sclerosis, this sign appeared earlier than the abnormal signal in the white matter. The patient being treated with rituximab was diagnosed postmortem and the pathology findings correlated with the MRI findings.
ConclusionThe finding of cortical hypointensity on T2-weighted gradient-echo MRI sequences seems to support the diagnosis of progressive multifocal leukoencephalopathy, regardless of the type of immunosuppression, so this finding should routinely assessed in patients suspected of having this disease.
La leucoencefalopatía multifocal progresiva (LMP) es una enfermedad desmielinizante del sistema nervioso central causada por la reactivación del virus JC. Esta encefalopatía oportunista se asocia mayormente a pacientes inmunodeprimidos con VIH en estadio III, y en los últimos años se ha asociado a tratamientos inmunosupresores como el natalizumab. La resonancia magnética (RM) tiene un papel importante tanto en el diagnóstico precoz como en el seguimiento de esta enfermedad. Recientemente, se han descrito en las secuencias eco de gradiente T2 (EGT2) y secuencias de susceptibilidad magnética (SWI) hipointensidades en las fibras-U y en la corteza adyacente a las lesiones de sustancia blanca características de la enfermedad.
ObjetivoNuestro objetivo es analizar la presencia y utilidad del signo de la hipointensidad cortical en secuencias EGT2 en relación con el diagnóstico de LMP, así como realizar una revisión bibliográfica sobre el tema.
Material y métodosEn este trabajo se analizan tres casos de LMP vistos en nuestro centro en 3 pacientes diferentes con inmunosupresión de distinto origen: uno con enfermedad por VIH en estadio III, otro con esclerosis múltiple en tratamiento con natalizumab y otro con artritis reumatoide en tratamiento con rituximab.
ResultadosEn los tres casos se observa en la RM el hallazgo de hipointensidad cortical adyacente a la lesión de la sustancia blanca en la secuencia EGT2. En la paciente con esclerosis múltiple, este signo fue más precoz que la alteración de señal en la sustancia blanca. El paciente en tratamiento con rituximab fue diagnosticado post mortem y se presenta una correlación radiopatológica.
ConclusiónLa hipointensidad cortical descrita en el EGT2 en los estudios de RM parece ser un hallazgo que apoyaría el diagnóstico de la LMP, independientemente del tipo de inmunosupresión, lo que nos hace plantear su inclusión de forma rutinaria entre los hallazgos a evaluar en RM en los pacientes con sospecha de LMP.
