Autism spectrum disorder (ASD) is a multifaceted neurodevelopmental disorder distinguished with behavioral disability which has diverse causative factors. It is extensively acknowledged that interaction with genetic, environmental, epigenetics, and lifestyle factors contribute to ASD. Growing pieces of evidences shows that any changes in the epigenetic process plays a major role in the onset of ASD pathophysiology. In this regard, this review is directed towards unraveling maternal and prenatal factors such as maternal age, obesity, dietary nutrients, etc. that may act up on epigenetic mechanisms such as any changes in DNA methylation or histone acetylation could be a probable etiological influencer for ASD. In this review, we discuss the impact of maternal factors such as maternal age, obesity, hormonal imbalances, and dietary nutrients on the developing fetus, as any problems in these factors especially during gestation are associated with higher risk of ASD. Further, this review examines the potential role of these factors on the epigenetic modifications on the developing embryo and its possible role as a etiology for ASD. However, more work in this front of epigenetics is still required, but according to various earlier studies has showed that better understanding of these epigenetic process will help us to prevent ASD.
Los trastornos del espectro autista (TEA) son un conjunto de trastornos del neurodesarrollo que se caracterizan por problemas conductuales provocados por diferentes factores. Muchos autores admiten que la interacción entre factores genéticos, medioambientales, epigenéticos y los relacionados con el estilo de vida favorecen la presencia de TEA. Existe cada vez más evidencia de que algunos cambios en el proceso epigenético tienen un papel primordial en el desarrollo de TEA. En este sentido, esta revisión pretende analizar factores maternos prenatales tales como la edad, obesidad y alimentación durante el embarazo, que puedan condicionar los mecanismos epigenéticos; cualquier cambio en la metilación del ADN o en la acetilación de la histona podría influir en la etiología de los TEA. Analizamos el impacto en el feto de tales factores maternos como la edad, obesidad, desequilibrios hormonales y alimentación durante el embarazo, ya que cualquier problema relacionado con los mismos, especialmente durante la gestación, se asocian a un mayor riesgo de TEA. En esta revisión también examinamos el papel potencial de estos factores en las modificaciones epigenéticas del embrión y su posible implicación en la etiología de los TEA. Sin embargo, sigue siendo necesario estudiar este campo de la epigenética, ya que según han demostrado estudios anteriores, un mejor conocimiento de estos procesos será de gran ayuda para la prevención de los TEA.
Autism is a neurological disorder which is considered as one of the most common childhood disorder, mainly affects the brain development and behavior which is highly heritable in nature and often displays with co-morbid phenotypes. The symptoms of autism are lack of social interactions, repetitive, or restricted behavior. The prevalence of ASD has been increased up to 1–2.5% since the last two decades.1 In the male-to-female ratio among the ASD, it is observed that this condition is three times more common among male than compared to females.2 According to the mounting evidence, it has been reported that during pregnancy various lifestyle factors might have a role in causing ASD in their offspring.3 Since the last decade, it has been witnessed that considerable global efforts have been taken to identify various factors associated with ASD pathogens is such as inflammation, metabolism, environmental, and genetics. Recently, the authors have reported that there is a strong influence of the exposure of particulate matter (PM) during early phases of pregnancy which might impact various molecular pathways and might cause ASD.4 Previously, we had conducted an observational study among the ASD patients, where we found a correlation between few environmental and lifestyle factors have an impact on fetal development causing the atypical pattern the phenotype of ASD.5 Even the environmental factors exposures could alter the epigenetic modifications such as methylation or histone acetylation resulting into gene expression variations. These epigenetic modifications generally starts from the sequence-specific proteins or RNAs. These changes might result into defaultive recruitment of the epigenetic writers or transferases which adds the small molecules like methyl or acetyl to the DNA or histone respectively.6 Growing evidences from human and animal studies show that early life environment, including in utero and during early post-natal life, may play a role in ASD and other neurodevelopmental disorders.7 In light of these growing pieces of evidence, we provide here an overview about the role of epigenetic factors as a possible etiology behind ASD. In the first section of this review, we aim to deliberate the possible maternal and prenatal factors as a possible role to cause ASD in the offspring. In the second section, we will look at the possible role of epigenetic changes in ASD. Finally in the third section, we will provide the information related to possible alterations in epigenetic mechanisms due to maternal lifestyle factors during pregnancy.
