Edited by: Dr. José Luis del Cura Rodríguez - Servicio de Radiodiagnóstico, Hospital Universitario Donostia, Donostia-San Sebastián, España
Last update: August 2022
More infoAlthough not necessary for the vast majority of ultrasound-guided procedures, intravenous contrast agents can be useful for procedures aimed at lesions that require contrast enhancement to be seen on ultrasonography.
Using contrast-enhanced ultrasonography to guide procedures has two drawbacks: first, because enhancement from ultrasound contrast agents is short lived, it is often necessary to plan several injections; second, because the needle is poorly seen on contrast-enhanced ultrasonography, a dual image display format is necessary.
Contrast-enhanced ultrasonography can be used for planning and monitoring diagnostic and therapeutic procedures, for guiding the procedures, and for follow-up. Using contrast-enhanced ultrasonography enables better results in both types of procedures; moreover, it can be used within cavities.
Aunque el contraste intravenoso no es necesario en la inmensa mayoría de los procedimientos realizados con guía ecográfica, su uso puede permitir realizar procedimientos en aquellas lesiones que solo se visualizan con ecografía con contraste.
Los problemas que tiene son dos: el tiempo limitado del realce producido por el contraste, que requiere con frecuencia planificar varias inyecciones, y la mala visualización de la aguja, que requiere el uso de doble ventana de visualización.
Puede ser usada en la planificación y el control de los procedimientos diagnósticos y terapéuticos, tanto en la guía del procedimiento, para monitorizar el alcance del tratamiento, como en los controles posteriores. Su uso permite mejorar los resultados de ambos tipos de procedimientos. Puede ser usada también intracavitariamente.
Ultrasound is undoubtedly the ideal technique for guiding procedures in radiology. It is cheap, widely available, does not use ionising radiation, enables real-time monitoring of needle or catheter positioning and allows versatile access to any lesion. In addition, ultrasound systems are portable and ultrasound requires less time than other techniques.1
However, the fundamental condition for performing a procedure with ultrasound guidance is that the lesion to be operated on is visible on ultrasound.1 This means that many lesions, especially in the abdomen, cannot be biopsied or treated with ultrasound monitoring.
In this context, although intravenous contrast is not necessary in the vast majority of ultrasound-guided procedures, it can allow access to lesions visualised on contrast-enhanced ultrasound and may even be useful in procedures in which lesions are better visualised with contrast.2–4
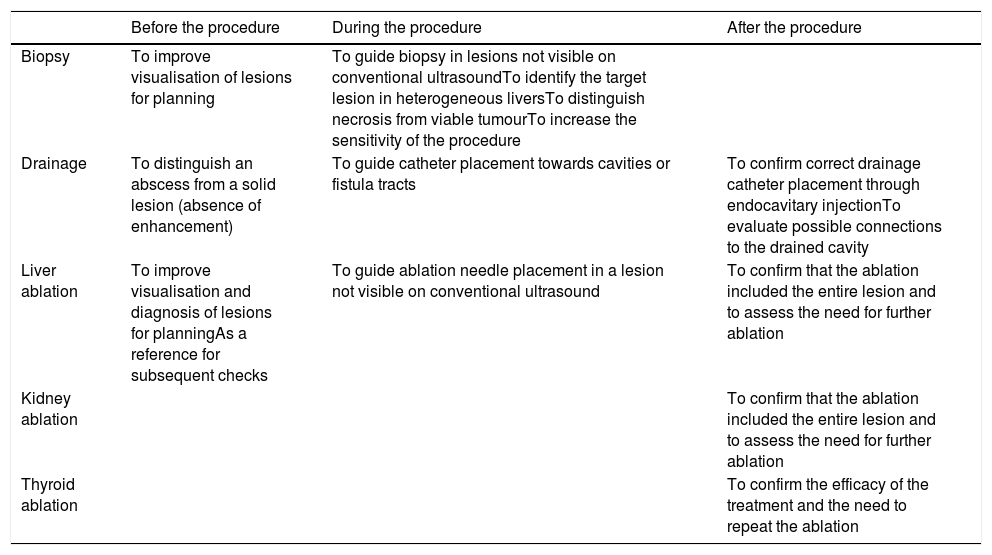
Contrast-enhanced ultrasound can also be used in therapeutic procedures — both during the procedure, to monitor the reach of the treatment, and after the procedure, in periodic follow-up checks5–13 (Table 1).
Uses of contrast-enhanced ultrasound in ultrasound-guided interventional radiology procedures.
| Before the procedure | During the procedure | After the procedure | |
|---|---|---|---|
| Biopsy | To improve visualisation of lesions for planning | To guide biopsy in lesions not visible on conventional ultrasoundTo identify the target lesion in heterogeneous liversTo distinguish necrosis from viable tumourTo increase the sensitivity of the procedure | |
| Drainage | To distinguish an abscess from a solid lesion (absence of enhancement) | To guide catheter placement towards cavities or fistula tracts | To confirm correct drainage catheter placement through endocavitary injectionTo evaluate possible connections to the drained cavity |
| Liver ablation | To improve visualisation and diagnosis of lesions for planningAs a reference for subsequent checks | To guide ablation needle placement in a lesion not visible on conventional ultrasound | To confirm that the ablation included the entire lesion and to assess the need for further ablation |
| Kidney ablation | To confirm that the ablation included the entire lesion and to assess the need for further ablation | ||
| Thyroid ablation | To confirm the efficacy of the treatment and the need to repeat the ablation |
Contrast-enhanced ultrasound can be used to guide diagnostic procedures such as biopsy and therapeutic procedures such as percutaneous tumour ablation. The technique for procedures in which contrast-enhanced ultrasound is used is similar to those in which it is not used.1 However, there are certain differences which depend on the need to inject contrast, the duration of ultrasound enhancement and the differences in visualisation in the contrast-enhanced ultrasound mode of the needles or catheters used compared to conventional ultrasound.
Specific problemsInjection of ultrasound contrast during the procedureThe enhancement caused by ultrasound contrast is limited in duration. This means that the time used for a specific procedure (biopsy, electrode or antenna placement in a lesion, etc.) is limited by the duration of the enhancement.
