Effective treatments that reduce relapses can diminish the impact on MS costs in the long-term. This study aims to estimate direct costs of RRMS relapses in Catalonia (Spanish region).
MethodsMulti-centre, prospective, cross-sectional observational study with retrospective data collection. Estimated costs: disease-modifying therapies (DMTs) and symptoms treatments, use of hospital and ambulatory resources, technical aids, transport and paid caregivers from the National Health System (NHS) and global perspectives.
ResultsOne hundered and forty (140) suitable patients were included to estimate direct costs (mean [SD] age: 40.7 [10] years; females: 71.5%; low EDSS score at relapse start: 77.5%). Mean total direct costs of relapse/patient were €4,541 (NHS) and €4,626 (global). A subanalysis was performed in patients with relapses lasting ≤90 days (100 patients), relapse total direct costs/patient were €4,989 (NHS) and €5,115 (global). Motor relapses presented a higher cost (a mean of €9,345/patient). Mean total direct cost/patient was higher when there was a DMT switch due to the relapse (€14,370 compared to €1,149), from a global perspective.
ConclusionMean direct costs of RRMS relapse/patient in Catalonia were €4,989 and €5,115 for NHS and global perspectives, respectively. The type of relapse and switching the DMT due to the relapse were associated with an increase in direct costs.
Los tratamientos que logran reducir los brotes en la esclerosis múltiple (EM) también pueden disminuir el coste de la enfermedad a largo plazo. El propósito de este estudio es realizar una estimación de los costes directos relacionados con la EM remitente-recurrente (EMRR) en Cataluña.
MétodosHemos realizado un estudio observacional, transversal, prospectivo y multicéntrico en el que hemos recogido los datos de forma retrospectiva. Para calcular los costes directos, consideramos los costes derivados del uso de fármacos modificadores de la enfermedad (FME) y tratamientos sintomáticos, recursos hospitalarios y ambulatorios, ayudas técnicas, transporte y cuidadores remunerados, tanto desde la perspectiva del sistema nacional de salud (SNS) como desde una perspectiva global.
ResultadosRecogimos datos de 140 pacientes cuya edad media (DE) era de 40,7 (10) años; de ellos, el 71,5% eran mujeres y 77,5% presentaron una puntuación baja en la EDSS al comienzo de los brotes. Los costes directos medios de los brotes fueron de 4541€ (SNS) y de 4626€ (costes globales) por paciente. Se realizó un subanálisis de los pacientes que sufrieron brotes de al menos 90 días de duración (100 pacientes), en los que los costes directos medios del brote fueron de 4989€ (SNS) y 5115€ (costes globales) por paciente. Las recaídas de las manifestaciones motoras supusieron un coste mayor (media de 9345€ por paciente). En términos globales, los costes directos medios por paciente fueron mayores cuando hubo que cambiar de FME debido a un brote (14 370€ frente a 1149€).
ConclusiónLos brotes de la EMRR en Cataluña tuvieron un coste directo medio de 4989€ por paciente para el SNS y de 5115€ por paciente a nivel global. El tipo de brote y el cambio de FME debido a un brote se relacionaron con un aumento de los costes directos.
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) with a typical age of onset among young adults (20–40 years of age). It is the most common non-traumatic cause of neurological disability among this age group.1
This disease has a substantial socioeconomic impact2 given that it affects young adults and its prevalence is increasing worldwide, specifically, 80–180 people diagnosed for every 100,000 inhabitants in Spain.3
In general, MS begins with a relapsing–remitting phase which tends to progress to the secondary progressive form.4 The main goal in the management of MS is to reduce the frequency of relapses and to slow disease progression.5 Relapsing–remitting multiple sclerosis (RRMS) is treated long-term with disease-modifying therapies (DMTs) that reduce clinical and radiological disease activity.6
However, published studies show that “first-line treatments” such as interferons and glatiramer acetate often do not adequately control the disease’s activity, and relapses and progression of the disease may continue.7–9 In addition, it has been observed that this disease has an early window of opportunity for treatment to influence the irreversible accumulation of neurological damage.10,11 Switching to a highly effective treatment (such as fingolimod, natalizumab or alemtuzumab, among others) at an early stage may have an effect on long-term clinical benefits7,12 controlling inflammation and neurodegeneration.13
Effective treatments can reduce the impact on MS costs and on patient’s quality-of-life, reducing the social impact of the disease.14 In order to assess effectiveness of these drugs, evaluating the costs associated with a relapse in MS patients is required.2
This study was aimed to estimate the direct costs per RRMS relapse in hospital environments in Catalonia from the Spanish national health system (NHS) and global (combining the costs of the NHS and those borne by the patient) perspectives.
Materials and methodsStudy designA multicentre, cross-sectional, observational study was carried out in Catalonia, Spain, collecting retrospective data from relapses in patients with RRMS. Patients were recruited prospectively from 13 participating health centres (Barcelona, Tarragona, Lleida and Girona), which were involved in monitoring patients for at least six months after the start of a relapse. Follow-up visits were not carried out.
A relapse was clinically defined as the appearance of a new neurological anomaly, or the worsening of a previous anomaly (that was stable or improving), with a separation of at least 30 days since the start of a previous clinical demyelinating event. The relapse must have been present for at least 24 hours in the absence of fever or infection. All the symptoms occurring within a period of 30 days were considered part of the same episode. For those patients who suffered more than one relapse during the period of the study, the data was gathered from the first relapse.
The start and end dates of the relapse were defined as the date the new neurological symptom appeared and the date it remitted and/or stabilised, respectively, according to the investigator’s clinical criteria.
Selection criteria for the study populationThe study size was calculated based on 290 patients to estimate the mean cost of a relapse of RRMS with a confidence level of 95%, a precision of ± €1,500, assuming a typical deviation for the cost of an MS relapse of €12,00015–17 and a repositioning rate of 15% for losses.
Eligible patients were aged 18–65 years, with a confirmed diagnosis of RRMS for at least 1 year, and an EDSS score (Expanded Disability Status Scale by Kurtzke) ≤7 points.18 All patients had at least one documented clinical relapse (reported since January 2013) and at least six months of follow-up. All participating patients provided their written informed consent.
Patients were excluded if they presented clinical signs of MS other than RRMS, were suffering from a relapse at the time of the visit or were participating in clinical trials.
The protocol and informed consent form were reviewed by the Clinical Research Ethics Committee (CREC) at the Hospital de Mataró, Catalonia, Spain.
Data collectionData collected from medical records included demographics, clinical information, previous DMT and non-DMT treatment, including corticosteroids, information corresponding to the relapse being studied (duration, type and EDSS score), as well as resources, selected based on the opinion of an expert physician,19 used due to the relapse. The unit costs associated with these resources were obtained from the health costs database eHealth.
Researchers recorded the data on an electronic case report form (eCRD) designed for this study. The integrity and accuracy of the data introduced was reviewed on eCRD according to the validation plan.
Analysis perspectivesCosts were estimated from two perspectives: NHS and global. The analysis from the NHS perspective considered all direct costs (medical and non-medical) paid by the NHS. The analysis from the global perspective also included the costs borne by the patients themselves (the part not reimbursed by the NHS).
CostsBoth direct medical costs (the use of hospital and ambulatory resources and drug treatments) as well as non-medical costs (technical aids, transport and paid caregivers) were taken into consideration.