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system caused by infection with the JC virus. This opportunistic encephalopathy is primarily associated with immunosuppressed patients with stage 3 human immunodeficiency virus (HIV) infection. In recent years, it has also been linked to treatment with natalizumab, a monoclonal antibody, as well as other immunomodulatory treatments.1,2
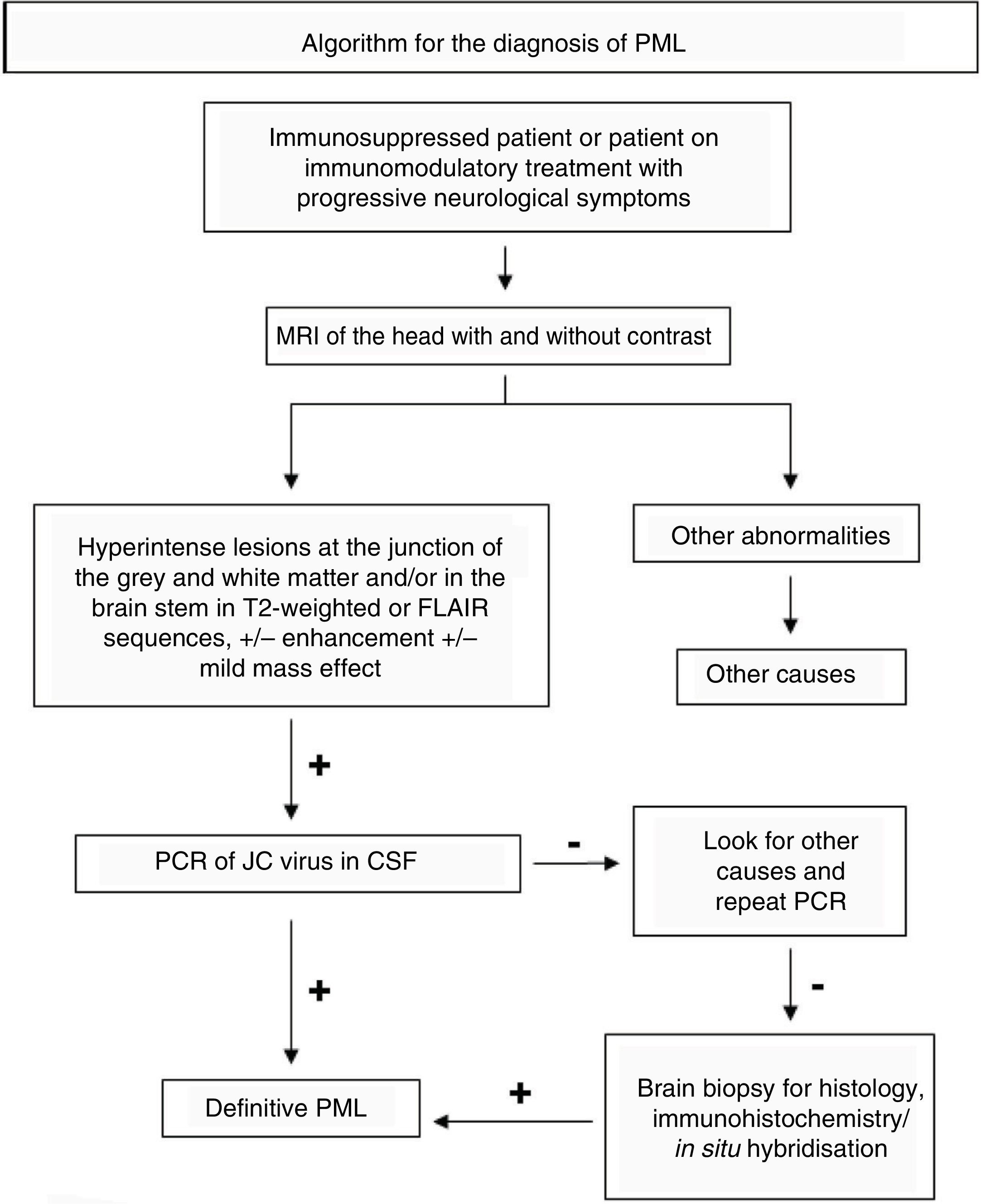
Magnetic resonance imaging (MRI) plays an important role both in early diagnosis and in follow-up of this disease. The American Academy of Neurology (AAN) has proposed diagnostic criteria for PML based on a combination of clinical data, radiological findings and the polymerase chain reaction (PCR) of the JC virus in cerebrospinal fluid (CSF) (Fig. 1). This demonstrates the great importance of MRI of the head in this context, based on the high sensitivity that this test shows in the early diagnosis of PML, even before signs and symptoms develop.3
Until not long ago, PML was considered a fatal disease. More recently, it has been shown that early diagnosis and treatment are associated with a more favourable prognosis in certain situations, such as when patients are treated with natalizumab.4,5 This means that improving diagnostic tools should be prioritised to achieve early diagnosis of PML with good specificity.
Characteristics of PML classically reported on MRI of the head6 are as follows:
- -
A single hyperintense area or multiple hyperintense areas with irregular borders on T2-weighted sequences that grow and converge as the disease progresses.
- -
Diffuse lesions, located in the white matter (WM), predominantly subcortical (involving the U-fibres), leaving the periventricular WM relatively intact.
- -
Lesions are predominantly frontal.
- -
Often, the corpus callosum and the parietal occipital region or the brain stem are involved.
- -
No mass effect or mild mass effect.
- -
Peripheral restriction in the initial phase in diffusion sequences.
Recently, several cases have been reported with hypointensities in the U-fibres and in the cortex adjacent to the WM lesions characteristic of the disease in magnetic susceptibility sequences (EGT2 and SWI).7–10
The aim of this study was to analyse the presence and usefulness of the hypointense cortical signal in EGT2 in relation to the diagnosis of PML. To do this, we analysed three cases of PML in patients with immunosuppression of different origins seen at our centre.
