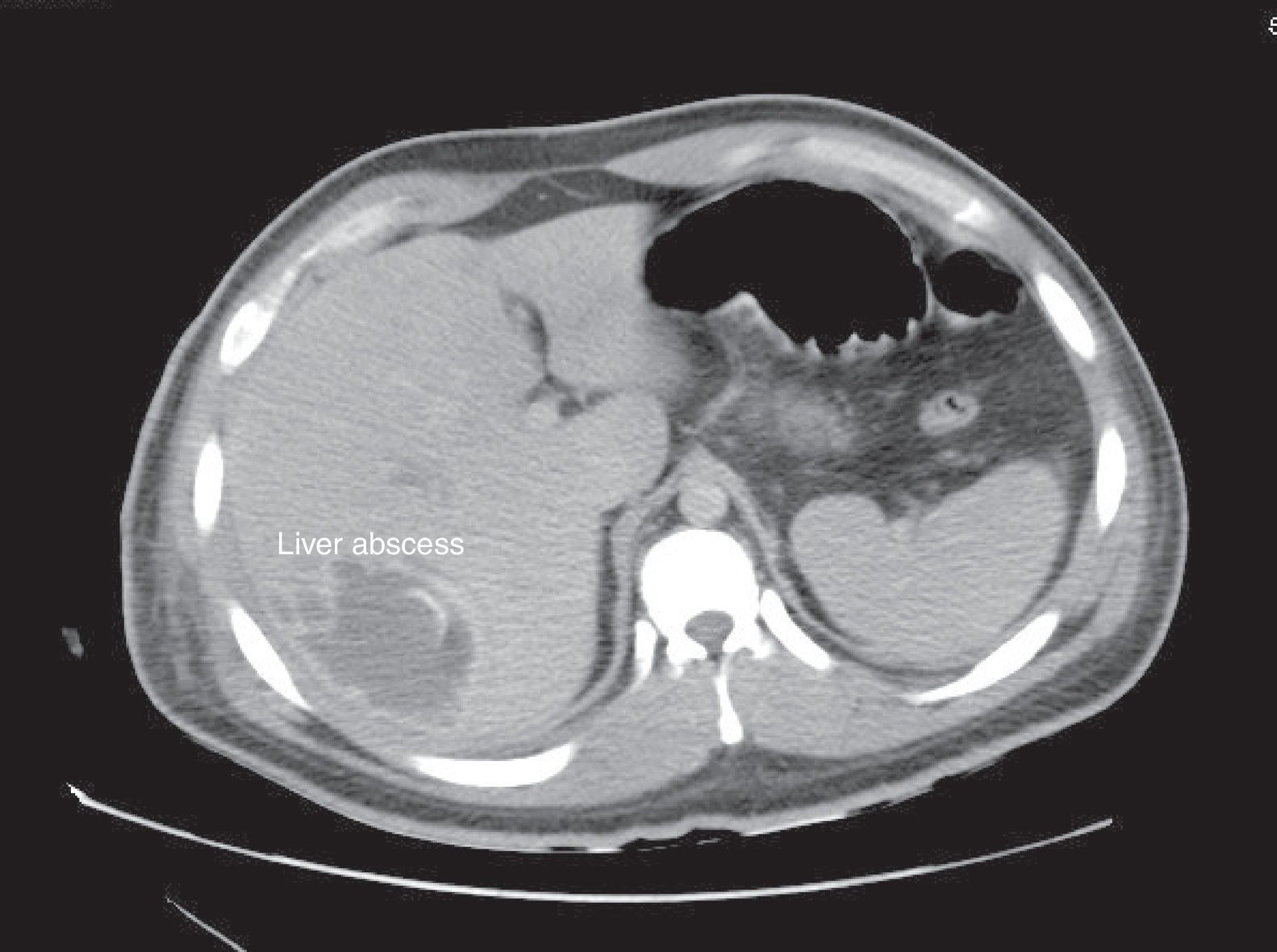
Amoebic abscesses (AA) are the most common cause of hepatic abscesses (HA) in the world and pyogenic abscesses (PA) in the western world. Complications of HA are sepsis, empyema by direct extension or abscess rupture.
ObjectiveTo determine the epidemiology and prognostic factors of complications in patients with hepatic abscess in northeastern Mexico.
Material and methodsPatients with diagnosis of hepatic abscess in the University Hospital “Dr. José Eleuterio González” between 2011 and 2015. The study has a retrospective design.