The medicines database of the General pharmaceutical Council of Spain (Bot PLUS)20 were consulted to obtain the unit costs of drug treatments, and the unit costs for hospital and ambulatory care resources were calculated using the eSalud medical costs database.19 Regarding transport, the unit cost for ambulances was consulted in the publicly available rates on eSalud19 and the unit cost per taxi, according to the fares for the Metropolitan area of Barcelona.21 The unit cost for technical aids was obtained from the orthoprosthetic services catalogue from the Catalan Health Service22 (Table S-1, additional material). The costs were expressed in 2017 Euro values which, according to Consumer Price Index variation (Statistics National Institute) might result 1–2% higher today.
Subanalysis and stratificationA subanalysis was carried out on patients whose relapse had a duration of ≤90 days. Within this analysis, the direct costs associated with the relapse were analysed according to the type of relapse, disability according to the patient's EDSS score (prior and during the relapse) and according to whether or not treatment was changed as a result of the relapse.
Statistical analysisA descriptive analysis of the variables collected was carried out. Quantitative variables were described using means, confidence intervals (CIs), medians and interquartile ranges (IQRs). The qualitative variables were analysed using absolute and relative frequencies.
The CI for costs were calculated using the bootstrapping technique for samples with replacement of the same size as the original sample.23,24 We carried out 10,000 simulations and use the percentiles 2.5 and 97.5 for distribution to determine the 95% CI.
All the analyses were carried out using SAS version 9.4 and R version 3.3.2 statistical programme.25
ResultsDescription of the study populationThirteen hospitals of the public healthcare system of Catalonia (nine sites in Barcelona, two in Tarragona, one in Girona and Lleida, respectively) providing active care to patients with MS, participated in the study.
A total of 153 patients with RRMS were enrolled (September 2017–December 2018). Of these, 151 met the clinical and sociodemographic description, and 140 were suitable for estimating direct costs. Despite not having reached the planned study size (290 patients), the precision achieved in estimating direct costs (€858.7–1,249.4) remained lower than that recommended in the protocol (±€ 1,500), it was a sufficient study size to evaluate the primary objective. Given the extreme values for the length of the relapse, for clinical criteria a subanalysis was carried out on those patients with a length of relapse below or equal to 90 days.26 This subanalysis included 110 patients with relapses lasting for ≤90 days, who met the sociodemographic and clinical descriptions, and of these, 100 patients had the necessary information to estimate costs (Figure - Supplementary material).
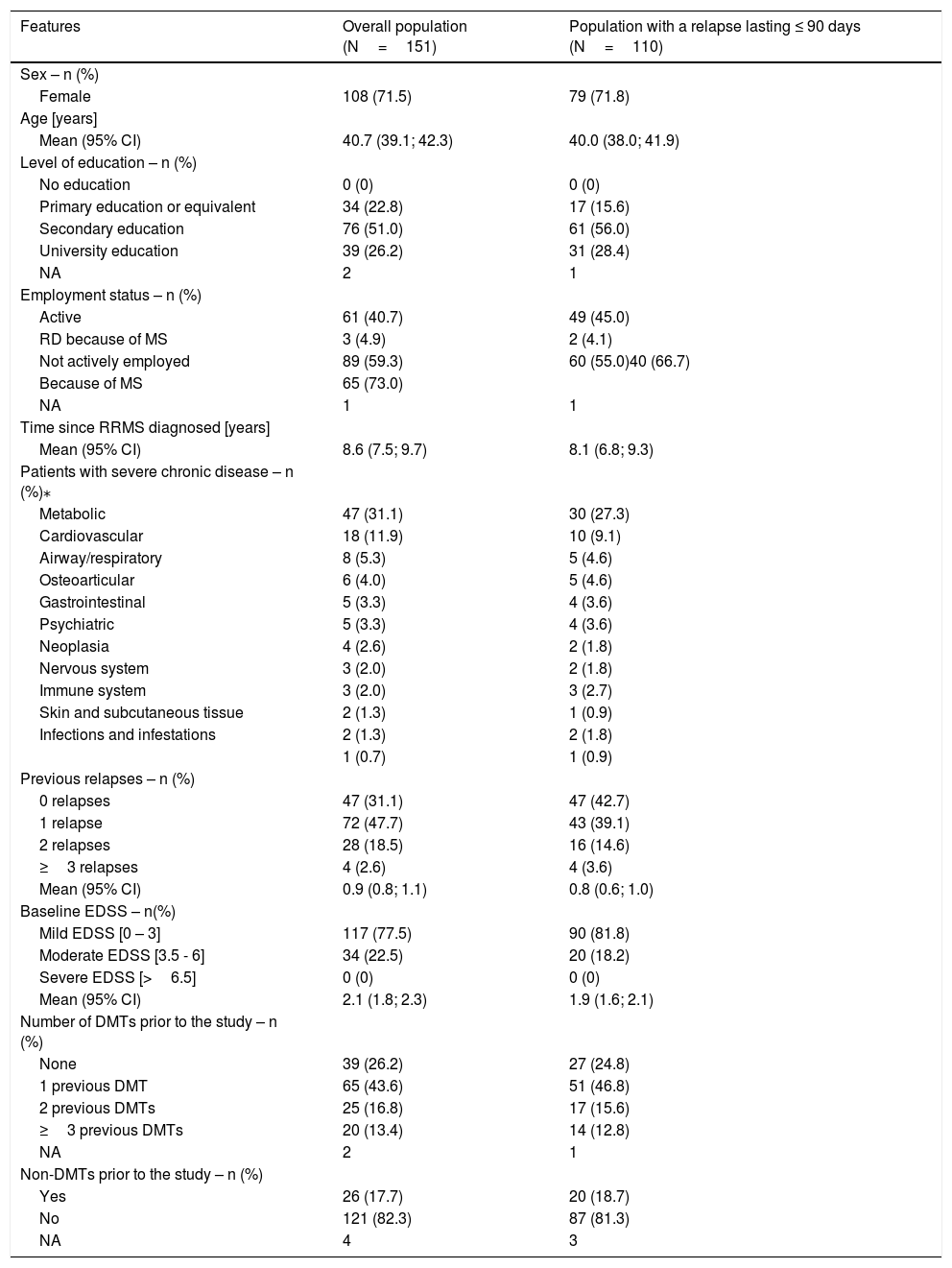
A total of 151 patients were included in the study. Their demographic and clinical characteristics are described in Table 1. Briefly, 71.5% were women, with a mean age of 40.7 years and a length of disease progression of 8.6 years from diagnosis. Around 40.7% of patients were in active employment, of whom 4.9% reported a reduction in daily working hours because of the MS and of the 59.3% patients not actively employed, 73% were due to the disease, as considered by the investigator.