Case 1The first patient was a 71-year-old man who had had stage 3 HIV disease for more than 20 years. His condition was not being managed and he was not on antiretroviral treatment (ART). He visited the A&E department due to general deterioration and gait disorder. An examination revealed marked weakness of the left upper limb. Laboratory testing showed an HIV viral load of 460,000 copies/ml, a CD4 count of 21 cells/mm³, positive PCR for JC virus in the CSF and PCR for JC virus in the urine of 3540 copies/ml.
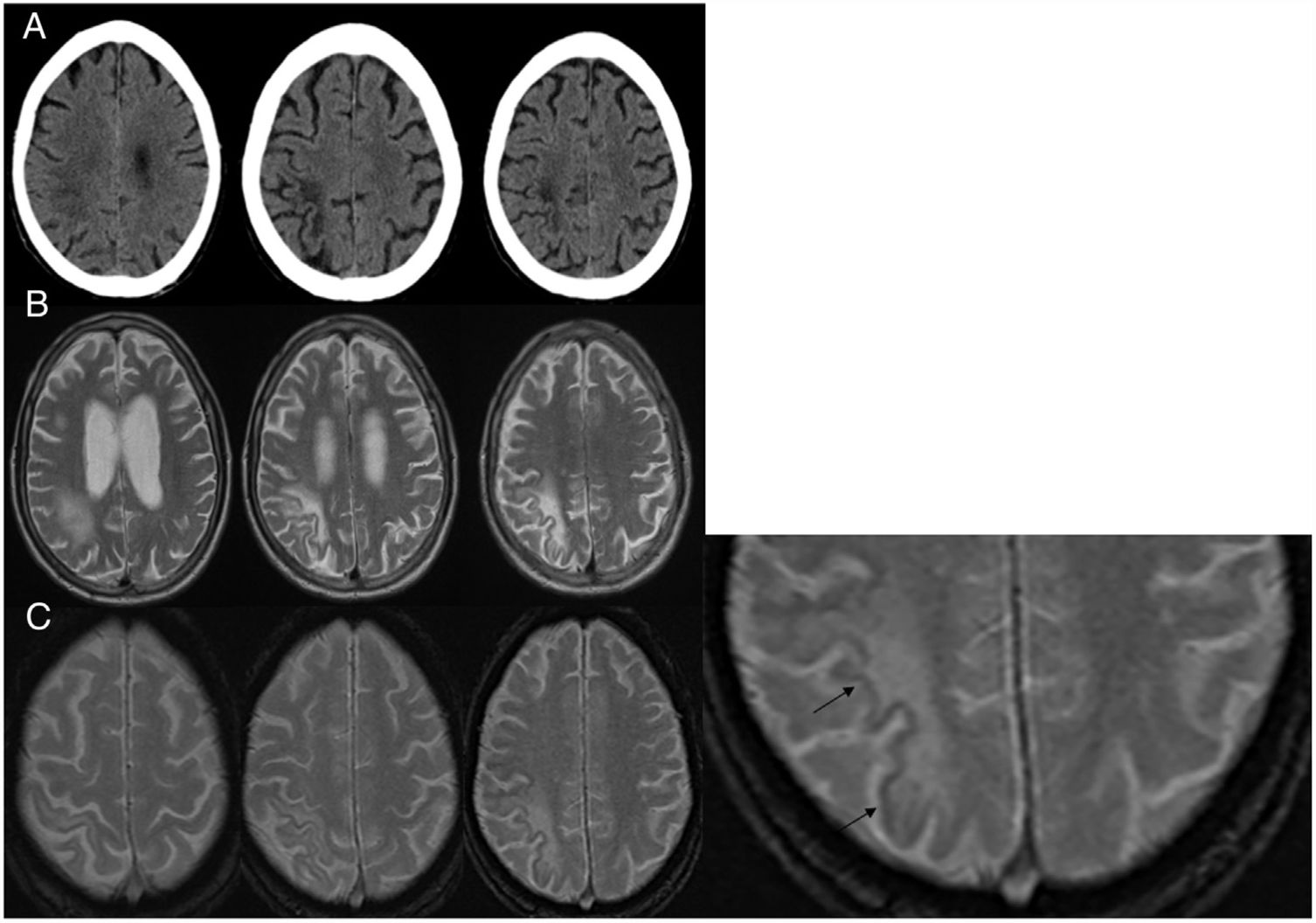
Imaging studies (Fig. 2) identified asymmetry between the two hemispheres of the brain, with hypodensity on CT and hyperintensity in T2 on MRI in the right parietal subcortical WM, leaving the adjacent cortex intact. This was consistent with PML given the patient's clinical context. In the EGT2 sequence, the signal of this preserved cortex was weaker than that of the adjacent areas and the contralateral cortex. ART was started and well-tolerated, and the patient was transferred to a long-term care centre. Subsequently, he followed an unfavourable clinical course and developed complete left hemiplegia. He underwent a repeat MRI of the head (not shown) with radiological worsening suggestive of immune reconstitution inflammatory syndrome (IRIS), so steroid treatment was started. At present, the infectious disease department is conducting regular follow-up of the patient.