Contrast is useful in two phases: in planning the procedure, to identify the lesion in question, and then in performing the procedure itself. Therefore, it is often necessary to plan at least two injections: one before and one during the procedure.
Sometimes the procedure lasts longer than the enhancement does; in such cases, it is a good idea to prepare more than one dose so a second dose (or successive doses) can be injected as needed. Alternatively, a continuous infusion can be used, especially in the second phase, although it is not essential. Some devices available on the market make it possible not only to perform this continuous contrast infusion but also to vary the injection speed.
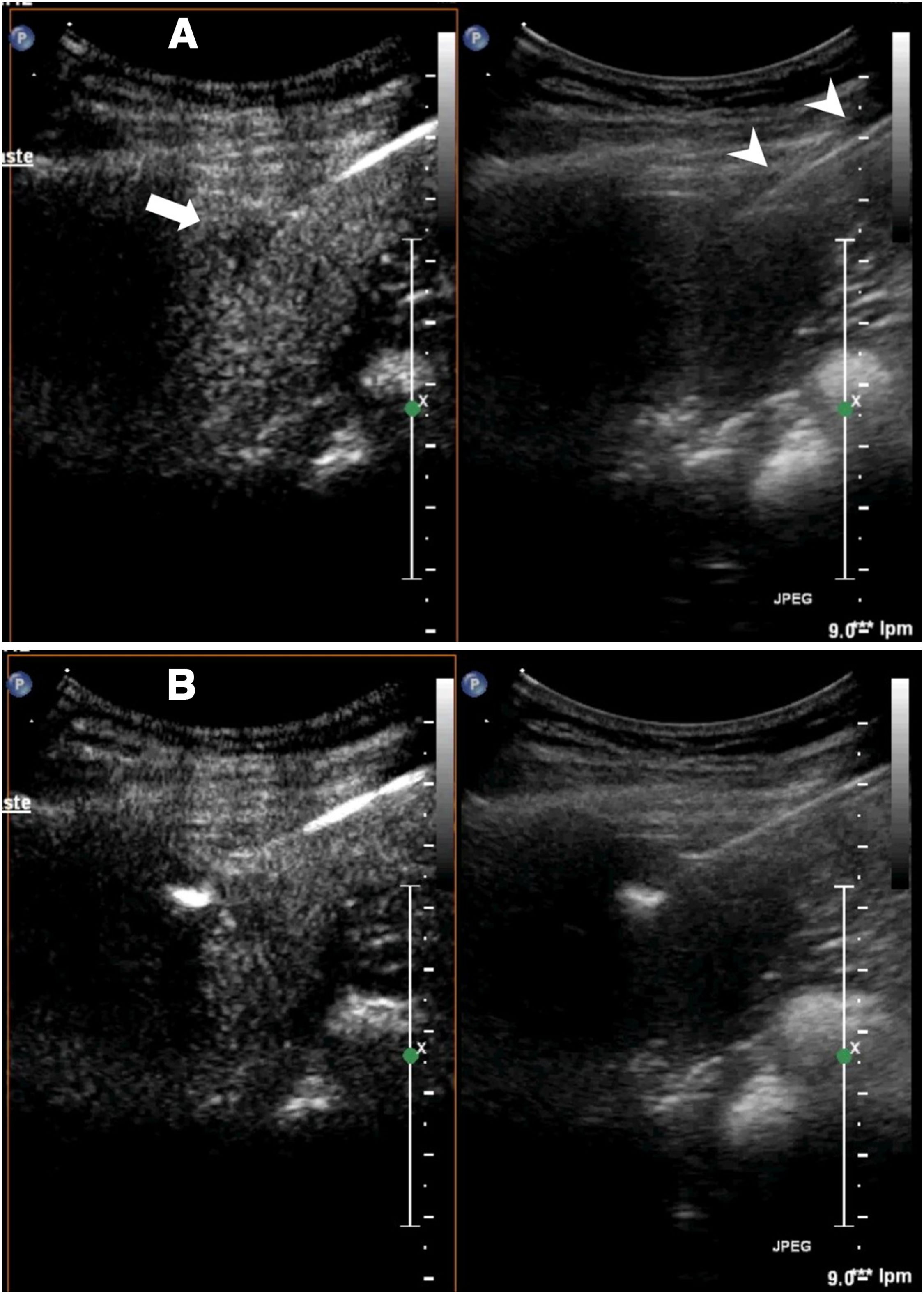
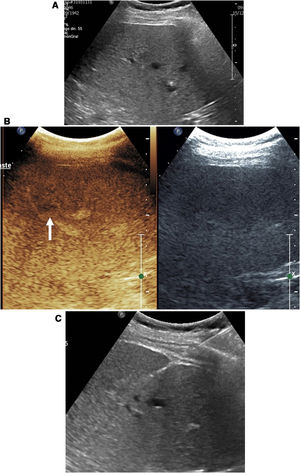
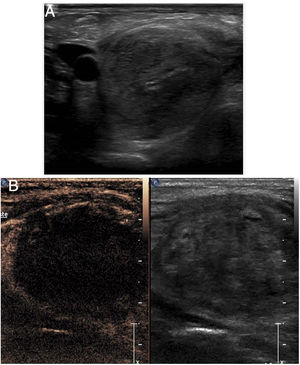
Visualisation of needles during the procedureIf contrast is used, the mode of examination to be used at least during the critical part of the procedure is the contrast mode. This mode entails certain difficulties in the performance of the technique. On the one hand, tissues that do not enhance are not visible on ultrasound. In addition, needle visualisation is much less precise than in conventional ultrasound. The needle often appears highly echogenic and seems thicker than it really is. Sometimes, the apparent position of the needle in relation to the target lesion is not always completely reliable (Fig. 1).
Biopsy of liver metastasis not visible on conventional ultrasound. A) The two-window image shows the lesion (arrow) visible in the contrast window. In that window, the biopsy needle is almost indistinguishable, but it is perfectly visible in the conventional ultrasound window. B) The biopsy was successfully completed.