Baseline sociodemographic and clinical features of the overall population and patient population with a relapse lasting ≤90 days.
| Features | Overall population (N=151) | Population with a relapse lasting ≤ 90 days (N=110) |
|---|---|---|
| Sex – n (%) | ||
| Female | 108 (71.5) | 79 (71.8) |
| Age [years] | ||
| Mean (95% CI) | 40.7 (39.1; 42.3) | 40.0 (38.0; 41.9) |
| Level of education – n (%) | ||
| No education | 0 (0) | 0 (0) |
| Primary education or equivalent | 34 (22.8) | 17 (15.6) |
| Secondary education | 76 (51.0) | 61 (56.0) |
| University education | 39 (26.2) | 31 (28.4) |
| NA | 2 | 1 |
| Employment status – n (%) | ||
| Active | 61 (40.7) | 49 (45.0) |
| RD because of MS | 3 (4.9) | 2 (4.1) |
| Not actively employed | 89 (59.3) | 60 (55.0)40 (66.7) |
| Because of MS | 65 (73.0) | |
| NA | 1 | 1 |
| Time since RRMS diagnosed [years] | ||
| Mean (95% CI) | 8.6 (7.5; 9.7) | 8.1 (6.8; 9.3) |
| Patients with severe chronic disease – n (%)⁎ | ||
| Metabolic | 47 (31.1) | 30 (27.3) |
| Cardiovascular | 18 (11.9) | 10 (9.1) |
| Airway/respiratory | 8 (5.3) | 5 (4.6) |
| Osteoarticular | 6 (4.0) | 5 (4.6) |
| Gastrointestinal | 5 (3.3) | 4 (3.6) |
| Psychiatric | 5 (3.3) | 4 (3.6) |
| Neoplasia | 4 (2.6) | 2 (1.8) |
| Nervous system | 3 (2.0) | 2 (1.8) |
| Immune system | 3 (2.0) | 3 (2.7) |
| Skin and subcutaneous tissue | 2 (1.3) | 1 (0.9) |
| Infections and infestations | 2 (1.3) | 2 (1.8) |
| 1 (0.7) | 1 (0.9) | |
| Previous relapses – n (%) | ||
| 0 relapses | 47 (31.1) | 47 (42.7) |
| 1 relapse | 72 (47.7) | 43 (39.1) |
| 2 relapses | 28 (18.5) | 16 (14.6) |
| ≥3 relapses | 4 (2.6) | 4 (3.6) |
| Mean (95% CI) | 0.9 (0.8; 1.1) | 0.8 (0.6; 1.0) |
| Baseline EDSS – n(%) | ||
| Mild EDSS [0 – 3] | 117 (77.5) | 90 (81.8) |
| Moderate EDSS [3.5 - 6] | 34 (22.5) | 20 (18.2) |
| Severe EDSS [>6.5] | 0 (0) | 0 (0) |
| Mean (95% CI) | 2.1 (1.8; 2.3) | 1.9 (1.6; 2.1) |
| Number of DMTs prior to the study – n (%) | ||
| None | 39 (26.2) | 27 (24.8) |
| 1 previous DMT | 65 (43.6) | 51 (46.8) |
| 2 previous DMTs | 25 (16.8) | 17 (15.6) |
| ≥3 previous DMTs | 20 (13.4) | 14 (12.8) |
| NA | 2 | 1 |
| Non-DMTs prior to the study – n (%) | ||
| Yes | 26 (17.7) | 20 (18.7) |
| No | 121 (82.3) | 87 (81.3) |
| NA | 4 | 3 |
95% CI: 95% confidence interval; DMT: disease-modifying therapy; EDSS: Expanded Disability Status Scale; MS: Multiple Sclerosis; n: number of patients in subgroup; N: number of patients; NA: not available; RD: reduced working day; RRMS: relapsing–remitting multiple sclerosis.
Before the start of the relapse, 77.5% of the patients presented mild disability (baseline EDSS 0-3). Twelve months prior to the start of the relapse covered by the study, 47.7% of the patients had experienced only one previous relapse. The mean number of relapses suffered per patient was 0.9 (95% CI: 0.8–1.1) (Table 1).
A total of 26.2% patients had not previously received any DMT while only 43.6% and 30.2% had previously received 1 or ≥2 or more DMTs, respectively (Table 1). A 45.6% of the patients had been treated with interferons (Table S-2, additional material). In addition, prior to the start of the relapse, 17.7% of patients had been treated with a non-DMT, the majority of them with anticonvulsant/antiepileptic drugs (Table 1).
In 27.8% (n=42) of the patients, the DMT was changed as a consequence of the relapse, of these, 12.7% (n=19) switched to first-line therapies (glatiramer acetate and interferons), and 5.3% (n=8) and 4.6% (n=7) switched to natalizumab and fingolimod, respectively. (Table S-2, additional material).
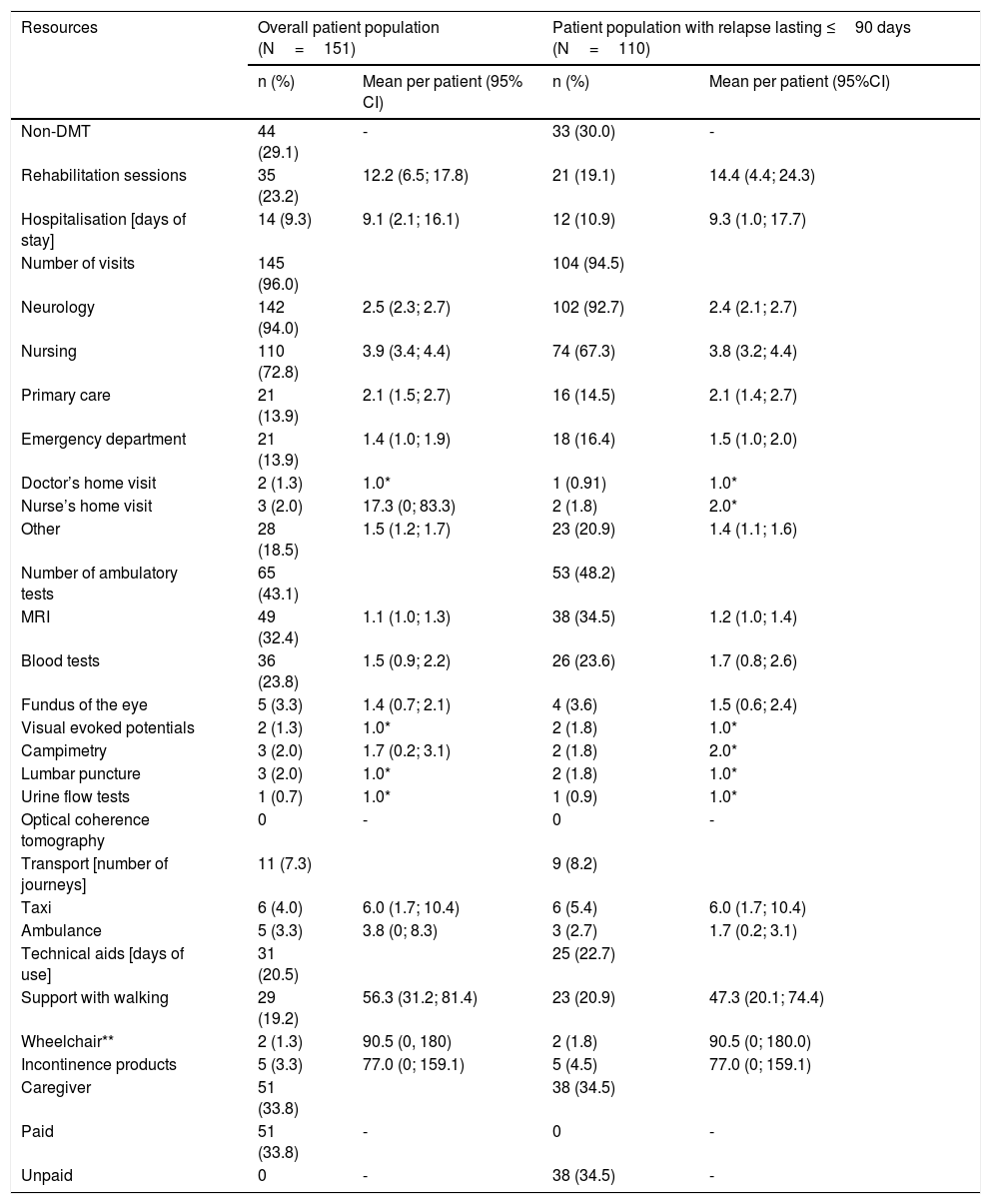
Analysis of resource useOverall, 145 patients (96.0%) attended medical visits as a result of the relapse, the majority of these were visits to neurologist (94.0%). Rehabilitation sessions were required by 23.2% (n=35) of the patients and 9.3% (n=14) were admitted to hospital due to the relapse (13.9% through the emergency department). A 43.1% (n=65) of the patients had to undergo some type of ambulatory procedure and 7.3% of patients (n=11) required additional transport (taxi or ambulance) (Table 2).