A) Computed tomography of the head without intravenous contrast in a patient with stage 3 HIV disease: hypodense areas are observed in the juxtacortical white matter in the right parietal region. B) Axial slices in T2-enhanced sequences: a hyperintense signal is identified in the parietal region involving U-fibres without a mass effect. C) Axial slices in EGT2 sequence: a border of hypointense cortical signal is observed adjacent to the white-matter lesions. The third image is enlarged and the hypointense border is marked with arrows.
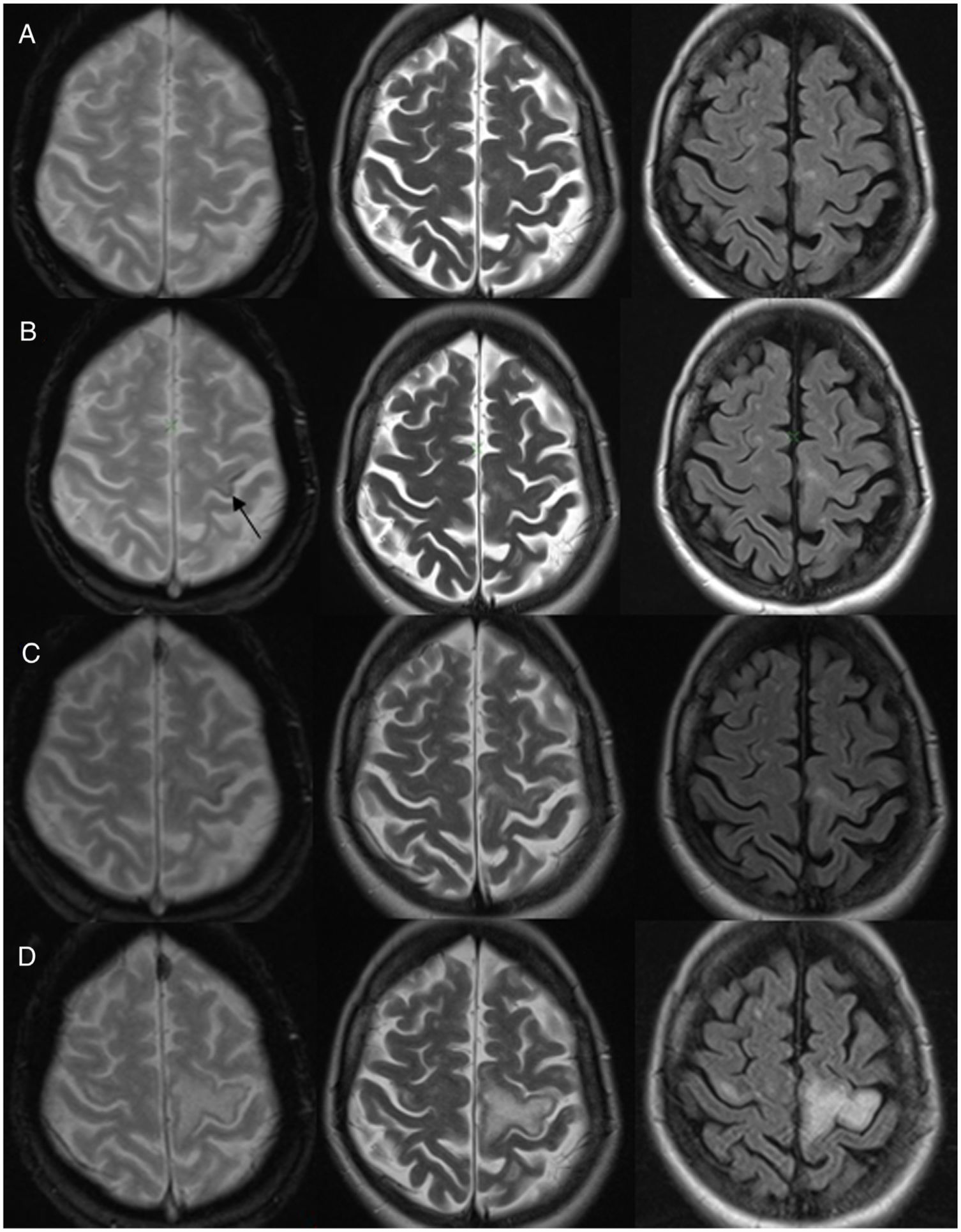
The second patient was a 45-year-old woman with a diagnosis of secondary-progressive relapsing–remitting multiple sclerosis being treated with natalizumab, with consistently negative PCR for JC virus and clinical and radiological follow-up every 3 months given her risk of developing PML. Following nine years of treatment, the patient came to our centre with gradual worsening over the course of several months of the mobility of her right hand and then her right lower limb. She underwent an MRI, which found no changes compared to previous studies (Fig. 3A).
A) Baseline magnetic resonance imaging (MRI) of the head in a patient with multiple sclerosis on treatment with natalizumab. Hyperintense lesions were observed in the white matter consistent with demyelination plaques, with no changes compared to prior studies. B) MRI seven months later: EGT2 showed a hypointense cortical signal in the left precentral gyrus (arrow). Weak hyperintense signal in the adjacent white matter in T2 sequences. C) MRI