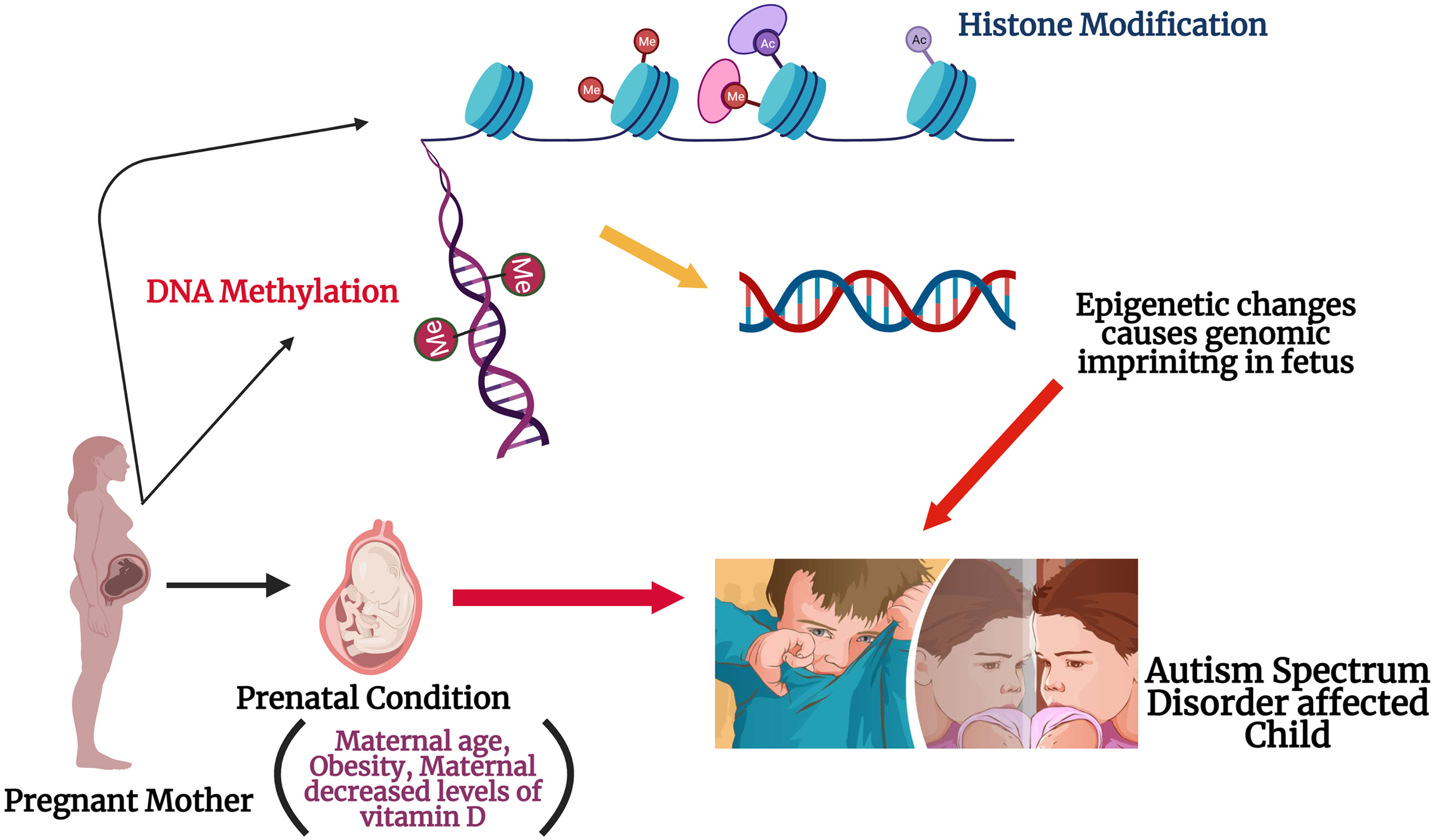
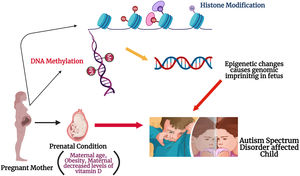
Epigenetics and ASDEpigenetic alterations are defined as impermanent and possibly inherited changes that regulate the gene expression through modifications to the shape and configuration of DNA, rather than nucleotide sequence. DNA methylation can be modified in many ways such as mutations,8 exposures to mother during pregnancy,9 or post-natal exposures to environmental factors10,11 and these studies provide a link between gene and environment in causing ASD. Epigenetic mechanisms regulates the prenatal development via maintaining the cell proliferation and differentiation, as well as tissue specification processes. A previous study conducted on human subjects showed that even childhood abuse could alter the DNA methylation patterns in the brain, resulting in epigenetic changes to the gene expression.12 Epigenetic processes and the interaction with maternal health conditions during pregnancy might help to explain the differences in gene expression patterns in ASD patients (Fig. 1). More research is still pending to find which maternal factors are responsible for the epigenetic changes seen in ASD, but many have been suggested: maternal age, maternal diet and intake of nutritional supplement, diabetes, and obesity especially during and before pregnancy. There are few findings from the genome-wide assays that epigenetic alterations and its related dysregulations, especially any changes in DNA methylation in ASD, but its needs even more research to understand completely.13 X-chromosome inactivation and imprinting along with DNA methylation might have a possible role in the field of ASD research.14 According to previous reports, the authors have stated that