Cavernous angiomas are cerebral vascular malformations that are usually congenital. These lesions are characterised as dynamic. The most common phenomenon in the course of these lesions is bleeding, which can result in significant fluctuations in their size and even lead to their disappearance. This article aims to describe the behaviour of a cavernous angioma in its natural history, documenting: (a) its de novo appearance, a very uncommon observation, and (b) its changes on imaging studies, where it grew progressively like an expanding lesion but had no clinical repercussions. On magnetic resonance imaging, atypical signs can orient the etiological diagnosis of cavernous angioma versus other alternatives: de novo appearance, fluid–fluid or air–fluid level, incomplete hypointense ring due to hemosiderin deposition, pseudotumour-like growth, pseudocyst-like or multiloculated shape, vasogenic oedema, mass effect, and size greater than 3cm.
El angioma cavernoso (AC) es una malformación vascular cerebral, generalmente congénita, cuya historia natural se caracteriza por ser dinámica. La hemorragia es el fenómeno evolutivo más común del AC, puede ocasionar importantes fluctuaciones del tamaño de la lesión e incluso provocar su desaparición. El objetivo de este artículo es comunicar el comportamiento evolutivo observado en un AC en el que pudo documentarse: a) su aparición de novo, un hecho muy infrecuente y b) una evolución radiológica, de crecimiento progresivo, a modo de lesión expansiva, pese a no traducir empeoramiento clínico. En la evolución por resonancia magnética (RM), la presentación de signos radiológicos atípicos puede orientar el diagnóstico etiológico de AC frente a otros alternativos: aparición de novo, nivel líquido-líquido o hidroaéreo, anillo hipointenso incompleto de hemosiderina, crecimiento seudotumoral, forma seudoquística o multiloculada, edema vasogénico, efecto de masa y tamaño mayor de 3cm.
Cavernous angioma (CA) is a generally sporadic and solitary congenital vascular malformation1,2 whose main manifestations are bleeding into the brain and epileptic seizures. Magnetic resonance imaging (MRI) with gradient echo sequence is the diagnostic technique of choice,3 as it shows characteristic “popcorn” images and the complete haemosiderin rim. Angiographically,4 like thrombosed aneurysms, capillary telangiectasia or thrombosed arteriovenous malformations (AVM), CA is a hidden malformation. CA are characterised as dynamic.5 The most common phenomenon in the course of these lesions is bleeding,6 which can result in significant fluctuations in their size and even lead to their disappearance.
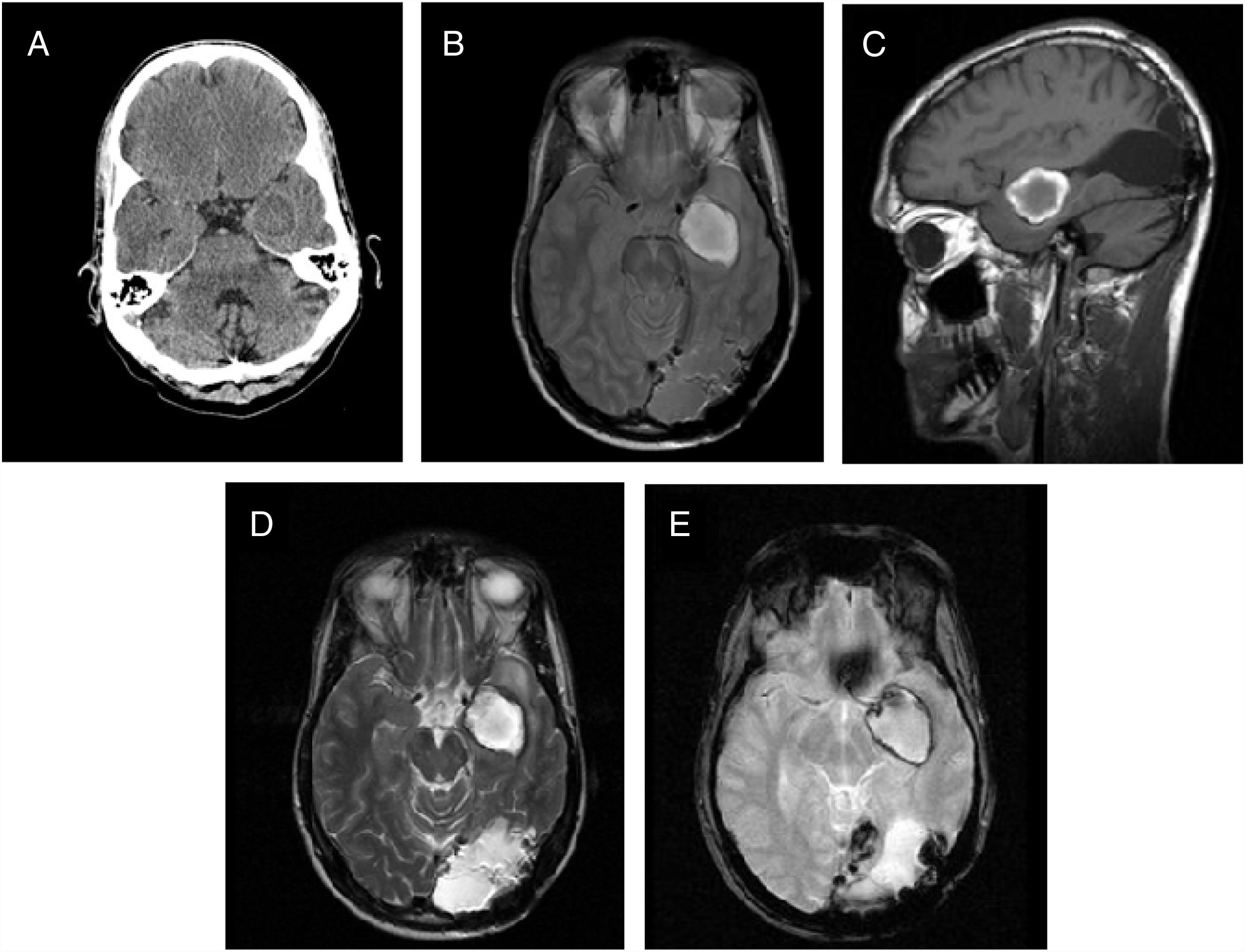
Case reportThis was a 35-year-old male, under follow-up since the age of 23, when he was treated for a left occipital AVM by embolisation and subsequent surgical excision. Three years before consulting our department he had a brain MRI as part of follow-up for the AVM which showed no abnormalities (Fig. 1A).