Use of resources associated with relapse in overall population and patient population with a relapse lasting ≤90 days.
| Resources | Overall patient population (N=151) | Patient population with relapse lasting ≤90 days (N=110) | ||
|---|---|---|---|---|
| n (%) | Mean per patient (95% CI) | n (%) | Mean per patient (95%CI) | |
| Non-DMT | 44 (29.1) | - | 33 (30.0) | - |
| Rehabilitation sessions | 35 (23.2) | 12.2 (6.5; 17.8) | 21 (19.1) | 14.4 (4.4; 24.3) |
| Hospitalisation [days of stay] | 14 (9.3) | 9.1 (2.1; 16.1) | 12 (10.9) | 9.3 (1.0; 17.7) |
| Number of visits | 145 (96.0) | 104 (94.5) | ||
| Neurology | 142 (94.0) | 2.5 (2.3; 2.7) | 102 (92.7) | 2.4 (2.1; 2.7) |
| Nursing | 110 (72.8) | 3.9 (3.4; 4.4) | 74 (67.3) | 3.8 (3.2; 4.4) |
| Primary care | 21 (13.9) | 2.1 (1.5; 2.7) | 16 (14.5) | 2.1 (1.4; 2.7) |
| Emergency department | 21 (13.9) | 1.4 (1.0; 1.9) | 18 (16.4) | 1.5 (1.0; 2.0) |
| Doctor’s home visit | 2 (1.3) | 1.0* | 1 (0.91) | 1.0* |
| Nurse’s home visit | 3 (2.0) | 17.3 (0; 83.3) | 2 (1.8) | 2.0* |
| Other | 28 (18.5) | 1.5 (1.2; 1.7) | 23 (20.9) | 1.4 (1.1; 1.6) |
| Number of ambulatory tests | 65 (43.1) | 53 (48.2) | ||
| MRI | 49 (32.4) | 1.1 (1.0; 1.3) | 38 (34.5) | 1.2 (1.0; 1.4) |
| Blood tests | 36 (23.8) | 1.5 (0.9; 2.2) | 26 (23.6) | 1.7 (0.8; 2.6) |
| Fundus of the eye | 5 (3.3) | 1.4 (0.7; 2.1) | 4 (3.6) | 1.5 (0.6; 2.4) |
| Visual evoked potentials | 2 (1.3) | 1.0* | 2 (1.8) | 1.0* |
| Campimetry | 3 (2.0) | 1.7 (0.2; 3.1) | 2 (1.8) | 2.0* |
| Lumbar puncture | 3 (2.0) | 1.0* | 2 (1.8) | 1.0* |
| Urine flow tests | 1 (0.7) | 1.0* | 1 (0.9) | 1.0* |
| Optical coherence tomography | 0 | - | 0 | - |
| Transport [number of journeys] | 11 (7.3) | 9 (8.2) | ||
| Taxi | 6 (4.0) | 6.0 (1.7; 10.4) | 6 (5.4) | 6.0 (1.7; 10.4) |
| Ambulance | 5 (3.3) | 3.8 (0; 8.3) | 3 (2.7) | 1.7 (0.2; 3.1) |
| Technical aids [days of use] | 31 (20.5) | 25 (22.7) | ||
| Support with walking | 29 (19.2) | 56.3 (31.2; 81.4) | 23 (20.9) | 47.3 (20.1; 74.4) |
| Wheelchair** | 2 (1.3) | 90.5 (0, 180) | 2 (1.8) | 90.5 (0; 180.0) |
| Incontinence products | 5 (3.3) | 77.0 (0; 159.1) | 5 (4.5) | 77.0 (0; 159.1) |
| Caregiver | 51 (33.8) | 38 (34.5) | ||
| Paid | 51 (33.8) | - | 0 | - |
| Unpaid | 0 | - | 38 (34.5) | - |
*SD: 0. ** The 95% CI has been truncated because the observation period was 180 days. 95% CI: 95% confidence interval; MRI: magnetic resonance imaging; N: number of patients; n: number of patients in subgroup.
Finally, 31 patients (20.5%) required technical aids and 51/151 patients assessed required the help of an unpaid caregiver (Table 2).
The mean total direct cost of the relapse per patient, calculated for the valid population in order to assess costs (n=140), was €4,541.2 (3,330.8–6,507.9) from the NHS perspective and €4,626.1 (3,418.8–6,623.7) for the global perspective.
Subanalysis of the population with relapses lasting ≤90 daysA total of 110 patients showed relapses lasting for ≤90 days and of them, 100 were suitable for cost analysis. Baseline characteristics of these subpopulation are described in Tables 1 and 2 shows the use of resources.
Description of the features of the relapseThe majority of patients (63.6%) presented mild disability at the time the relapse was detected, according to EDSS score (mean [95% CI]: 3.1 [2.8–3.3]). The mean (95% CI) duration of the relapse was 27.7 (24.1–31.3) days, and sensory type relapses were the most common (39.1%) followed by motor (24.5%) and brainstem (21.8%) relapses (Table S-3, additional material).
During the relapse being studied, 34 patients (30.9%) switched DMT due to the relapse, a half of them were previously treated with glatiramer acetate (15.6%). Of the patients who required a change of DMT, 35.3% switched to a second-line treatment (fingolimod or natalizumab) (Table S-2, additional material). Regarding the non-DMTs drug changes, 22 patients (20.0%) changed treatment during the observation period, from these only 5.5% corresponded to symptomatic treatments (14.6% initiated methylprednisolone) (Table S-2, additional material).
Direct costsThe cost analysis was carried out on 100 suitable patients among the population with relapses lasting ≤90 days. The average total direct cost (95% CI) per patient was €4,988.8 (3,505.5–7,440.5) and €5,115.2 (3,632.2–7,583.9) from the NHS and global viewpoints, respectively. In general, the direct costs were very similar for both perspectives, apart from the rehabilitation sessions, technical aids and transport to the healthcare or rehabilitation centre, which were higher (mean increase of €85.2, €30.8 and €8.8, respectively) from the global perspective.