maternal age-related DNA methylation alterations have a strong connection between aging and DNA methylations. Further, these types of changes has also been practically detected in mouse oocytes and human.15 Maternal obesity is associated with increased risks of pregnancy outcomes and childhood obesity, even it has been reported that molecular mechanism has a sensitivity in their progeny's epigenome due to the maternal obesity.16 The folate has a major role to do during the pregnancy period, as it has a major role in DNA methylation because it is the major donor of methyl for the process.17 Alterations in the expression of imprinted genes during pregnancy can influence fetal and neonatal phenotype.18 Therefore, a discussion of potential epigenetic etiologies of ASD necessarily involving the health condition and both maternal and prenatal factors is necessary to understand the onset of this disorder.
Epigenetic modifications due to maternal influencers: The figure illustrates the possible role of maternal factors in modifying the epigenetic process during the gestation. Histone modification might occur either via acylation or methylation at the histone tail during the fetal neurodevelopment in womb. DNA methylation could occur due to replacement of DNMT 3a and 3b enzymes location in the embryo. These possible alterations in the could cause genomic imprinting in the fetus and might be a reason for ASD onset in the offspring.
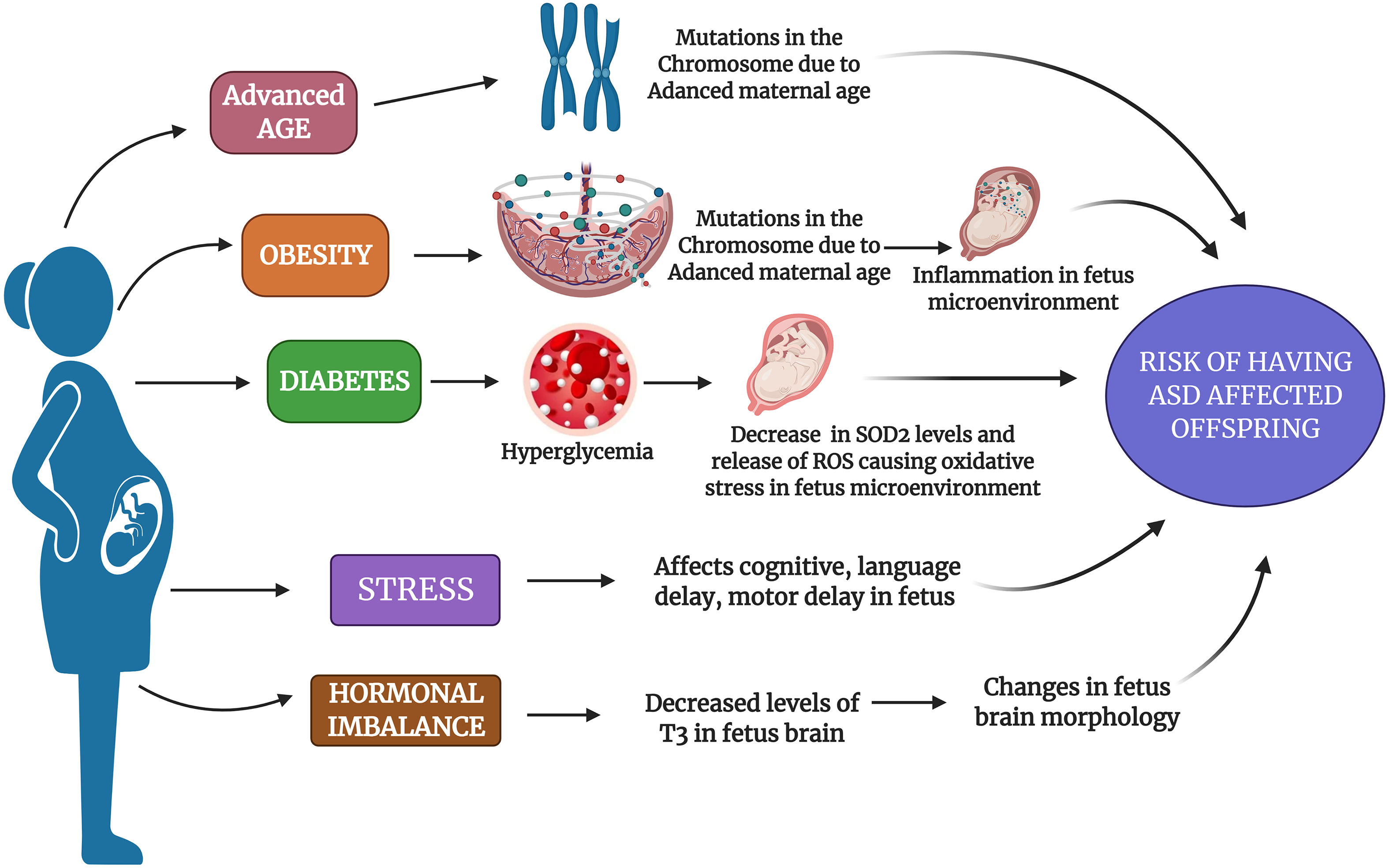
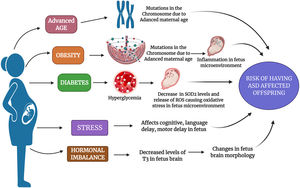