nine months from baseline: increase in T2 hyperintense signal in left precentral gyrus. Hypointense cortical signal showed no changes. Radiological suspicion of progressive multifocal leukoencephalopathy. D) MRI after one year: worsening of the left T2 hyperintense signal with signal abnormality in the contralateral precentral gyrus. This follow-up study shows no hypointense signal in EGT2.
A subsequent MRI (Fig. 3B) showed a hypointense cortical signal in the left precentral gyrus in EGT2 and a weak hyperintense subcortical signal in T2-weighted sequences. At that time, PCR for JC virus remained negative and the patient continued treatment with natalizumab.
However, the patient showed gradual clinical worsening and was therefore admitted for study. Another MRI (Fig. 3C) revealed progression of the lesions, which supported the suspicion of PML. PCR for JC virus was repeated and this time it was positive. As a result, treatment with natalizumab was suspended.
The patient started treatment with mefloquine and mirtazapine for PML. She exhibited clinical and radiological worsening. The follow-up study did not show a hypointense cortical signal (Fig. 3D). The patient developed IRIS, which was treated with corticosteroids. Subsequently, her PML was stabilised.
Case 3The third patient was a 92-year-old woman with rheumatoid arthritis on treatment with rituximab for the past 5 years. She visited the A&E department with a clinical picture of general deterioration for the past 2 months. She was found to have sensory–motor syndrome of the right limbs and cognitive impairment.