ResultsA total of 150 patients were reviewed, the most common symptoms were abdominal pain and fever. The etiology was 74 pyogenic, 28 amoebic, and unidentified in 48 patients. The most common agent was Klebsiella pneumoniae. Conservative management was given to 16 (17.3%) cases. The mortality was 18 patients (12%). The prognostic variables of complication were abdominal pain, respiratory rate, ALT>154IU/L, hemoglobin less than 10g/dL, presence of a perforated abscess, and performing a second procedure. Mortality and hospitalization increased in the presence of complications.
DiscussionA prevalence of 20% of diabetes mellitus was observed in our patients. Mortality of PA, when associated with K. pneumoniae, ranges from 6 to 17%.
ConclusionsThe predominant etiology remains pyogenic despite being an endemic country for amoebiasis. In our study, because of lower morbidity and mortality rate, the first choice of treatment was puncture of the abscess.
Hepatic abscesses are not rare. Amoebic (AA) are the most common cause of hepatic abscesses worldwide. In the western world, pyogenic (PA) are more common.1 A predisposing factor is diabetes mellitus, which has been linked to 15% of patients with PA.2
AA are caused when the trophozoite of the Entamoeba histolytica migrates to the liver via portal, destroying liver tissue. We can observe cellular waste instead of pus, with little to no inflammatory exudate. PA is a form of intra-abdominal pathology; between 30 and 40% are of a biliary origin, or secondary to appendicitis, diverticulitis, or inflammatory intestinal disease.
Its usual presentation includes fever, weight loss, and abdominal pain. PA is commonly polymicrobial. However, Klebsiella pneumoniae, Escherichia coli are often predominant.3 Diagnosis is performed with imaging studies, such as ultrasound (US) or a CT scan. Injuries are usually located in the right lobe of the liver.4 In the case of AA, it is confirmed with blood tests. Alkaline phosphatase usually increases in 90% of PA cases.
Treatment for AA is metronidazole for 5–10 days. In the case of PA, broad-spectrum antibiotics are recommended, with suggestive coverage for anaerobes. Not all hepatic abscesses must be drained, only the bigger ones with over 5cm in diameter. Small abscesses are usually resolved with the administration of antibiotics. A liver abscess which is not drained will resolve over a longer period, thanks to antibiotics. Surgical intervention is necessary if treatment fails, or if there is a subjacent disease that requires surgical treatment.
Hepatic abscess complications include sepsis, empyema (as a direct extension or rupture of the abscess in the pleural cavity), or peritonitis (due to an abscess rupture, and pleuropericardial spillage). Pyogenic hepatic abscess has a poor diagnosis when it is multiple, or has a late diagnosis. Mortality rates before the new treatments were 77%.5 Nowadays, mortality is 1–3%6 in AA and 10% in PA.3
ObjectiveTo determine the epidemiology and prognostic factors of complications in patients with hepatic abscesses in northern Mexico.
Materials and methodsWe requested the charts of patients over 18 years old, diagnosed with hepatic abscess between 2011 and 2015, from the Statistics Department at the University Hospital “Dr. José Eleuterio González”. The CIE-10 code used was K75.0. The study had a retrospective design. Exclusion criteria were the absence of a chart.
Multiple variables were determined, such as the patient's general data, personal pathological and non-pathological records, symptomatology, vital signs, laboratory tests, cultures, antibiotic therapy, treatment, and follow-up until discharge.
The diagnosis of liver abscess was based on the medical records, clinical profile, imaging studies, and laboratory results. It was considered amoebic when there was the presence of an indirect hemagglutination higher than 1:32. The rest were considered pyogenic.
For statistical analysis we divided the patients in a group with complications (NCG) either surgical or medical and the group without complications (CG).
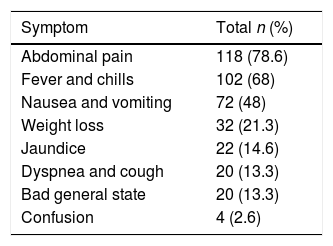
ResultsDescriptive analysisA total of 156 patients were obtained; 6 patients were eliminated due to a lack of clinical charts. Out of the 150 patients reviewed, 96 (64%) were men, and 54 (36%) were women, with a mean age of 47 years. Mean hospital stay was 43 days. Within their personal non-pathological history, a total of 58 (38.6%) were smokers, and 53 (34.6%) consumed alcohol chronically. Moreover, their personal pathological history included 30 (20%) patients with type 2 diabetes mellitus, 20 (13.3%) patients with systemic arterial hypertension, 6 (4%) patients with hepatitis B, 6 (4%) HIV positive patients, and 2 patients with hepatitis C (1.3%). Regarding their clinical symptoms, the most common are described in Table 1. Vital signs and lab work are described in Table 2.