Childbearing in the later stages of life is becoming more common nowadays, especially due to the lifestyle factors, women who are more carrier-oriented, financial status, fertility issues, and many more because of which these women are more to deliver progeny with some kind of neurodevelopmental problems.19 Mothers who are pregnant at older age can have many complications during their pregnancy such as premature birth, low birth weight, labor complications, surgical delivery, and chromosomal defects.20 Studies have reported that mothers with older age have various chromosomal defects leading to a heightened risk for delivery complications like uterine muscle dysfunction and low blood supply.21 The consequence of increased parental age is an entrenched element of risk for the chromosomal anomaly, like Down syndrome and neurodevelopmental conditions including schizophrenia, Attention Deficit Hyperactivity Disorder (ADHD), and ASD.22 According to growing evidence, it has been proved that as the maternal age increases, the mother is exposed to various kinds of environmental toxins which induce damage in DNA as well as hypermethylation and germline mutations which might be a reason for the occurrence of ASD in the progeny.23 Genetic predispositions is high in ASD mainly due to mutations in X chromosome which might be passed on from one generation to other (Fig. 2).24 In a previous experimental study, the authors have confirmed that the women who were aged more than 40 years during the pregnancy had 51% higher risk of having ASD affected children than compared to women who were 25 to 29 years during their pregnancy.25 However, all these evidences prove that higher parental age has the probability of developing de novo effects such as autism or any other neurodevelopmental conditions in their children.26–28 In spite of this, more confirmation is still required to provide men and their partners with precise advice regarding the risks of delayed fatherhood and motherhood.