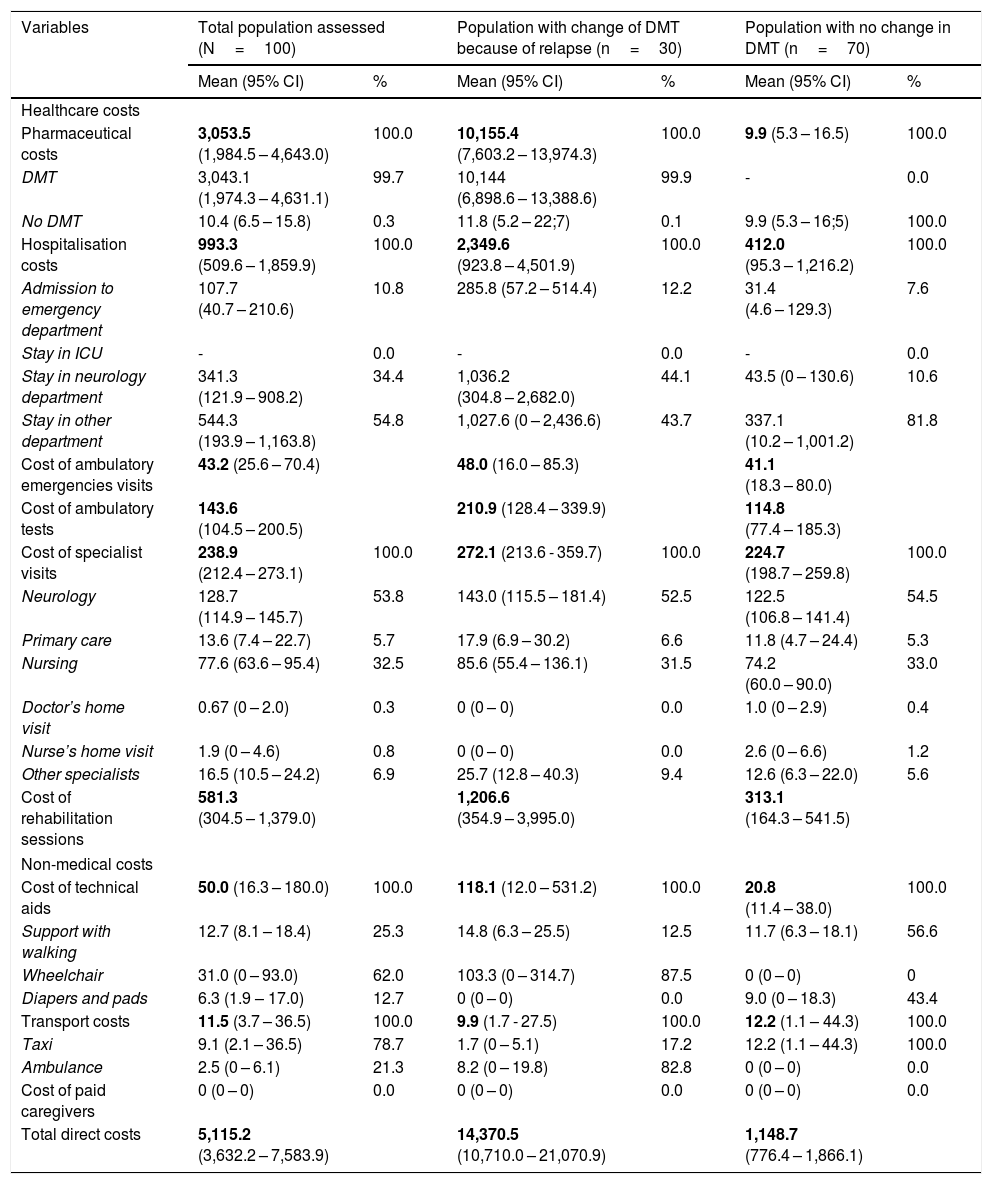
Stratified cost analysisDirect costs according to change in DMT: of the 100 patients suitable for estimating costs, 30 switched DMT as a consequence of the relapse. Table 3 shows the distribution of costs per patient from the global perspective, separated into the population requiring a change of DMT because of the relapse and those who did not require a change in DMT. For patients who switched DMT, the mean total direct cost was €14,370.5/patient (10,710.0–21,070.9), while for the population with no DMT change the mean total direct cost was €1,148.7/patient (776.4–1,866.1). The bulk of these costs was for drugs for those patients who switched DMT, and for hospitalisation for those patients who did not require a change in DMT because of the relapse.
Distribution of mean (Standard Deviation) direct costs, €/patient, for the studied populations according to the overall perspective.
| Variables | Total population assessed (N=100) | Population with change of DMT because of relapse (n=30) | Population with no change in DMT (n=70) | |||
|---|---|---|---|---|---|---|
| Mean (95% CI) | % | Mean (95% CI) | % | Mean (95% CI) | % | |
| Healthcare costs | ||||||
| Pharmaceutical costs | 3,053.5 (1,984.5 – 4,643.0) | 100.0 | 10,155.4 (7,603.2 – 13,974.3) | 100.0 | 9.9 (5.3 – 16.5) | 100.0 |
| DMT | 3,043.1 (1,974.3 – 4,631.1) | 99.7 | 10,144 (6,898.6 – 13,388.6) | 99.9 | - | 0.0 |
| No DMT | 10.4 (6.5 – 15.8) | 0.3 | 11.8 (5.2 – 22;7) | 0.1 | 9.9 (5.3 – 16;5) | 100.0 |
| Hospitalisation costs | 993.3 (509.6 – 1,859.9) | 100.0 | 2,349.6 (923.8 – 4,501.9) | 100.0 | 412.0 (95.3 – 1,216.2) | 100.0 |
| Admission to emergency department | 107.7 (40.7 – 210.6) | 10.8 | 285.8 (57.2 – 514.4) | 12.2 | 31.4 (4.6 – 129.3) | 7.6 |
| Stay in ICU | - | 0.0 | - | 0.0 | - | 0.0 |
| Stay in neurology department | 341.3 (121.9 – 908.2) | 34.4 | 1,036.2 (304.8 – 2,682.0) | 44.1 | 43.5 (0 – 130.6) | 10.6 |
| Stay in other department | 544.3 (193.9 – 1,163.8) | 54.8 | 1,027.6 (0 – 2,436.6) | 43.7 | 337.1 (10.2 – 1,001.2) | 81.8 |
| Cost of ambulatory emergencies visits | 43.2 (25.6 – 70.4) | 48.0 (16.0 – 85.3) | 41.1 (18.3 – 80.0) | |||
| Cost of ambulatory tests | 143.6 (104.5 – 200.5) | 210.9 (128.4 – 339.9) | 114.8 (77.4 – 185.3) | |||
| Cost of specialist visits | 238.9 (212.4 – 273.1) | 100.0 | 272.1 (213.6 - 359.7) | 100.0 | 224.7 (198.7 – 259.8) | 100.0 |
| Neurology | 128.7 (114.9 – 145.7) | 53.8 | 143.0 (115.5 – 181.4) | 52.5 | 122.5 (106.8 – 141.4) | 54.5 |
| Primary care | 13.6 (7.4 – 22.7) | 5.7 | 17.9 (6.9 – 30.2) | 6.6 | 11.8 (4.7 – 24.4) | 5.3 |
| Nursing | 77.6 (63.6 – 95.4) | 32.5 | 85.6 (55.4 – 136.1) | 31.5 | 74.2 (60.0 – 90.0) | 33.0 |
| Doctor’s home visit | 0.67 (0 – 2.0) | 0.3 | 0 (0 – 0) | 0.0 | 1.0 (0 – 2.9) | 0.4 |
| Nurse’s home visit | 1.9 (0 – 4.6) | 0.8 | 0 (0 – 0) | 0.0 | 2.6 (0 – 6.6) | 1.2 |
| Other specialists | 16.5 (10.5 – 24.2) | 6.9 | 25.7 (12.8 – 40.3) | 9.4 | 12.6 (6.3 – 22.0) | 5.6 |
| Cost of rehabilitation sessions | 581.3 (304.5 – 1,379.0) | 1,206.6 (354.9 – 3,995.0) | 313.1 (164.3 – 541.5) | |||
| Non-medical costs | ||||||
| Cost of technical aids | 50.0 (16.3 – 180.0) | 100.0 | 118.1 (12.0 – 531.2) | 100.0 | 20.8 (11.4 – 38.0) | 100.0 |
| Support with walking | 12.7 (8.1 – 18.4) | 25.3 | 14.8 (6.3 – 25.5) | 12.5 | 11.7 (6.3 – 18.1) | 56.6 |
| Wheelchair | 31.0 (0 – 93.0) | 62.0 | 103.3 (0 – 314.7) | 87.5 | 0 (0 – 0) | 0 |
| Diapers and pads | 6.3 (1.9 – 17.0) | 12.7 | 0 (0 – 0) | 0.0 | 9.0 (0 – 18.3) | 43.4 |
| Transport costs | 11.5 (3.7 – 36.5) | 100.0 | 9.9 (1.7 - 27.5) | 100.0 | 12.2 (1.1 – 44.3) | 100.0 |