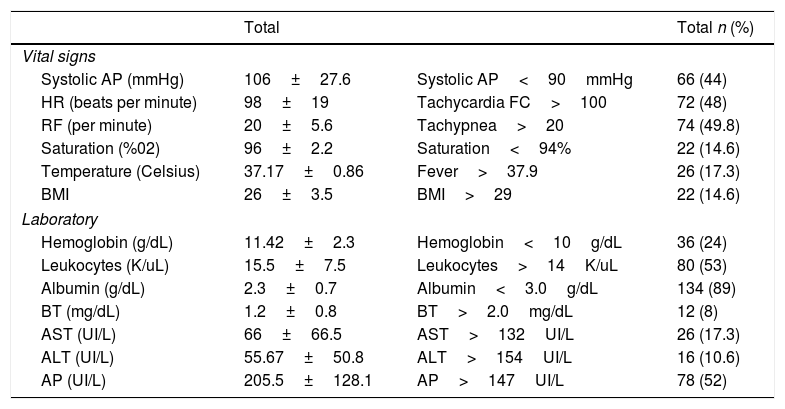
Vital signs and laboratory tests of patients with liver abscesses.
| Total | Total n (%) | ||
|---|---|---|---|
| Vital signs | |||
| Systolic AP (mmHg) | 106±27.6 | Systolic AP<90mmHg | 66 (44) |
| HR (beats per minute) | 98±19 | Tachycardia FC>100 | 72 (48) |
| RF (per minute) | 20±5.6 | Tachypnea>20 | 74 (49.8) |
| Saturation (%02) | 96±2.2 | Saturation<94% | 22 (14.6) |
| Temperature (Celsius) | 37.17±0.86 | Fever>37.9 | 26 (17.3) |
| BMI | 26±3.5 | BMI>29 | 22 (14.6) |
| Laboratory | |||
| Hemoglobin (g/dL) | 11.42±2.3 | Hemoglobin<10g/dL | 36 (24) |
| Leukocytes (K/uL) | 15.5±7.5 | Leukocytes>14K/uL | 80 (53) |
| Albumin (g/dL) | 2.3±0.7 | Albumin<3.0g/dL | 134 (89) |
| BT (mg/dL) | 1.2±0.8 | BT>2.0mg/dL | 12 (8) |
| AST (UI/L) | 66±66.5 | AST>132UI/L | 26 (17.3) |
| ALT (UI/L) | 55.67±50.8 | ALT>154UI/L | 16 (10.6) |
| AP (UI/L) | 205.5±128.1 | AP>147UI/L | 78 (52) |
SD: standard deviation; BP: blood pressure, HR: heart rate; RF: respiratory frequency; BT: total bilirubin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; AP: alkaline phosphatase; BMI: body mass index.
In 130 patients, abscess samples were sent for cultures (Figure 1), while 20 patients were without a sample. Results showed a total of 58 positives and 72 negatives. Of the positives, 19 had K. pneumoniae, 16 had E. coli, 7 had Enterococcus faecalis, 4 had Streptococcus agalactiae and Streptococcus sanguinis, 4 had Coagulase-negative staphylococcus, 4 had Morganella morganii, and 4 had Streptococcus spp. A total of 30 hemocultures were performed, obtaining 8 positives and 22 negatives. Of the positives, 2 had Staphylococcus, 4 had M. morganii, and 2 had E. faecalis.
Regarding the imaging studies performed, US and CT scans were conducted in 44 patients, only US on 54 patients, and only TAC on 32 patients (Figure 1). Abscesses were located on the right side in 84 (56%) patients, on the left side in 40 (26%), and on both sides in 26 (17.3%). The number of reported abscesses was 1.27±1.04. There was 1 abscess reported in 108 (72%) patients, 2 in 20 (13.3%), and 3 or more in 18 (12%). Average size was 3.96cm±4.7; the largest reported size was 18cm. There were 8 (5.4%) reported with a thickened abscess wall, 26 (17.3%) with edge highlighting, 8 (5.4%) with pneumobilia, and 4 with gas present (2.6%). Subcapsular rupture occurred in 22 (14.6%) patients. There were 2 cases of portal thrombophlebitis reported.