Various maternal factors as a risk factor for ASD: The figure depicts various maternal factors such as advanced age, obesity, diabetes, stress, and hormonal imbalance as a causative risk factor for ASD. The figure depicts the possible mechanism that could be altered due to mentioned factors in a pregnant mother which increases her risk to have an ASD-affected offspring.
Maternal obesity during gestation or before pregnancy is mainly related with an elevated risk of neurodevelopmental disorders in their offspring, because there is a common assumption that obese pregnant mothers babies will have an unfavorable effects especially due to the intrauterine environment conditions. Obesity during pregnancy has some connection with ASD risk, as there is a positive correlation between pre-conception body mass index (BMI) and ASD risk in children along with diabetes.29,30 It has been reported that the amount of weight gained especially during or before pregnancy has detrimental health effects for both mother and fetus, mainly the fetus neurological system might get affected.31 In an ASD subjects’ case–control study which was conducted in California, it was observed that mothers who were obese before their pre-natal period had a 67% of higher risk for having ASD affected kids.32 Many studies have reported that obese mothers have an increased risk of having ADHD affected, intellectual disability (IQ < 70), low cognitive test, and higher risk of neurodevelopmental disorders.33–35 Previous studies have pointed out that obesity leads to inflammation in the placenta which may result into fetal systemic inflammation and cytokine abnormalities especially in the children with ASD (Fig. 1).36,37 Similarly a study by Edlow et al. (2014) showed that apolipoprotein D which is important for fetal neurodevelopment gets disrupted due to maternal obesity, and this might be a reason for neurodevelopmental issues in a child.38 Extending this research, a review in 2018 summarized the risk of maternal weight on autism and other neurodevelopmental disorders.39 Thus, these pieces of evidence prove that maternal obesity does have a major role in causing ASD in their progeny, but more in-depth research is needed to find a solution for it.
Maternal diabetesPregnant women are most usually (15% to 87.5%) affected with diabetes which is commonly known as gestational diabetes (GDM). This happens mainly due to glucose intolerance especially during the onset of pregnancy.40 Diabetes during pregnancy has been allied with various health issues and risk such as miscarriage, macrosomia, fetal abnormalities, neurodevelopmental issues in the fetus.41–43 Previously, it has been stated that there is a direct correlation among the gestational diabetes and risk for ASD in the progeny.44 The reason for diabetes to play a role behind causing ASD was been discovered by Wang et al. (2019) that the hyperglycemia-mediated persistent oxidative stress and superoxide dismutase 2 (SOD2) gets suppressed in the offspring of the mothers who had diabetes during their pregnancy (Fig. 1).45 In an epidemiological study, the authors have revealed that mothers who had either type 1 diabetes mellitus (TDM1) or type 2 diabetes mellitus (TDM2) OR GDM found to have a link for ASD risk in their offspring.46 Also in a recent meta-analysis study, the authors concluded that there is a high probability that mothers who had diabetes during their pregnancy had 62% higher risk to have ASD affected baby when compared to their non-diabetic mothers.47 Hence, these studies have shed a light for the researchers to conduct more productive research especially to analyze the link between diabetes causing ASD affected babies.