| Taxi | 9.1 (2.1 – 36.5) | 78.7 | 1.7 (0 – 5.1) | 17.2 | 12.2 (1.1 – 44.3) | 100.0 |
| Ambulance | 2.5 (0 – 6.1) | 21.3 | 8.2 (0 – 19.8) | 82.8 | 0 (0 – 0) | 0.0 |
| Cost of paid caregivers | 0 (0 – 0) | 0.0 | 0 (0 – 0) | 0.0 | 0 (0 – 0) | 0.0 |
| Total direct costs | 5,115.2 (3,632.2 – 7,583.9) | 14,370.5 (10,710.0 – 21,070.9) | 1,148.7 (776.4 – 1,866.1) | |||
95% CI: 95% confidence interval; DMT: disease-modifying therapy; ICU: intensive care unit; N: number of patients; n: number of patients in subgroup.
Bold text signifies the mean direct cost per patient, expressed in euros (standard deviation).
Direct medical costs - calculations
Visits to emergencies, ambulatory tests, medical visits and rehabilitation sessions: multiplying the natural units used by the associated unit cost.
Hospitalisation: multiplying the days of stay in each of the departments by the corresponding cost for each department and then a summary of costs for each department was calculated. When the patient was admitted to the emergency department <24 hours, it was recorded as visit to the emergency department. If this stay was ≥24 hours, it was recorded as a full day stay in the emergency department (the same cost per unit was used for both perspectives).
Any added costs or changes to the cost of drug treatments (DMTs and non-DMTs) as a consequence of the relapse were evaluated. The cost was obtained multiplying total dose during the length of treatment by the respective unit cost of the drug. If the patient continued with the drug after the relapse, end of treatment date was considered the date the relapse ended.
Direct non-medical costs – calculations
Cost of technical aids was obtained by multiplying the number of days the patient required help by unit cost. In cases of use of incontinence products due to the relapse, it was assumed four diapers per day.
Ambulance transport costs: the number of journeys was multiplied by the unit cost. Taxi costs: the number of kilometres each journey was multiplied by total number of journeys and multiplied by the unit cost.
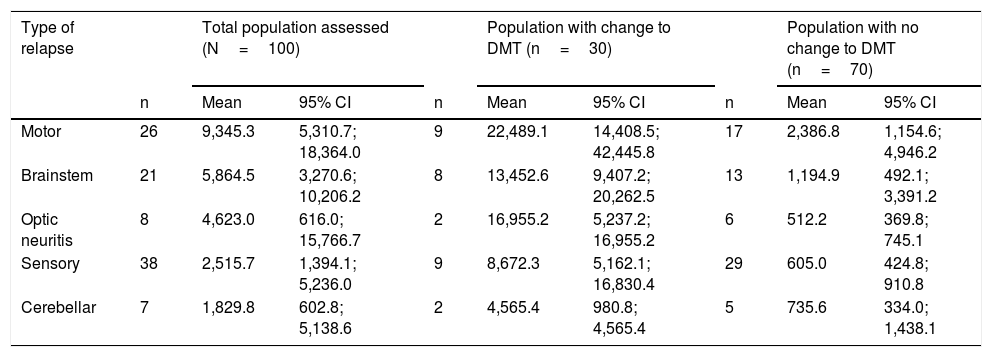
Direct costs according to type of relapse: Table 4 shows costs according to the type of relapse for the different populations. The costs were higher for motor and the brainstem relapses (mean of €9,345.3 [5,310.7–18,364.0] and €5,864.5/patient [3,270.6–10,206.2], respectively). Considering the subgroups, an increase in costs was seen for all types of relapses in those patients who switched DMT due to the relapse.
Direct costs (€/patient) for the various populations according to type of relapse.
| Type of relapse | Total population assessed (N=100) | Population with change to DMT (n=30) | Population with no change to DMT (n=70) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | n | Mean | 95% CI | |
| Motor | 26 | 9,345.3 | 5,310.7; 18,364.0 | 9 | 22,489.1 | 14,408.5; 42,445.8 | 17 | 2,386.8 | 1,154.6; 4,946.2 |
| Brainstem | 21 | 5,864.5 | 3,270.6; 10,206.2 | 8 | 13,452.6 | 9,407.2; 20,262.5 | 13 | 1,194.9 | 492.1; 3,391.2 |
| Optic neuritis | 8 | 4,623.0 | 616.0; 15,766.7 | 2 | 16,955.2 | 5,237.2; 16,955.2 | 6 | 512.2 | 369.8; 745.1 |
| Sensory | 38 | 2,515.7 | 1,394.1; 5,236.0 | 9 | 8,672.3 | 5,162.1; 16,830.4 | 29 | 605.0 | 424.8; 910.8 |
| Cerebellar | 7 | 1,829.8 | 602.8; 5,138.6 | 2 | 4,565.4 | 980.8; 4,565.4 | 5 | 735.6 | 334.0; 1,438.1 |
Costs according to overall perspective. Caution with interpreting date where the n is reduced. 95% CI: 95% confidence interval; DMT: disease-modifying therapy; N: number of patients; n: number of patients in subgroup.