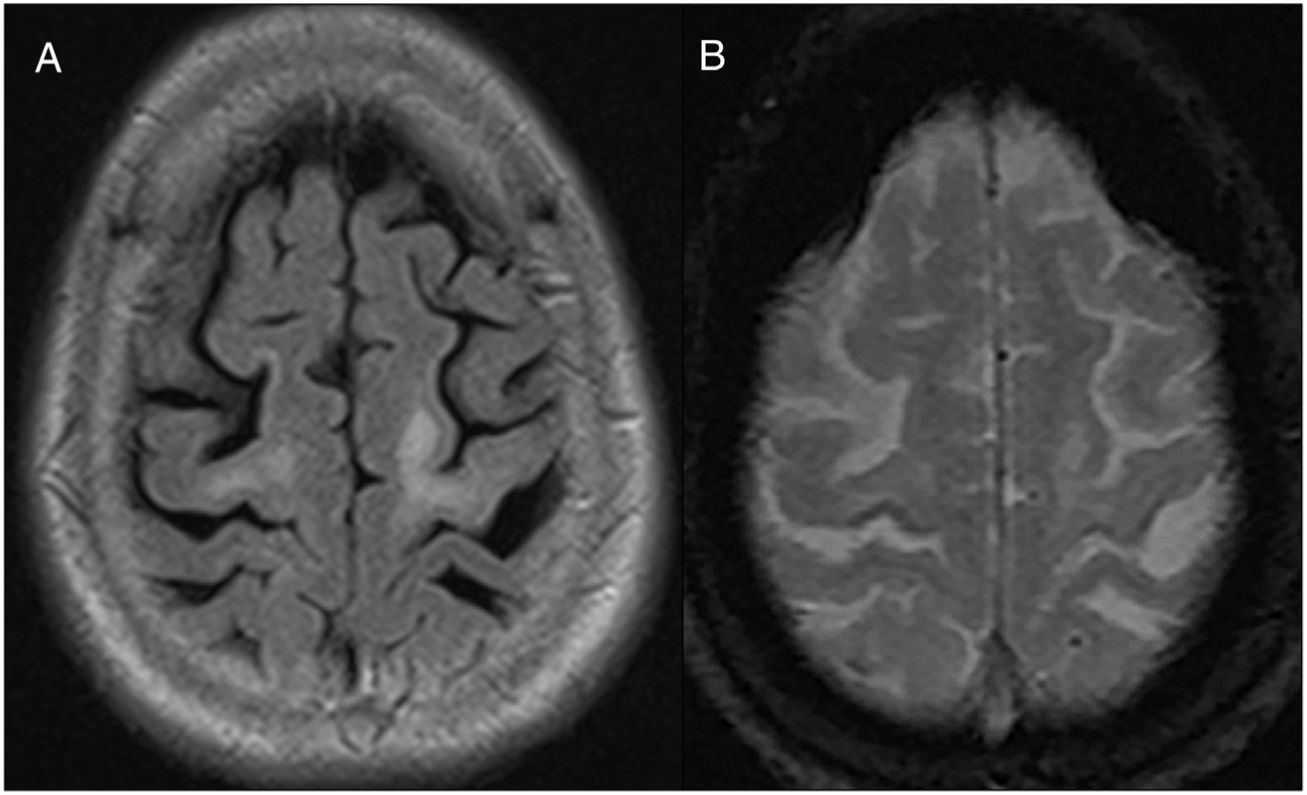
She underwent an MRI of the head on admission (Fig. 4), which revealed right parietal cortical focal atrophy and indeterminate leukopathy, probably of vascular aetiology. The patient showed clinical deterioration and died seven months later. PML was diagnosed in the post mortem study. Retrospective analysis of the MRI identified a hypointense cortical/subcortical border in the bilateral precentral gyrus (Fig. 4B).
Patient with rheumatoid arthritis on treatment with rituximab. A) FLAIR sequence showing a hyperintense signal in the bilateral juxtacortical white matter with major involvement of the left precentral gyrus. B) EGT2 sequence with a hypointense cortical/subcortical border in the U-fibres adjacent to the white-matter lesions in the bilateral precentral gyrus.