Maternal stressPre-natal stress is associated with neurodevelopmental issues in progeny.37 Various retrospective studies have observed that maternal stress especially during pregnancy has augmented the risk of having some issues in the offspring including neurodevelopmental problems, language delay, cognitive issues, behavioral problems and many more (Fig. 1).48–50 Even few previous animal model studies have reported that stimulation of stress on rodents or primates during pregnancy could affect the behavioral symptoms in their offspring.51–53 The studies which focused on the maternal stress interpreted that mothers during pregnancy who were exposed to situations like disharmony in family, stressful life events; natural calamities were associated to have increased risk for ASD affected children.54–56 Even Robert et al., (2014) had conducted an experimental study and reported that there is an intergenerational association among the childhood abuse or stress as well as risk of ASD in their future offspring.57 In a recent study, the authors observed that the any mutations in SLC6A4 accompanied with prenatal stress might have a significant role in inducing the risk of ASD in their offspring.58 According to a previous study, it has been reported that increased levels of stress during pregnancy can be a risk factor for ASD development in the offspring when compared to stress-free environment during pregnancy.59 It is also found that the severity of ASD was significantly increased with multiple parental stressful events in life. In spite of controlling various sociodemographic and obstetric variables, pre-natal stress was an important factor in ASD severity.60 A higher peak of stressors during 25‑28 weeks of gestation was found to be associated with autism; hence prevention of stress during pregnancy may benefit both offspring and maternal mental health.61 Thus, it is important to maintain the psychological stress of mothers during pregnancy to maintain a normal developmental and behavioral outcome of an offspring.
Hormonal imbalancesHormones such as estriol, estradiol, estrone, estrogen, progesterone, androgen, steroid, testosterone, and thyroid are essential for the healthy development of a fetus.62 In a previous study, it was found that unconjugated estriol (uE3), maternal serum alpha-fetoprotein (MSAFP), and human chorionic gonadotropin (hCG) imbalance were related with autism in offspring.63 Also, according to the ‘extreme male brain’ theory, it says that males are having more risk to acquire autism due to the exprosure of androgen hormone, especially during the fetus is growing in in-utero region.64 Hans Asperger had quoted that the autistic individual has a utmost variation of male intelligence, thus in ASD kids male pattern, behavior, and tendencies are extremely intensified.65 In a recent study, the authors observed that the steroid hormones especially the estrogen hormone analyzed during the 14‑16 weeks of gestation found that the offspring had later diagnosed with ASD; also when the ASD children were tested for all four forms of estrogen hormone showed an increased levels in each form.66
Similarly, maternal progesterone level also has a pivotal role in fetal development, but mother's over-exposure to progestin induces autism-like behavior in the fetus, as it suppresses the levels of estrogen receptor β in the brain.67,68 Most women who would have had fertility issues might have taken estrogen hormone during their therapy, while there are reports which suggest that excessive levels of estrogens has pathological role in various neurodevelopmental disorders such as schizophrenia, ASD, and ADHD.62 An increased risk of having an autistic kid was seen in severe maternal hypothyroxinaemia with thyroid peroxidase (TPO) or thyroglobulin antibodies negative mothers.69 As the maternal hypothyroxinemia results in low T3 levels in the brain of a fetus especially during 8‑12 weeks of pregnancy; this may lead to autism due to changes in the morphology of the brain; hence treating maternal hypothyroidism may lower the risk of ASD in the offspring (Fig. 1).70 Even in an animal model (rat) study, the authors have found that disturbances or low supply of T3 hormone levels during pregnancy can contribute to halting of proper neurogenesis which might contribute to behavioral disturbances as one of the noted symptom in ASD.71 Hence, hormonal impact from both maternal and pre-natal stages of pregnancy should be studied to enlighten the crucial role of hormones in causing ASD.
Impact of folate in epigenetic modificationsFolic acid (FA) is an essential vitamin that is needed for the proper physiological process of the human body. Folate is necessary for proper development of fetus neural development and metabolism, thus ensuring that the pregnant women is having appropriate amount of FA nutrition is supplied in her diet.72 Various reports suggest that the group of B-vitamins has a major role in metabolism of one-carbon as well as in regulation of DNA methylation and synthesis.73–75 In a previous study, it was shown that the low levels of folate has a wide role in ASD pathogenesis.76 A study with 837 subjects found that the intake of FA in the first trimester was greater in typical development (n = 278) when compared with mothers with ASD children.77 In many neurodevelopmental disorders, it was found that the reduced form of folate known as folinic acid can reverse the neurologic and other developmental disorders when given in high dosages.78 Global methylation or any changes in epigenetics mainly occurs due to any fault in its main methyl donor which is the 5-methyl-tetrahydrofolate. This happens mainly due to any variations in the folate levels or problem on metabolism of folate and these changes might also trigger ASD.79,80 Also during the folate metabolism, the MTHFR enzyme has a major role as it chemically reduces the 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate which acts as primer donor during the methylation process from homocysteine to methionine.81 These studies provide us a clue that folate deficiency associated with any polymorphism in MTHFR might alter the DNA methylation process.