For this reason, it is advisable to use dual visualisation during the phase of the procedure in which the needle is inserted and directed towards the lesion. The conventional window, although of lower resolution than standard ultrasound, allows the needle to be monitored and complements the contrast image to accurately identify the actual position of the needle during the procedure.
Contrast-enhanced ultrasound as an aid in biopsyBiopsy of lesions not visible on conventional ultrasoundIn oncology, the most sensitive and most commonly used imaging methods for both diagnosis and staging of lesions are computed tomography (CT) and magnetic resonance imaging (MRI). Positron emission tomography/computed tomography (PET/CT) must often be added to these.
In modern medicine, biopsy has become a very in-demand tool, not only for diagnosis of lesions but also for identification of therapeutic targets in oncology treatments. In theory, CT and MRI would be the most suitable techniques for guiding the biopsy of a lesion. However, the versatility of ultrasound, its multiplanar capabilities and the option to perform procedures in real time and in a much simpler manner have rendered ultrasound the most suitable technique for these procedures when a lesion is visible on ultrasound.1
However, it may happen that a lesion that has been detected by CT or MRI is not visible on ultrasound. In that case, alternatives include using techniques that fuse images from ultrasound and CT or MRI, or performing CT-guided biopsy.
As long as the lesion is visible using contrast-enhanced ultrasound, however, it is still possible to perform biopsy with ultrasound guiding the technique in the contrast mode. The use of contrast-enhanced ultrasound has been confirmed to enable biopsies of lesions not visible on conventional ultrasound with a technical success rate of 86–88.5%14–16 (Fig. 1).
In fact, when conventional ultrasound is capable of identifying a lesion, but access to that lesion is complex or dangerous, contrast-enhanced ultrasound sometimes enables identification of other lesions not visible in the baseline image that can be biopsied more simply or safely.
Procedure planningThere are two main reasons for inadequate samples being taken in biopsies: the target is not reached (this is the most common problem in small lesions), and necrotic areas of lesions are sampled (this particularly occurs in large lesions). Another problem arises when the liver is heterogeneous; in this case, it may be difficult to identify the lesion to be biopsied. This is especially common in patients with cirrhosis.17
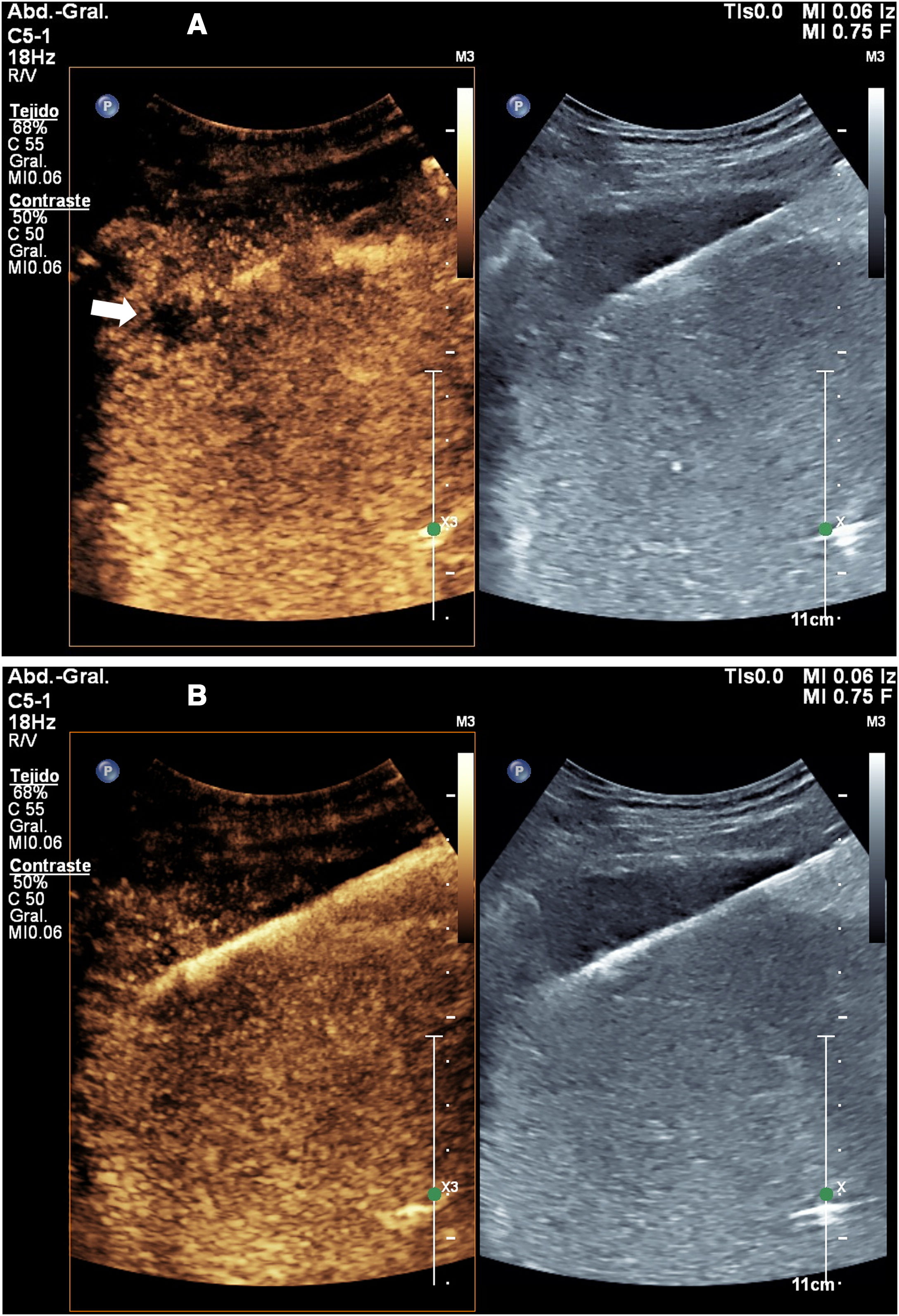
In the latter two problems, contrast-enhanced ultrasound may be useful. In the planning of liver biopsy, it increases the reliability of the technique by 5–8%, with a greater effect on malignant lesions18,19 (Fig. 2).