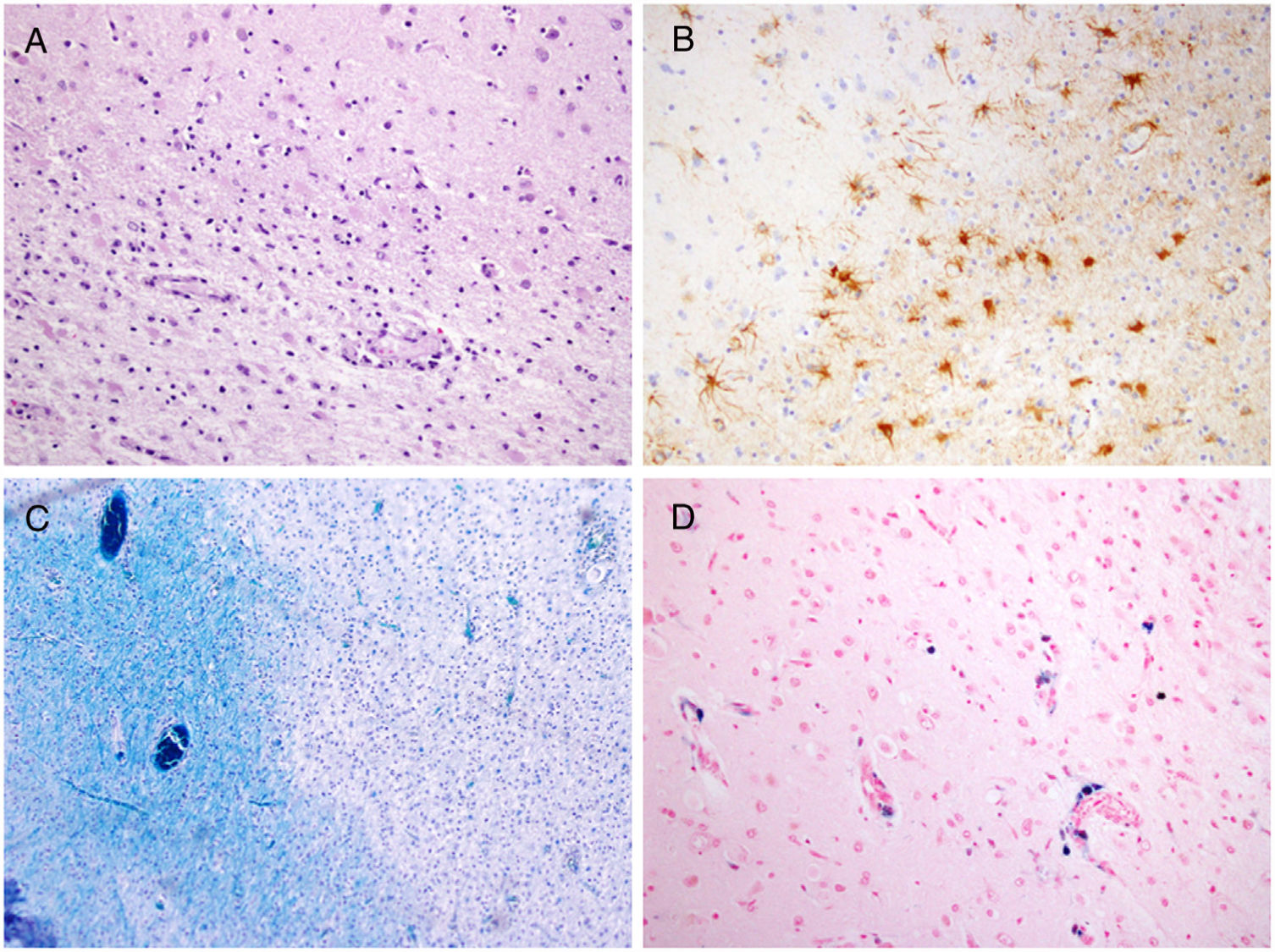
The pathology study (Fig. 5) confirmed precentral and parietal cortical atrophy with the presence of lymphohistiocytic inflammatory infiltrates. Proliferation of astrocytes (glial cells) was observed at the cortical and subcortical level, with areas of demyelination. Perls' Prussian blue staining was used to identify iron deposited in perivascular spaces and inside macrophages in the precentral cortex (Fig. 5D). Immunohistochemistry studies showed glial cells positive for protein p53 and JC virus (SV40), which was subsequently confirmed by PCR.
A) Sample of the cortical/subcortical junction with degeneration of U-fibres (haematoxylin–eosin, ×20). B) Proliferation of astrocytes in areas of demyelination (glial fibrillary acidic protein, ×20). C) Demyelination of white matter (Luxol fast blue, ×20). D) Iron deposition in perivascular spaces and inside macrophages — Perls' Prussian blue staining (haematoxylin–eosin, ×20) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
The above three cases of patients with PML have the shared characteristic of a recently reported hypointense cortical signal in EGT2 sequences.