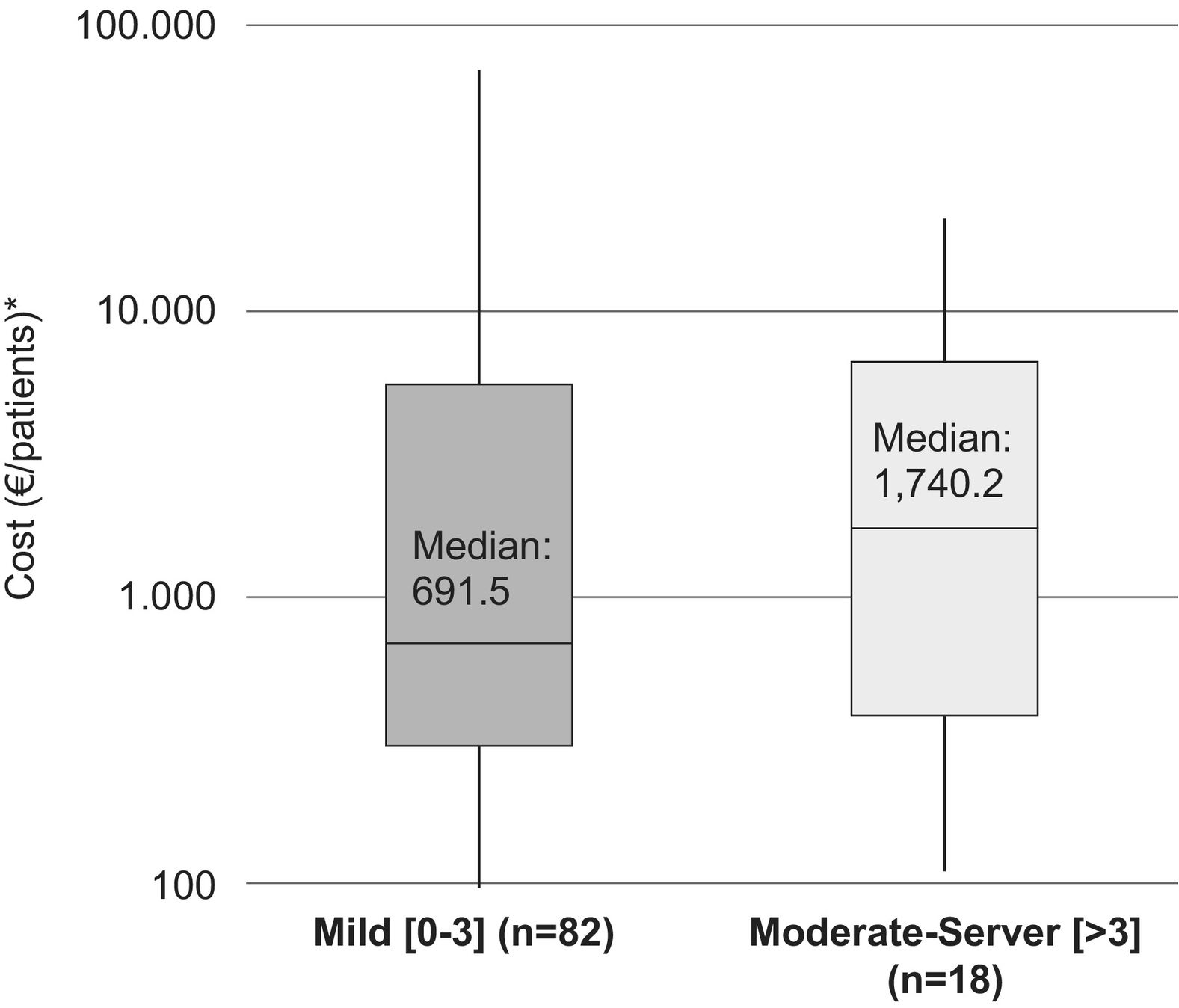
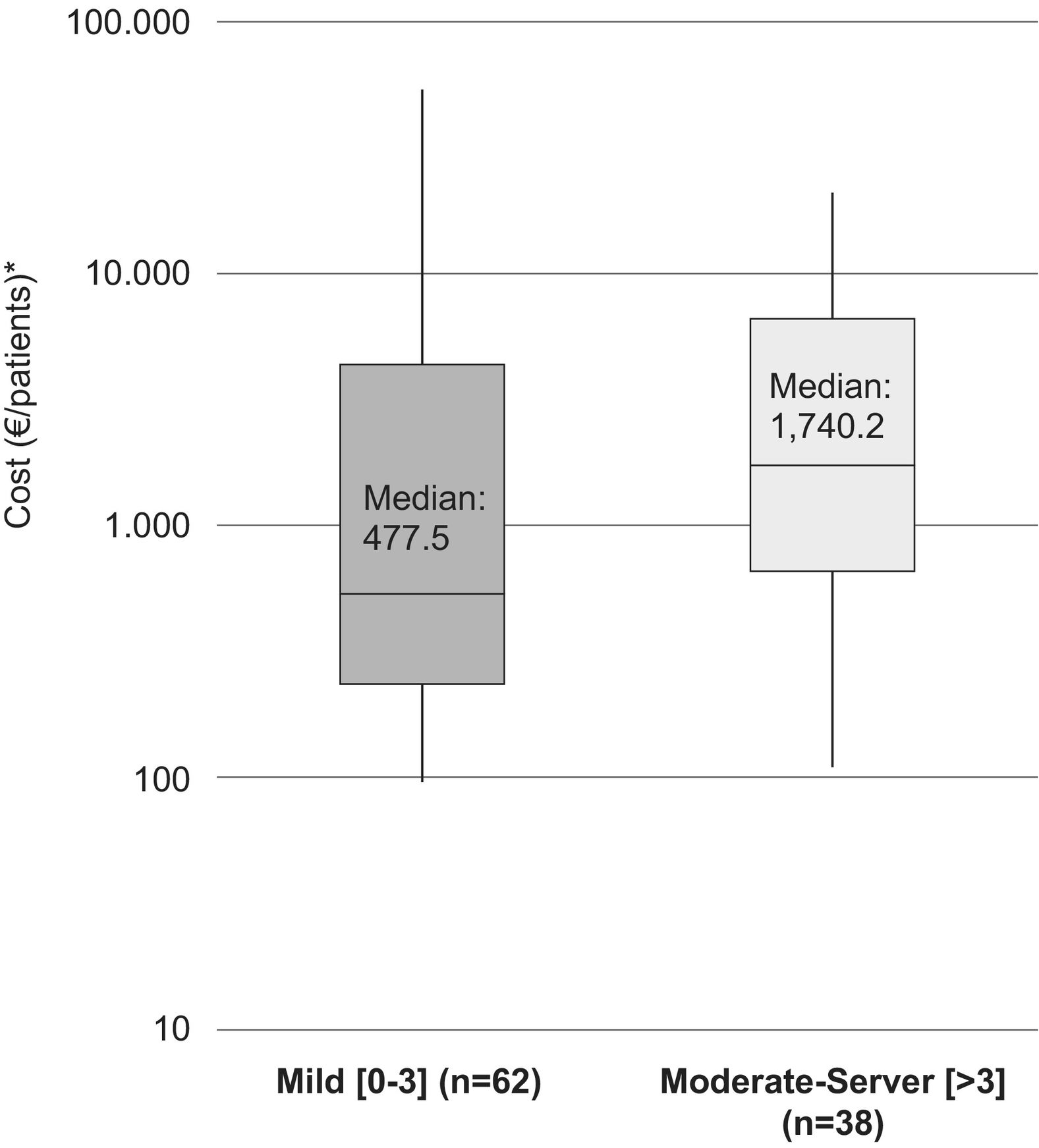
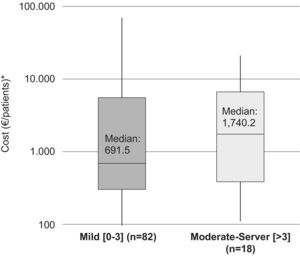
The median (IQR) for the direct costs of a relapse, for patients with a moderate–severe baseline disability (EDSS >3), was €1,740.2/patient (385.5–6,651.4) (medical costs €1,707.1 [358.4–6,569.9]/patient and non-medical costs €0 [0–18.1]/patient). In addition, the median (IQR) for the cost of a relapse in patients with mild disability (EDSS: 0–3) was €691.5 (302.0–5,544.7)/patient (medical costs €614.4 [302.0–5,485.8]/patient and non-medical costs €0 [0–20.3]/patient) (Fig. 1).