Previous imaging study and study at diagnosis. (A) T2-enhanced axial magnetic resonance imaging (MRI) of brain: absence of parenchymal abnormalities or other lesions suggestive of cavernous angioma (CA). Post-surgical changes in the posterior fossa. (B) Emergency computed tomography of brain three years after image (A): a left mesial-temporal hypodense nodule of de novo appearance adjacent to the internal carotid artery (ICA) and middle cerebral artery (MCA), 1.7cm in size, with a hyperdense level in its interior in relation to haematoma. (C) T1-weighted sagittal MRI. (D) T2-weighted axial MRI. (E) GRE T2-weighted axial MRI. Nodular lesion adjacent to MCA and ICA, 12mm transverse diameter (T)×15mm anterior/posterior (AP)×13mm craniocaudal (CC), with haemorrhagic lesion in subacute stage and fluid–fluid level. (F) Digital subtraction cerebral angiography: the left ICA was selectively catheterised, and rectification of said artery was observed at the intracranial level, without pathological staining (angiographically occult lesion).
The patient went to Accident and Emergency with epileptic seizures. Computed tomography (CT) of his brain (Fig. 1B) showed a left mesial-temporal hypodense nodule with hyperdense halo and slight blood level. This lesion was not evident on the brain MRI from three years previously. He was admitted to Neurology and had a new brain MRI (Fig. 1C–E), whose findings pointed to the differential diagnosis between CA, with atypical acute bleeding, and a thrombosed aneurysm. Cerebral angiogram (Fig. 1F) then showed that the lesion was angiographically occult.
He was prescribed antiepileptic drugs which dealt with the seizures, but within two months the seizures started to progressively reoccur, and more frequently. The radiological study was repeated (CT, Fig. 2A; MRI, Fig. 2B–E), showing significant changes in the left mesial-temporal lesion: signs of a pseudotumour-like growth due to a subacute haematoma, oedema and significant mass effect. Given the poor control of the epileptic seizures despite optimisation of the medical treatment, elective surgery on the lesion was indicated. Preoperative MRI, performed five months later (Fig. 3A–D) showed a large decrease in the size of the haemorrhagic lesion, not, however, accompanied by relevant clinical changes, and radiological signs typical of CA.
Follow-up images at two months. (A) Brain computed tomography (CT). (B) T1-weighted axial magnetic resonance imaging (MRI). (C) T1-weighted sagittal MRI. (D) T2-weighted axial MRI. (E) GRE T2-weighted axial MRI. Increase in the size of the left temporal/basal hypodense nodular image, with well-defined borders; has tripled in size. The growth had occurred due to a subacute haematoma with haemosiderin halo which caused significant oedema and mass effect on the cerebellar peduncle and the left middle cerebral artery.
Peri-surgical brain magnetic resonance imaging (MRI) study. (A) T1-weighted axial MRI. (B) T1-weighted sagittal MRI. (C) T2-weighted axial MRI. (D) GRE T2-weighted axial MRI. Notable in the preoperative study five months later was the decrease in the size of the haemorrhagic lesion, with slightly polylobulated and typical “popcorn-like” borders: well-defined nodule, with mixed intensity lobes that contained blood at different stages, and a complete hypointense peripheral ring corresponding to haemosiderin deposits. (E) Post-surgical follow-up T2-weighted axial MRI at eight months after surgery showing complete resection of the cavernous angioma.