Liver metastases in which ultrasound-guided biopsy was indicated. A) When ultrasound was performed, the lesion was not clearly identified, and so a decision was made to perform contrast-enhanced ultrasound. B) Contrast-enhanced ultrasound enabled the lesion to be identified (arrow). C) Once the lesion was identified, it was successfully targeted in biopsy.
Ultrasound contrast is an excellent tool for biopsy planning, as it identifies necrotic areas of the tumour, which are those that do not enhance. The biopsy can then target the areas that do enhance. In some cases, it also identifies new lesions which may be more accessible to biopsy.2,4
Improving the sensitivity of biopsyUltrasound contrast, used both before and during the procedure, has demonstrated its usefulness for increasing the sensitivity of biopsy and decreasing the number of punctures needed in each procedure.19 A prospective study of 171 liver tumours confirmed that in the group in which contrast-enhanced ultrasound was used to guide biopsy, a significantly higher sensitivity was achieved compared to the group in which conventional ultrasound was used (96.5% versus 81.5%). This sensitivity was most improved in cirrhotic livers (95.2% versus 75%), lesions poorly visualised on conventional ultrasound (100% versus 66.6%) and lesions exceeding 6cm (97.8% versus 82%). The reason in the former two cases is that the contrast enables better identification of lesions, whereas in the latter case the contrast helps to avoid necrotic areas.17
This increase in biopsy sensitivity with ultrasound contrast has also been shown in the lungs, the neck and musculoskeletal tumours, especially sarcomas, and particularly occurs in large lesions, malignant lesions and metastatic lesions with a significant necrotic component.4,20–23
Contrast-enhanced ultrasound as an aid in ablation proceduresPercutaneous ablation treatments are increasingly being pursued as non-surgical options, and sometimes even first-line options, in the treatment of tumours of the liver, kidneys, thyroid gland, bone, adrenal glands, prostate and uterus. Either CT or ultrasound can be used to guide these procedures. Cryoablation, given its specific characteristics, must be CT-guided, but for the rest of the techniques, radiofrequency ablation and microwave ablation, ultrasound guidance is preferred for access to the lesion, for the same reasons as in biopsy.1
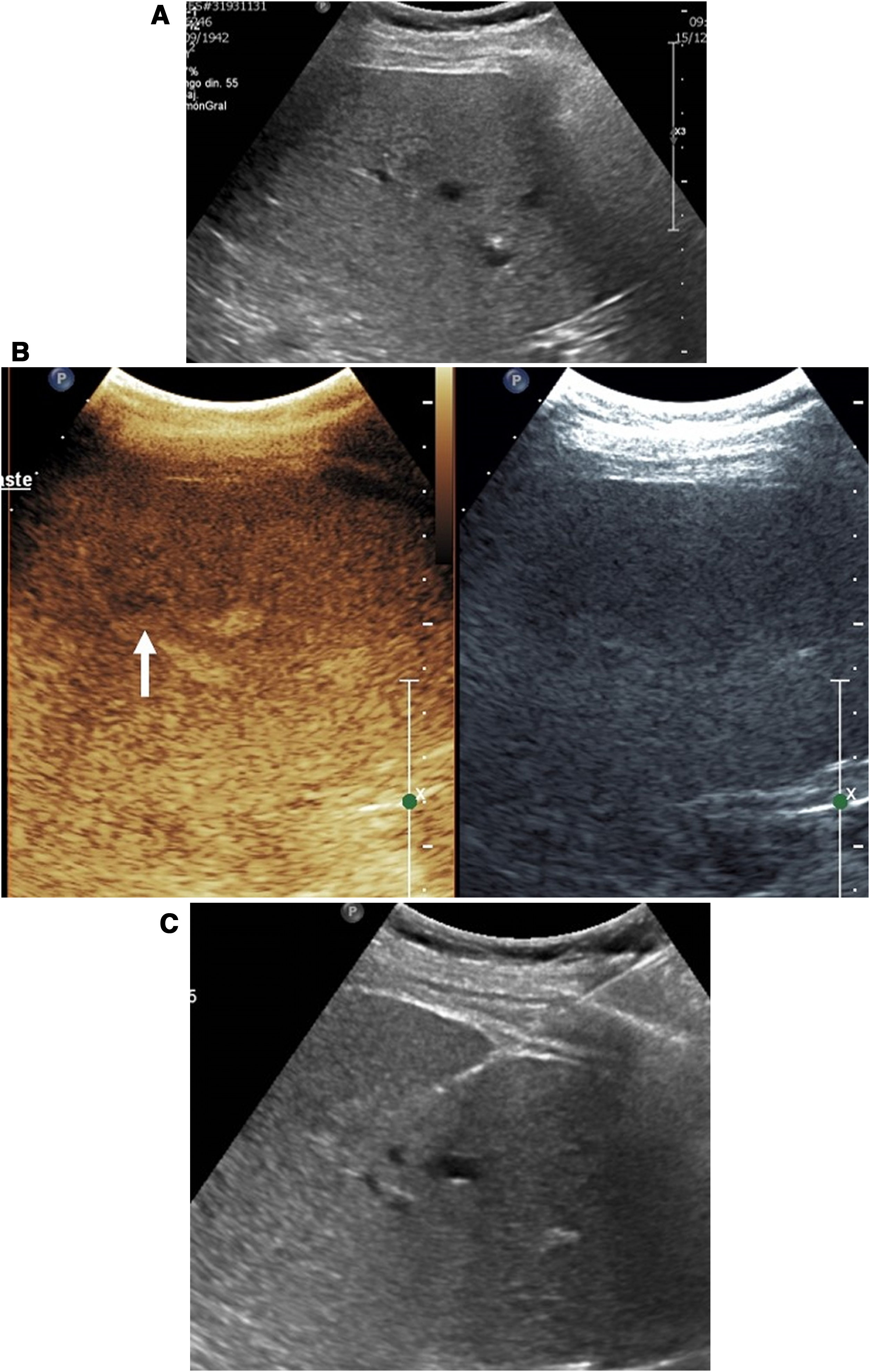
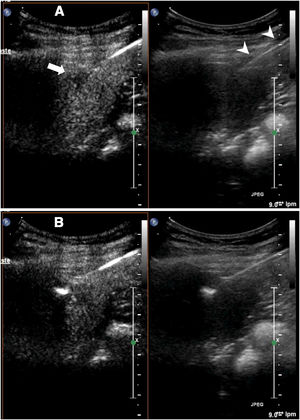
Contrast-enhanced ultrasound can be used in ablation, as in biopsy, to guide procedures in lesions poorly visualised on conventional ultrasound (Fig. 3).