As for the etiology, 74 patients were determined to be pyogenic in origin by imaging studies, while 28 patients with an amoebic etiology were confirmed by laboratory. In 48 patients, the etiology was not identified by imaging and presented a negative seroameba. Of the 74 patients with pyogenic abscess, the etiology was biliary pathology in 41 (55.4%) patients, diverticulosis in 16 (21.6%) patients, appendicitis in 12 (16.2%) and liver tumor in 5 (6.7%).
Regarding the antibiotics used, ceftriaxone with metronidazole was used in 128 (85%) cases. Imipenem and vancomycin were used in 22 (14.6%) cases. The duration of antibiotic therapy was 11.21±6.8 days.
The treatment performed was conservative in 16 (10.66%) cases, a percutaneous puncture was performed in 94 (62.66%) cases, surgical treatment by laparoscopy or open surgery was performed in 34 (22.6%), and 6 (4%) patients required puncture with subsequent surgical treatment. From the punctures, an average of 268±253ml was drained. Only 2 patients from the NCG underwent puncture or aspiration without drainage placement.
Among the complications of hepatic abscess presented, a total of 24 (16%) of the cases were septic shock and 24 (16%) were right pleural effusion. Other complications were: portal thrombosis, vena cava thrombosis, thoracoabdominal fistula, gastric perforation, pancreatitis, peripancreatic abscess, and multiple intra-abdominal collections. Patients with pleural effusions due to thoracoscopy had to be operated on again; 12 patients had to undergo a second percutaneous drainage. Days spent in the intensive care unit were 1.27±7.09. A recurrence of 18 (12%) patients treated percutaneously or conservatively was reported.
The mortality rate reported was due to septic shock in 18 (12%) cases. Of the patients with ruptured abscesses, the toll was 6 (27%) of the 22 patients. No deaths were reported in the 28 cases of amoebic abscesses.
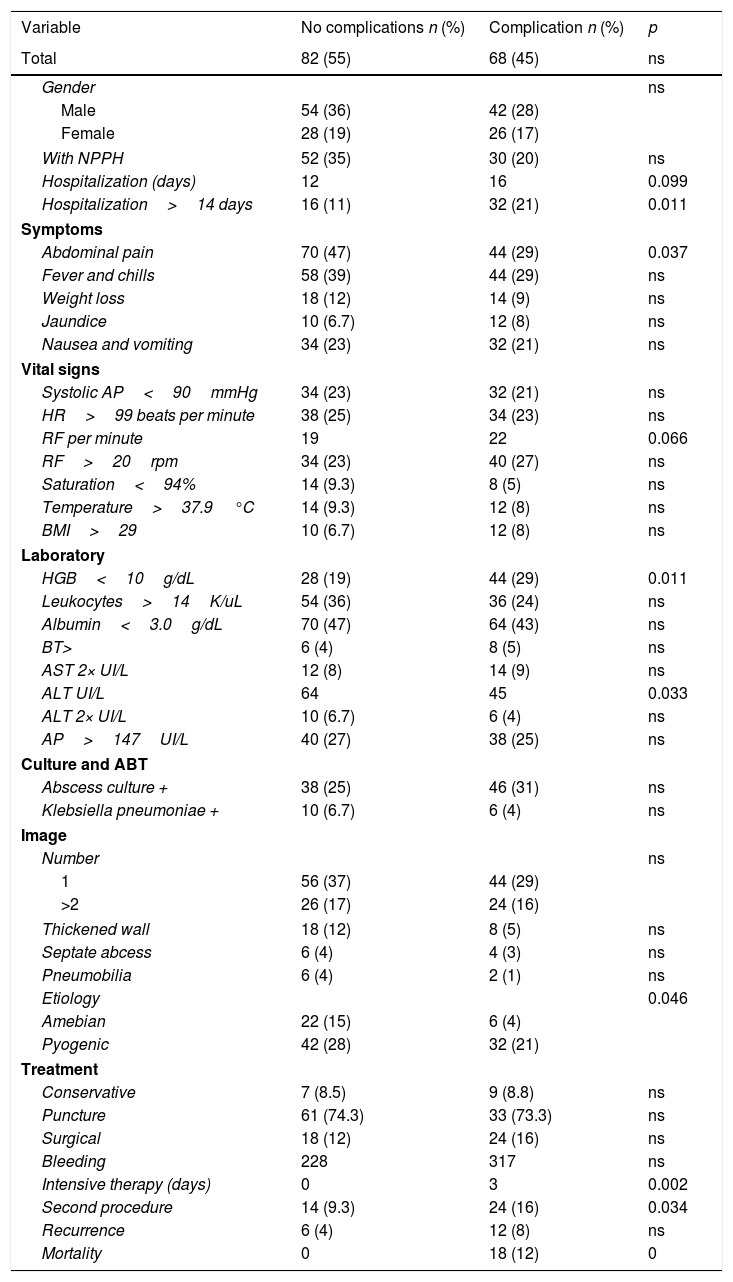
The statistical analysis was carried out by initially comparing the group that had suffered some medical or surgical complication to the group that did not present complications (Table 3). Patients with septic shock, disseminated intravascular coagulation, coronary syndrome, renal or respiratory failure, pleural effusion, and metastatic infection were included in this former group. Abdominal pain (p<0.05), respiratory rate, ALT>154IU/L, hemoglobin less than 10g/dL, the presence of a ruptured abscess, the performance of a second procedure, mortality rate, and hospitalization days were found to be statistically significant variables.