After complete surgical resection of the lesion, the pathology diagnosis was CA. The frequency of seizures decreased over the course of the first year of follow-up and no neurological deficits appeared. The post-surgery follow-up MRI at eight months showed no residual cavernoma (Fig. 3E).
DiscussionThe purpose of this article is to document the unusual behaviour of a CA for two reasons. Firstly, the de novo appearance of the lesion, as they are usually congenital. Secondly, the fact that MRI showed it to have developed with pseudotumour-like behaviour due to the appearance of recurrent bleeding, without clinical signs.
The de novo formation of a CA is a rare phenomenon which has been described in patients with familial forms or who have received whole-brain or stereotactic radiotherapy.1 Our patient did not have these risk factors and this sporadic occurrence of CA is therefore exceptional.
In the literature we reviewed3 on the natural history of CA, several risk factors for haemorrhage, growth and aggressiveness are described: multiple or familial forms; clinical presentation before the age of 35; being female; infratentorial location, especially in the brainstem; being larger than 1cm in size; and partial excision. However, these data come from numerous studies whose results are very heterogeneous and difficult to extrapolate. A systematic review and meta-analysis5 of 25 studies evaluating the natural history of lobar and brainstem CA found no conclusive predictors of bleeding. However, according to another systematic analysis,6 prior bleeding was the main risk marker for having a new haemorrhage (odds ratio [OR] 3.73, 95% confidence interval [CI] 1.26–11.1; p=0.02). In addition, recurrence of bleeding was more common in the first 2–3 years and in brainstem lesions.
There are two suspected underlying mechanisms that can cause bleeding in CA2,7:
- •
Extralesional: this is the most common mechanism and is due to the rupture of peripheral caverns which would cause a low-flow bleed with a tendency to resorption. This mechanism would explain the transitory nature of some lesions. The risk of recurrence is low but can be life-threatening in brainstem lesions.
- •
Intralesional: this is less common and is caused by the rupture of adjacent caverns. In general, growth is not aggressive. However, recurrent bleeding can increase the cavernomatous matrix8 instead of collapsing it, leading to large expansion without previous evidence of haemorrhages and, as with a neoplastic lesion, having a pseudotumour-like clinical presentation with an increase in the frequency of epileptic seizures, acute elevation of intracranial pressure and transtentorial herniation. This behaviour has also been described in paediatric patients with cerebellar CA,9 which raises the differential diagnosis with cystic tumours such as pilocytic astrocytoma.
According to a prospective study10 on the dynamism of these lesions documented by MRI (114 CA in 68 patients), the size of the lesion does not seem to be related to either the likelihood of bleeding or the symptoms. Although 35% and 43% of CA grew in follow-up periods of one and two years respectively, the clinically relevant bleeding in those periods was only 3.1% per patient-year. In the first year, 55% decreased in size, 35% grew and 10% remained stable. The case we present here exemplifies the conclusions of this study: changes in the volume and MRI appearance of CA are common, often in relation to haemorrhages without clinical symptoms, and the general tendency is for them to slowly decrease.
With neuroimaging findings of a space-occupying lesion, the following radiological signs should make us suspect a diagnosis of CA (with recent haemorrhage) versus other alternatives1,8,9:
- •
The appearance of a de novo lesion larger than 3cm in size.
- •
A fluid–fluid or air–fluid level inside.
- •
Loss of the complete hypointense haemosiderin ring.
- •
Parenchymal haemorrhagic lesion with hyperintense peripheral halo in T1.
- •
A pseudocyst shape, with a well-defined capsule appearance.
- •
Vasogenic oedema.
- •
Mass effect.
- •
Progressive pseudotumour-like growth with elevated intracranial pressure.
In conclusion, CA should be considered in the differential diagnosis of newly occurring intraparenchymal lesions, and they can undergo a dramatic increase in size. Serial radiological assessment helps us make the definitive diagnosis of CA and predict its re-bleeding potential, which is the main risk factor for a new haemorrhagic complication.
Authorship- 1.
Responsible for the integrity of the study: CMRS.
- 2.
Study conception: FHF.
- 3.
Study design: FHF.
- 4.
Data acquisition: CMRS.
- 5.
Data analysis and interpretation: EL.
- 6.
Statistical processing: NA.
- 7.
Literature search: EL, CMRS.
- 8.
Drafting of the article: CMRS.
- 9.
Critical review of the manuscript with intellectually relevant contributions: FHF, TS.
- 10.
Approval of the final version: FHF, TS.
The authors declare that they have no conflicts of interest.
We would like to thank Drs Juan David Molina Nuevo and Hernán Sandoval Valencia for their contributions to the diagnostic and therapeutic process with this patient.
Please cite this article as: Romero-Sánchez CM, Hernández-Fernández F, Lozano E, Segura T. Correlato clínico-radiológico de un angioma cavernoso de novo con comportamiento seudotumoral. Radiología. 2020;62:243–247.