Ablation of liver metastasis not visible on conventional ultrasound. A) The lesion (arrow) is only visible in the contrast window. In the conventional ultrasound window, the ablation needle is better identified (arrow tips). B) The ablation has begun. Echogenic bubbles can be seen to form at the tip of the needle.
It can also be used as a diagnostic tool both in immediate evaluation of the outcome of the ablation and in post-treatment follow-up of lesions to detect relapses.
LiverTreatment planningAs mentioned in reference to biopsy, contrast-enhanced ultrasound is capable of increasing the sensitivity of ultrasound in detecting liver lesions, which is also useful for treatment. Patients evaluated with contrast-enhanced ultrasound prior to ablation have lower rates of relapses and incomplete ablations than patients evaluated with ultrasound alone. Tumour edges are better detected following administration of ultrasound contrast, and the relationship of the tumour to surrounding structures can be better identified, making it easier to adapt the treatment strategy and reduce the risk of complications.24,25
In addition, a prior contrast-enhanced ultrasound examination serves as a reference for subsequent checks using this technique — both checks done during the ablation procedure and post-ablation checks.
During ablationFor the same reason, contrast-enhanced ultrasound can be used to guide electrode or antenna placement in a lesion not visible on ultrasound without contrast. The technique for accessing the lesion is the same as the technique described for biopsy, although in this case, needle placement is easier as the needle is thicker and therefore more visible. The needle is inserted when the lesion is visible and guided towards the lesion26–29 (Fig. 3).
Guidance with contrast-enhanced ultrasound has been shown to increase the efficacy of ablation compared to guidance with conventional ultrasound, as it decreases the number of sessions needed to achieve complete ablation and increases the single-session complete ablation rate from 84% to 94%.26,27
At the end of ablationContrast-enhanced ultrasound is useful for confirming, at the end of the procedure, whether the ablation volume is sufficient and includes all the target areas. The use of contrast contributes functional information that conventional ultrasound, based on morphology, does not. Necrosis of the tissues causes their microvascular network to be destroyed and not take up ultrasound contrast.5–7 This is the key sign in this application of contrast. It also enables detection of complications, such as infarctions caused by injury of vessels as a result of treatment.
This use poses specific difficulties, since a cloud of bubbles forms during ablation and renders the interpretation of the examination much more difficult. Therefore, it is a good idea to wait at least 10min or so for these bubbles to disappear before injecting contrast.
This is useful for evaluating whether additional ablations are needed to complete the treatment, thus increasing the efficacy of the technique.5–7 It is used less often, given the difficulties described, but it is particularly indicated when the electrode or needle has become displaced during the ablation or when there is a nearby vessel that may cool the treated area and leave areas of untreated tumour.
If further treatments are performed, additional doses of contrast can be administered to re-evaluate the results.
Follow-up of treated lesionsThe Response Evaluation Criteria in Solid Tumours (RECIST) guidelines are not suitable for locoregional treatment due to the limited relationship between necrosis and tumour size. After thermal ablation, completely necrotic tumours may remain unchanged in size, whereas tumours that contract may be partially viable. For hepatocellular carcinoma, the imaging indicator of complete ablation is the disappearance of any intralesional enhancement. This same criterion is applied to contrast-enhanced ultrasound.2,7–10,30
Although contrast-enhanced ultrasound can be reliable in detecting local recurrence or incomplete ablation in a treated nodule, CT and MRI have a substantial advantage: they provide an overall view of the liver, enabling detection of tumours beyond the treated area and even beyond the liver itself. Therefore, they cannot be replaced in the follow-up of tumours after ablation.
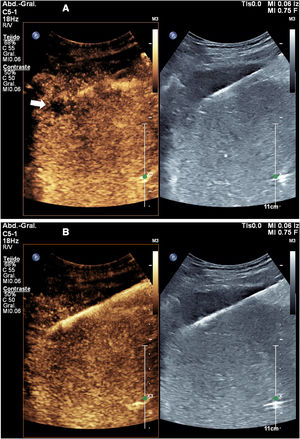
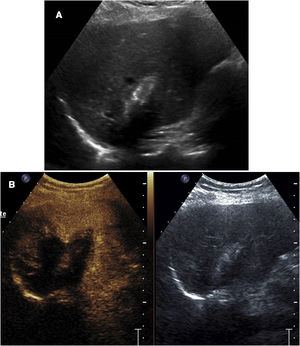
We have found it useful to perform contrast-enhanced ultrasound 24h after ablation, since this enables identification of the outcome of the ablation, visualisation of the actual treatment volume, comparison of that volume to the theoretical volume, identification of insufficiently treated areas and diagnosis of some complications, especially vascular ones (Fig. 4). The information obtained in this examination has enabled us to modify our ablation technique and adapt it to each specific circumstance, thus achieving better outcomes and, in some cases, detecting insufficiently treated lesions requiring further procedures.
Follow-up check 24h after microwave ablation of a liver metastasis. A) Ultrasound shows the characteristic parallel-line image of the microwave ablation tract. The extent of the treated area cannot be determined. B) Contrast-enhanced ultrasound enables identification of the coagulated area, which does not enhance.