Maternal factors & epigenetics – mechanism of actionDuring development, dynamic changes in epigenetic markers such as DNA methylation, DNA hydroxymethylation, post-translational covalent modifications (e.g., addition of methyl or acetyl groups to histone residues), and numerous types of short and long RNAs regulate the transcriptional activity of genes as cells acquire specific functions. Major cellular processes such as cell metabolism, survival, proliferation, and activity does get regulated due to this epigenetic mechanisms. Various studies have also reported that during the embryonic period any alterations in DNA methylation, histone modifications, microRNAs might lead to metabolic disorders in their later life.82,83 The formation as well as transfer of phenotypes to the next generation mainly depends on three mechanisms which are gene expression, chromatin accessibility, and DNA replication.84 The tenacious epigenetic changes due to maternal and pre-natal factors could result into irregularities in early development/health/ may be a reason for any disease in future in the human beings. In the process of DNA methylation, the addition of methyl group in the cytosine base which is commonly present in the CpG islands within the regulatory sites of gene promoter region act as a pivotal regulator of the gene expression.85 Hence, a controlled regulation of these DNA methylation is necessary for the proper development of the fetus.86 Therefore, in any environmental exposures during the gestational or neonatal period, the epigenome gets susceptible to mutations and this could impact the normal process of DNA methylation or histone modifications in the offspring.87 These alterations are mainly due to environmental factors especially by controlling the phenotypes of gene expression.88 The DNA methylation enzymes such as DNMT 3a and DNMT 3b are responsible for the proper functioning of methylation during fetal development. The DNMT 3a is expressed from the beginning of primordial stage while the DNMT 3b is expressed on primary follicle stage only. The studies have stated that any dysfunction in the mothers such as maternal age, obesity, or stress, etc. will alter the levels of these enzymes and inadequate methylation resulting into imprinting in embryo and future offspring, which could be a reason for neurodevelopmental disorders like ASD. Another possible role of maternal obesity in epigenetic changes is when the mother is obese during her pregnancy, there is an excess accumulation of macrophages and lipids which leads to increased levels of oxidative stress in placenta.89 Such kind of morphological changes in placenta could alter the epigenetic processes like DNA methylation, DNA hydroxymethylation, histone acetylation, micro-RNA, and eventually resulting into improper fetal brain reprogramming during pregnancy.
ConclusionAs ASD is a lifelong childhood affected neurological condition,90 we need a proper awareness of this particular disorder to avoid the social stigma faced by affected individuals and their families. Epigenetics and its related technologies when it comes to ASD is still in its toddler stage.91,92 As it has been established that inhibition of improper DNA methylation and histone deacetylation could successfully reverse the abnormal gene expression in a given disease condition.93 However, in this review, few studies strongly show significant findings about maternal health and lifestyle practices and its influence on the epigenetic process do play a major role in causing and onset of ASD in offspring. The findings suggest that ASD has a complex interconnection between genetic, epigenetic, maternal lifestyle, and socioeconomic factors especially during pregnancy which could disrupt the normal epigenetic and neurodevelopment process in the fetus. Therefore, a mechanistic understanding about how these various factors associated during pregnancy phase interact in the onset of ASD may provide important insights into the possible therapeutic approaches for this condition. Hence, we suggest that these findings may provide unique leads for further research and to discover the mechanisms underlying on the onset of ASD.
FundingThis study was supported by Science and Engineering Research Board (SERB) Early Career Research (ECR) Award funded by the Government of India, New Delhi (Grant No. ECR/2016/ 001688).
Declaration of interestNone.
The authors would like to thank Bharathiar University for providing the necessary infrastructure facility and the Science and Engineering Research Board (SERB; ECR/2016/001688), Government of India, New Delhi for providing necessary help in carrying out this review process.