Statistical analysisbetween complicated versus uncomplicated patients with hepatic abscesses.
| Variable | No complications n (%) | Complication n (%) | p |
|---|---|---|---|
| Total | 82 (55) | 68 (45) | ns |
| Gender | ns | ||
| Male | 54 (36) | 42 (28) | |
| Female | 28 (19) | 26 (17) | |
| With NPPH | 52 (35) | 30 (20) | ns |
| Hospitalization (days) | 12 | 16 | 0.099 |
| Hospitalization>14 days | 16 (11) | 32 (21) | 0.011 |
| Symptoms | |||
| Abdominal pain | 70 (47) | 44 (29) | 0.037 |
| Fever and chills | 58 (39) | 44 (29) | ns |
| Weight loss | 18 (12) | 14 (9) | ns |
| Jaundice | 10 (6.7) | 12 (8) | ns |
| Nausea and vomiting | 34 (23) | 32 (21) | ns |
| Vital signs | |||
| Systolic AP<90mmHg | 34 (23) | 32 (21) | ns |
| HR>99 beats per minute | 38 (25) | 34 (23) | ns |
| RF per minute | 19 | 22 | 0.066 |
| RF>20rpm | 34 (23) | 40 (27) | ns |
| Saturation<94% | 14 (9.3) | 8 (5) | ns |
| Temperature>37.9°C | 14 (9.3) | 12 (8) | ns |
| BMI>29 | 10 (6.7) | 12 (8) | ns |
| Laboratory | |||
| HGB<10g/dL | 28 (19) | 44 (29) | 0.011 |
| Leukocytes>14K/uL | 54 (36) | 36 (24) | ns |
| Albumin<3.0g/dL | 70 (47) | 64 (43) | ns |
| BT> | 6 (4) | 8 (5) | ns |
| AST 2× UI/L | 12 (8) | 14 (9) | ns |
| ALT UI/L | 64 | 45 | 0.033 |
| ALT 2× UI/L | 10 (6.7) | 6 (4) | ns |
| AP>147UI/L | 40 (27) | 38 (25) | ns |
| Culture and ABT | |||
| Abscess culture + | 38 (25) | 46 (31) | ns |
| Klebsiella pneumoniae + | 10 (6.7) | 6 (4) | ns |
| Image | |||
| Number | ns | ||
| 1 | 56 (37) | 44 (29) | |
| >2 | 26 (17) | 24 (16) | |
| Thickened wall | 18 (12) | 8 (5) | ns |
| Septate abcess | 6 (4) | 4 (3) | ns |
| Pneumobilia | 6 (4) | 2 (1) | ns |
| Etiology | 0.046 | ||
| Amebian | 22 (15) | 6 (4) | |
| Pyogenic | 42 (28) | 32 (21) | |
| Treatment | |||
| Conservative | 7 (8.5) | 9 (8.8) | ns |
| Puncture | 61 (74.3) | 33 (73.3) | ns |
| Surgical | 18 (12) | 24 (16) | ns |
| Bleeding | 228 | 317 | ns |
| Intensive therapy (days) | 0 | 3 | 0.002 |
| Second procedure | 14 (9.3) | 24 (16) | 0.034 |
| Recurrence | 6 (4) | 12 (8) | ns |
| Mortality | 0 | 18 (12) | 0 |
NPPH: non-pathological personal history; BP: blood pressure; HR: heart rate; RF: respiratory frequency; BMI: body mass index; HGB: hemoglobin; AST: aspartate amino transferase; ALT: alanine aminotransferase; PA: alkaline phosphatase; ABT: antibiotic therapy.