In performing contrast-enhanced ultrasound of a lesion that has been treated, the entire volume of each tumour subjected to ablation should be evaluated and this volume should be compared to the pre-treatment volume. It is useful to use dual-contrast/non-contrast imaging.
In early post-ablation evaluation (within the first 30 days), enhancement can be seen around the necrotic region; this is similar to CT findings. This perilesional hyperaemic halo is normal and should not be interpreted as residual viable tumour.2,7–10,30
Metastatic lesions, for their part, are difficult to evaluate as they show either no enhancement or brief enhancement, which in general does not permit a relapse to be distinguished from an area of ablation.29 In these lesions, contrast-enhanced ultrasound is not useful for follow-up checks.
KidneysPercutaneous ablation is a treatment option in kidney tumours, specifically renal cell carcinoma. It is particularly indicated in T1a tumours (<4cm). It yields excellent outcomes, with a treatment success rate of around 90%. The American Urological Association even recommends it as a first-line option in patients with a disease with high surgical risk, and in patients with only one kidney.31
Before ablationAs kidney tumours take up contrast in a similar way to the kidney parenchyma (except for papillary and chromophone tumours), contrast-enhanced ultrasound is less helpful in kidney ablation procedure planning. It may be useful, however, to perform a contrast-enhanced ultrasound prior to ablation so that it can serve as a reference in subsequent checks.
During the procedureContrast-enhanced ultrasound of the kidneys has the same problems as contrast-enhanced ultrasound of the liver, due to the appearance of air bubbles as a result of treatment. However, it may be useful, as in the liver, for confirming at the end of the procedure whether the ablation volume is sufficient and includes all the target areas. Some authors have demonstrated an increase in the therapeutic efficacy of ablation when contrast-enhanced ultrasound is used in this way. Again, it is necessary to let at least 10min elapse before performing the examination to allow the gas produced by the ablation to disappear.4,10,32,33