The origin of the weak signal intensity in magnetic susceptibility sequences in PML remains unclear. Both diamagnetic materials (calcifications) and paramagnetic materials (iron, haemosiderin and deoxyhaemoglobin) are hypointense in these sequences. However, the literature notes that this finding is due to an accumulation of iron.7,8
PML often causes lesions in the subcortical white matter involving U-fibres (myelinated fibres at the junction of the grey and white matter).
It is known that oligodendrocytes in these areas are often infected with JC virus and that iron is present in high concentrations inside the oligodendrocytes and myelin sheaths at the cortical/subcortical junction.11 In addition, an increase in iron deposits in the basal ganglia and the thalamus has also been reported in patients with multiple sclerosis.12 Many potential causes of this accumulation of iron in MS have been suggested, such as: blood–brain barrier dysfunction; decreased iron clearance attributable to axon dysfunction; and deregulation of iron transport proteins.13 It is believed that similar mechanisms could be involved in the excessive accumulation of iron in PML.14 This iron deposition has also been reported as a hyperintense signal in T1 sequences.15
There have been isolated cases with pathological correlation,14,15 in which infiltration of macrophages and gliosis have been reported, without the presence of iron having been determined. In the case presented in our series with a post mortem study, iron was seen to be deposited in the perivascular spaces and inside macrophages using the Perls' Prussian blue staining in the precentral cortex, the area corresponding to the hypointense signal visible on MRI (Fig. 5D).
Cases have been reported in the literature of PML with this finding in EGT2 and SWI sequences, not only in patients with PML and HIV or being treated with natalizumab, but also in patients with immunosuppression of another origin, such as treatment with rituximab,7,10,14 as in one of the cases presented above.
Hodel et al.14 conducted a retrospective observational study that enrolled 12 patients with PML on treatment with natalizumab, five patients with PML due to other causes and 55 patients with MS and without PML. All of the patients with PML except for one showed at least one hypointense area in EGT2 or SWI, including the deep or cortical grey matter. No changes were observed in the magnetic susceptibility sequences in any of the patients with MS without PML. In addition, cerebral magnetic susceptibility showed changes in the presymptomatic phase of PML, whereas findings were subtle when other MRI sequences such as FLAIR were used.
There is evidence affirming that hypointensity in U-fibres is independent of the clinical course of the disease, as it is already present in an early phase in many cases,3,8,9,14 as in the case of our second patient. This demonstrates the importance of an early MRI if PML is clinically suspected, as well as the value that must be attached to this finding. A hypointense cortical signal should probably be incorporated into the diagnostic radiological criteria for PML on MRI, taking into account that it may be the first radiological finding. We therefore propose including a magnetic susceptibility sequence in the routine MRI study if PML is suspected.
The cases presented in this article support the notion that PML may develop regardless of the type of immunosuppression, as well as the notion that the hypointense cortical signal may appear in EGT2 sequences in the presymptomatic stage of the disease. However, more extensive studies are needed to properly assess this finding.
Authorship- 1.
Responsible for the integrity of the study: PLS, NAA, VZH, CBS and TCG.
- 2.
Study conception: TCG.
- 3.
Study design: PLS and TCG.
- 4.
Data collection: PLS, NAA, VZH, CBS and TCG.
- 5.
Data analysis and interpretation: PLS, NAA, VZH, CBS and TCG.
- 6.
Statistical processing: Not applicable.
- 7.
Literature search: PLS and NAA.
- 8.
Drafting of the paper: PLS and NAA.
- 9.
Critical review of the manuscript with intellectually significant contributions: TCG, CBS and VZH.
- 10.
Approval of the final version: PLS, NAA, VZH, CBS and TCG.
The authors declare that they have no conflicts of interest.
Please cite this article as: López Sala P, Alberdi Aldasoro N, Zelaya Huerta MV, Bacaicoa Saralegui MC, Cabada Giadás T. Hipointensidad cortical en secuencias eco de gradiente T2 en la leucoencefalopatía multifocal progresiva. Radiología. 2020;62:59–66.