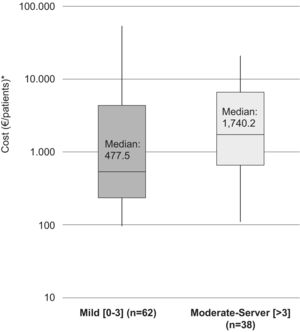
The median (IQR) direct cost increased with increasing disability at the time of the relapse. For patients with a mild disability (EDSS: 0–3) it was €477.5 (210.4–5,357.6)/patient (medical costs €477.5 and non-medical costs €0) while for patients with moderate and severe disabilities (EDSS: >3) it was €1,740.2 (517.0–7,786.7)/patient (medical costs €1,707.1 [432.6–7,786.7]/patient and non-medical costs €40.3 [0–66.1]/patient (Fig. 2).
DiscussionOur findingsThis multicentre study provides an update to the current understanding of the burden of MS on both, the NHS and the global perspectives. The main finding from our study is the mean direct cost of an RRMS relapse in Catalonia (Spanish Region). The mentioned cost, estimated per patient, was €4,541.2 from the NHS perspective and €4,626.1 from the global perspective. In the population with relapse lasting for ≤90 days, as usually seen in daily practice, the mean direct cost of the relapse was €4,988.8/patient and €5,115.2/patient for the NHS and global perspectives, respectively. The costs were very similar for both populations from the two perspectives.
As secondary findings, we were able to show an increase in mean direct costs for all types of relapses in those patients who switched DMT due to the relapse compared with those who did not require a change.
Comparison with other studiesThe total direct costs of €5,115.2 estimated per relapse and per patient (according to the global perspective) in our study are notably higher than those collected during previous Spanish studies.2,26,27 To our knowledge, in Catalonia, the cost of a MS relapse was previously studied at Bellvitge University Hospital in terms of direct, indirect and intangible costs. The estimated direct cost per patient for a relapse was €1,498.5,2 lower than the cost reported in studies carried out in other countries,15–17 while the mean total cost per relapse was €3,048.8 per patient.2 This increase in cost may be due to the inclusion of the cost associated with switching the DMT or adjusting the treatment prescription as a consequence of the relapse. Although, DMTs are not treatments for relapses per se, and therefore usually they are not taken into account in estimating the cost of a relapse, we consider that switching DMT is a direct consequence of a new MS relapse and should be considered. Therefore, the cost arising from the mentioned change forms part of the direct medical cost of a relapse.
In other studies, where the main objective was to estimate the total cost of the disease, the cost of relapse was calculated using the difference between the total cost between patients who had suffered a relapse compared with those who had not experienced one.28,29 The results of these estimations were not easily comparable with our study since they included the indirect costs in the estimation. Nevertheless, the costs were still lower (€2,75828 and €2,05029) since in neither case did they include the cost attributable to a change in DMT.
When we carried out a breakdown of costs between patients requiring a change in DMT and those who did not, we saw that the total direct cost of relapse for the population with no change was €1,148.7/patient, closer to the direct costs of relapse requiring hospital (€1,499) and ambulatory management (€1,538) described by Casado et al.,2 as well as €1,524 for the direct cost of a relapse calculated by Gubieras et al.27
Therefore, it should be noted there is a significant increase in cost when a relapse triggers a change in patient's treatment prescription. Specifically, it was observed in the population who switched DMT that the cost increased to €14,370.5/patient, almost 10 times higher than that for the population where a change was not required. In addition, breaking down the population according to DMT allowed us to observe a clearly differentiated distribution of costs where not only the pharmacological cost increased due to the use of DMT, but also the use of resources such as hospitalisation, rehabilitation and technical aids increased.
This study also provides a new element, the estimation of cost according to the type of relapse. The highest cost corresponded to those with motor involvement (€9,345.3/patient). Regarding the estimation of costs according to the patient's disability (baseline and at the time of the relapse) it can be seen that in both cases, a higher degree of disability, both at the outset and caused by the relapse, leads to an increase by double or triple the cost. The study by Granell et al.,26 which estimated the direct cost of a relapse according to its severity, is in line with our study. Granell obtained a cost per relapse per patient comparable to the costs per patient in our study.
LimitationsWe should highlight the limitations restricting our work such as the small sample size (N=151), even if it was a sufficient study size to evaluate the primary objective according to the protocol design, and its retrospective nature. In order to prevent any possible bias by the investigator when choosing a relapse to assess, the choice was limited to collecting the data from the first relapse in patients who had suffered more than one relapse. In addition, the interpretation of the subgroup analyses must be treated with caution, considering the reduced number of patients.
We also observed that 34% of patients required assistance from unpaid caregivers. The analysis carried out did not take into account the cost associated with this use of resources which some authors included as part of the non-medical direct costs. Gubieras et al.27 estimated that the annual cost of informal caregivers was €777/patient for patients with a mild disability and €26,986/patient for more severe patients and, therefore, an increased cost could also be expected in our study if this concept had been included in the accounting calculation.
Other published studies have also analysed the indirect and intangible costs associated with relapses but our analysis has not considered these. These costs can involve up to 50% (indirect) and 18% (intangible) of the total cost of a relapse.2
Despite its limitations, this study estimates, for the first time, the direct cost of a relapse taking into account whether or not this relapse leads to a DMT switch. The availability of new DMTs and the fact that these drugs are not exclusively prescribed for a relapse would explain why previous studies have not considered this added cost. We consider that a relapse that consequently leads to a change in prescription or DMT creates a greater impact on the use of resources compared with a relapse not involving any change. This impact must be reflected by a higher cost. In addition, this study also allowed us to understand the difference in costs according to the type of relapse, which may be useful when it comes to considering the most effective solution.
ConclusionThis study shows the substantial financial impact of an RRMS relapse in Catalonia and considers, for the first time, the direct cost of relapse taking into account whether this relapse causes a switch of the DMT or not. The results of the stratified samples show that relapses involving a change of DMT have a higher impact on patients because of the increased use of resources on a general level, influencing the direct costs. In the future, it would be convenient to study the overall financial impact of disability in RRMS and, consequently, the potential benefit of DMTs on reducing this impact. The early use of highly effective treatments, which decrease relapses, would involve a reduction in the financial burden of the disease in the long-term.
Compliance with ethical standardsThis study was approved by the Clinical Research Ethics Committee/Independent Ethics Committee. It was carried out in accordance with the principles of the Declaration of Helsinki and complied with the standards of Good Clinical Practice (GCP)30 and the Good Epidemiological Practice.31 All participating patients provided their informed consent.
Conflict of InterestVC participated as a clinical expert in the study and declares no conflict of interest in relation to the manuscript. The rest of the authors declare no conflicts of interest in relation to this manuscript.