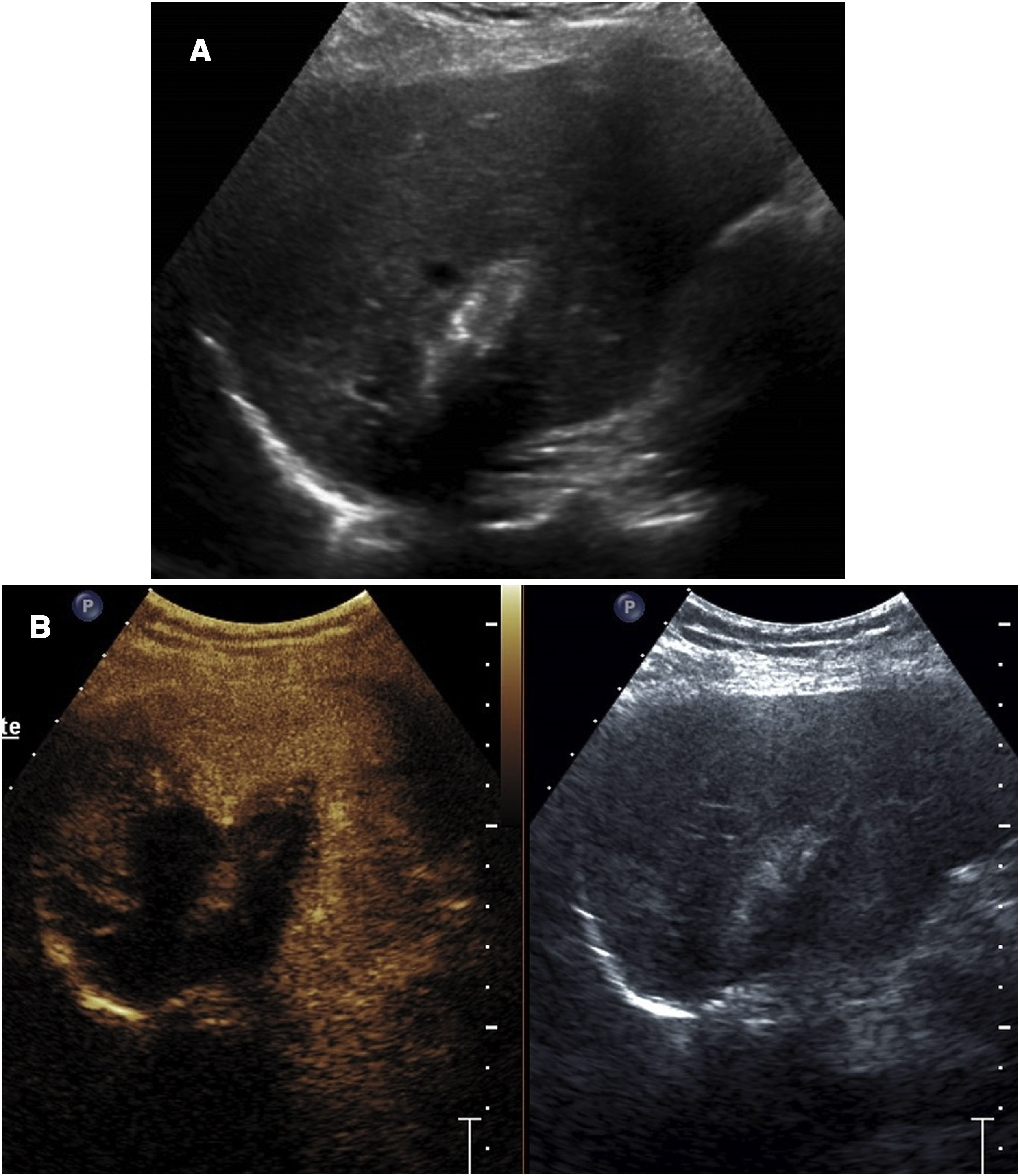
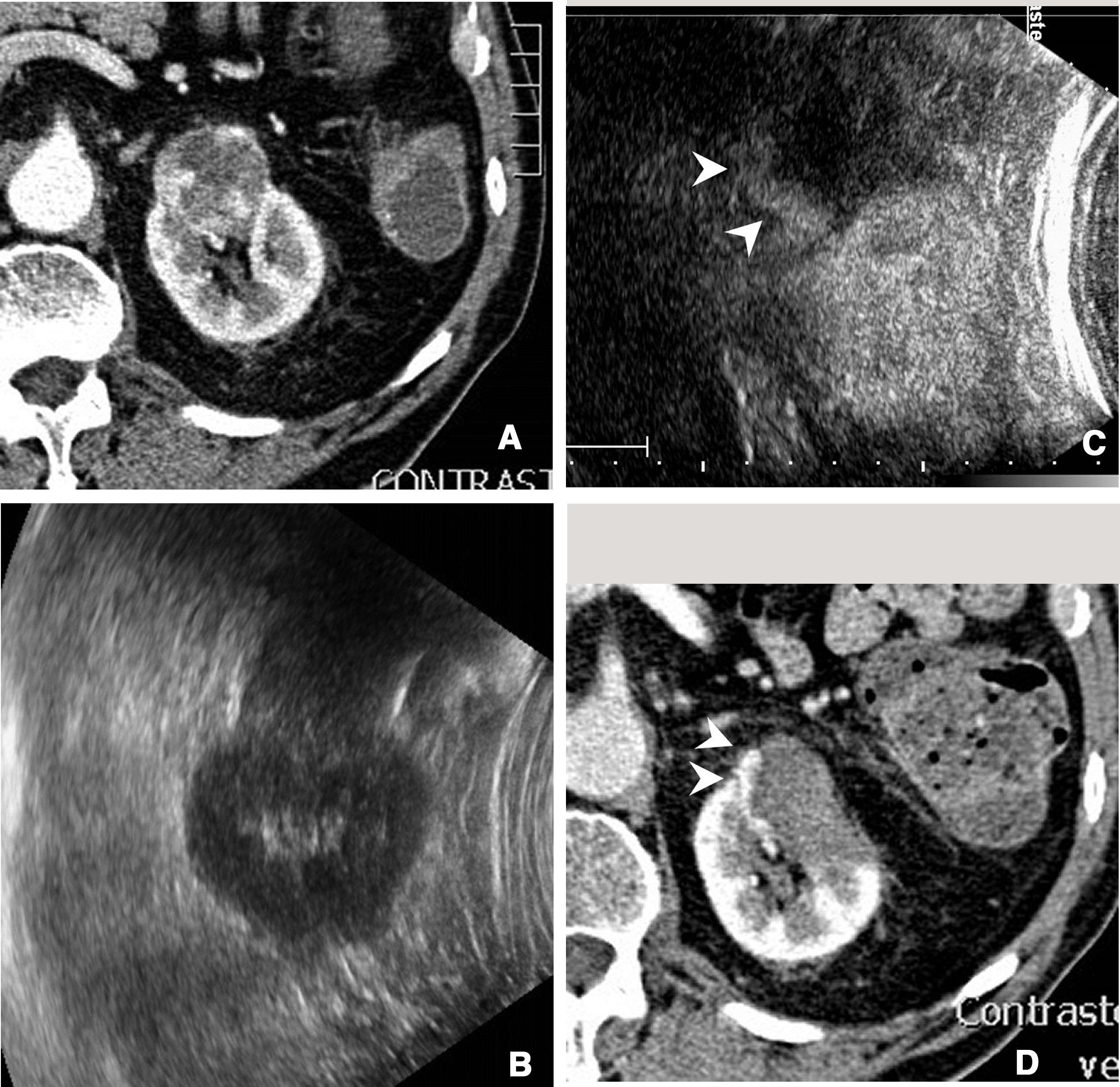
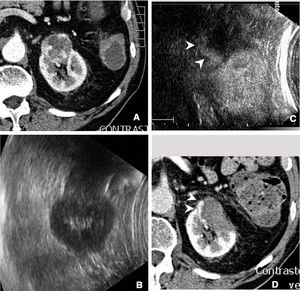
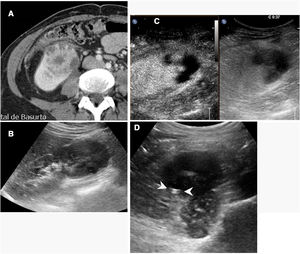
In follow-upContrast-enhanced ultrasound in the kidneys is mainly useful in the initial evaluation the day after ablation is performed33 (Fig. 5). Used in this way, it can provide early evidence of incomplete ablations.
Incomplete ablation of renal carcinoma. Computed tomography (A) and ultrasound (B) of the kidney showing a solid tumour. A decision was made to pursue treatment with radiofrequency ablation. C) Contrast-enhanced ultrasound performed 24h after ablation showed a crescent of uptake on the periphery of the treated tumour (arrow tips), indicating incomplete ablation. D) The tomography subsequently performed showed exactly the same finding (arrow tips).
In subsequent follow-up, ultrasound with contrast has a concordance rate of 80–100% with CT and MRI results in detecting relapses, although a certain trend towards false positives has been reported.4,10–13,32,33 In any case, as patients with kidney tumours often present multiple metachronic kidney tumours, contrast-enhanced ultrasound cannot be used as a replacement for CT or MRI in routine post-ablation follow-up checks, although it can be used to replace some of the checks.
Thyroid glandIn recent years, there has been a growing interest in ablation treatments in the thyroid gland. They are used above all as an alternative to surgery to decrease volume in benign lesions. Radiofrequency ablation and laser ablation are the techniques most commonly used for this purpose.
Contrast-enhanced ultrasound is the ideal examination for evaluating the outcome of thyroid ablation (Fig. 6). The thyroid gland is easily accessible to ultrasound examination, and contrast provides information on treatment success, both immediately and in subsequent checks.4,34 We use it as a reference technique (along with examination of lesion volume) for the follow-up of treated lesions, with one examination scheduled every three to six months for two years.
Intracavitary usesIntracavitary injection of ultrasound contrast may be a useful alternative to radiographic procedures with contrast injection, especially in ultrasound-guided procedures.