Additionally, another statistical analysis was carried out comparing the surgery group versus the conservative treatment group and puncture, finding only the abscess rupture, days of hospitalization, elevated alkaline phosphatase, and days of intensive therapy as statistically significant variables (p<0.05).
DiscussionHepatic abscesses are rare, but according to international literature, whenever they occur, it is predominantly among males over 60 years of age.7 In our country, the average age is much earlier, at 47 years old, which could be explained considering intestinal infection by E. histolytica is endemic in Mexico.8 Among our patients, a 20% prevalence of diabetes mellitus was observed, similar to that reported by other authors.9 The presence of diabetes increases mortality in these patients.
The clinical diagnosis is difficult to perform since the symptoms are usually vague and nonspecific. Regarding of our patients’ symptomatology, the main symptoms present in more than half of them were abdominal pain and fever. The pain is due to hepatic subcapsular distension that causes the abscess to grow.
Leukocytosis was predominant in 53% of these patients. Also, 89% of patients presented decreased serum albumin due to the state of hepatopathy or infection they presented. Abscess growth time may be related to hypoalbuminemia, reflecting the duration of liver abscess growth associated with failure in hepatic synthesis. Despite these alterations, they were not significant for the hepatic function test in the majority of the patients.
Importantly, almost half of the patients were unstable, hypotensive, tachycardic, and tachypneic. The advanced stage of the disease may be due to the self-medication culture of our population, and the lack of resources in the area to get a timely diagnosis for all these patients. Early diagnosis is essential; delay is related to an increase in mortality.10,11
The etiology of the abscesses was pyogenic and amoebic, with an approximate ratio of 3:1. The origin of the pyogenic abscesses was mostly identified as a biliary origin, without being able to identify any additional abdominal pathology in the imaging studies. Currently, this trend has begun to change due to better antibiotic treatments. The anatomy of the portal system explains the high incidence of right lobe hepatic abscesses in our study and other international ones.12 We report 24 cases of empyema associated with liver abscess.
Management with systemic antibiotics has only been proven as an effective treatment for pyogenic abscesses less than 3cm.13 Management with ceftriaxone and metronidazole was the conventional treatment, without reporting resistance in the antibiograms to these medications.
Of the cultures obtained, around half of them were negative. This is explained by antibiotic treatments before the puncture. In our study K. pneumoniae was the most commonly isolated pathogen. A considerable increase in the isolation of K. pneumoniae in the United States has been reported.14 In Taiwan, it is the most commonly isolated pathogen,15 with the previous most commonly isolated being E. coli. The prevalence of K. pneumoniae with more nonspecific clinical profiles may explain the delay in diagnosis and the most advanced stages of our patients. Mortality of PAs when associated with K. pneumoniae range from 6 to 17%.16
Being a retrospective study, one of the main limitations was not being able to discern the clinical judgment of the surgeon for the decision to give or not a conservative, minimally invasive, or open surgery. Percutaneous therapy continues evolving. Different reports indicate the high morbidity and mortality that exists in patients who are managed with open surgery as a primary treatment.17
The paradigm in the treatment has changed, and percutaneous drainage is now the initial treatment for drainage of the abscesses.18,19 Open surgery is reserved for patients with septated abscesses and those greater than 5cm.20 In 8 patients who were offered conservative or percutaneous management, these options failed, and open surgery was performed. A fourth of all our cases were treated with open surgery.
The prognostic variables of complication in these patients coincide with the importance of not minimizing any case of hepatic abscess and highlighting that the initial clinical picture may or may not be determinant in the prognosis of these patients. Abdominal pain, respiratory rate, ALT>154IU/L, hemoglobin less than 10g/dL, the presence of a ruptured abscess, and the performance of a second procedure significantly affected the complication rate. At the same time, complications significantly affected the mortality rate and hospitalization days of 18 (12%) of our patients.
ConclusionThe late presentation is characteristic in our environment. The predominant etiology remains pyogenic, despite being an endemic country for amoebiasis. K. pneumoniae is the pathogen mainly isolated from liver abscesses.
In our study, the usual treatment was puncture of the abscess, with good results, this being the first choice of treatment due to the lower morbidity and mortality. The predictive variables of presenting complications are the presence of abdominal pain and tachypnea in their initial exploration. ALT>154IU/L, hemoglobin less than 10g/dL, the presence of a ruptured abscess was also statistically significant as a predictor.
FundingNo funding was received for this study.
Conflict of interestThe authors declare that there are no conflicts of interests regarding the publication of this article.