The problem with intracavitary use lies in the fact that contrast, at a concentration for vascular use, creates a sound screen that obscures the posterior planes and impedes visualisation of structures. Therefore, it should be used in a more dilute form, with a concentration similar to that used in ultrasound cystography. The concentrations that should be employed must be determined taking into account the need to maintain contrast visualisation for the duration of the procedure, which may be long. For this reason, it is preferable that they are not in the lower ranges of what is described in the scientific literature to balance the duration of visualisation and the absence of shadow. We use 0.1ml of contrast per 10ml of saline.4,35–37
Cholangiography and percutaneous nephrostomyThe use of ultrasound contrast in endoscopic retrograde cholangiography has been described.35 Though not a radiological procedure, it opens up the possibility of the use thereof in radiological biliary interventional procedures.
In percutaneous nephrostomy, it reportedly enables confirmation of needle or catheter positioning, evaluation of the site of obstruction and assessment of catheter-related complications.4,36
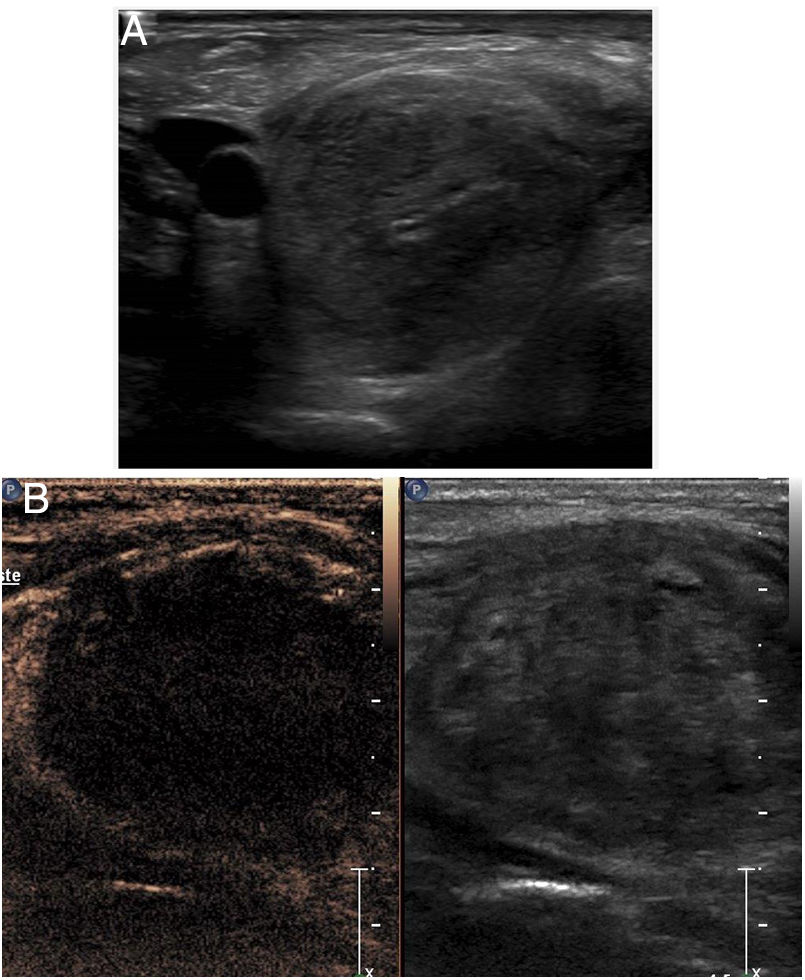
Drainage of abscessesContrast-enhanced ultrasound enables guidance of percutaneous placement of a drain in cases in which it is difficult to distinguish between a cavity and adjacent tissue (Fig. 7). It allows abscessed areas to be identified with confidence, as these areas do not enhance, and the catheter can be guided towards the non-enhancing area using the contrast mode.
Right kidney abscess. A) Computed tomography shows a heterogeneous image in the lower pole of the kidney, with fluid areas. A decision was made to perform percutaneous drainage. B) The ultrasound shows a hypoechoic lesion, but does not clearly identify the fluid area, complicating the guidance of the procedure. C) Contrast-enhanced ultrasound identified the abscess area, which did not enhance, and guided the procedure towards it. D) The catheter (arrow tips) is inside the cavity. The patient recovered quickly.
In addition, as in nephrostomy, it has been reported that direct injection of ultrasound contrast in an abscess facilitates confirmation of proper needle or catheter positioning during the percutaneous drainage procedure and enables evaluation of any connections between cavities in complex abscesses.4,37
We have used it to detect fistula tracts, especially with the gastrointestinal tract, in abscesses with a sustained high flow rate after a week of drainage. This procedure, however, can be done more economically by injecting radiological contrast. We have used this as well (this application cannot be pursued with any other technique) to enhance a fistula so that cyanoacrylate could be injected into it with the help of ultrasound guidance in order to seal it.
ConclusionsThe use of contrast-enhanced ultrasound makes it possible to broaden the indications for ultrasound-guided procedures to include many lesions not visible on conventional ultrasound.
It also aids in the planning and follow-up of diagnostic and therapeutic procedures, improves the sensitivity of biopsies and the efficacy of therapeutic procedures, and allows for an immediate check of the outcome of ablations.
The use of contrast-enhanced ultrasound should be a standard procedure in any unit that performs ultrasound-guided procedures, especially in the abdomen.
Authorship- 1
Responsible for study integrity: JLC.
- 2
Study concept: JLC.
- 3
Study design: JLC.
- 4
Data collection: JLC, GC, RZ, IK.
- 5
Data analysis and interpretation: N/A.
- 6
Statistical processing: N/A.
- 7
Literature search: JLC, GC.
- 8
Drafting of the manuscript: JLC, GC.
- 9
Critical review of the manuscript with intellectually significant contributions: RZ, IK.
- 10
Approval of the final version: JLC, GC, RZ, IK.
The authors declare that they have no conflicts of interest.
Please cite this article as: del Cura JL, del Cura G, Zabala R, Korta I. Ecografía con contraste en procedimientos ecoguiados. Radiología. 2022;64:277–288.