SARS-Cov2 is currently causing a persistent Covid-19 pandemic, which poses a risk of causing long-term cardiovascular sequels in the population. The viral mechanism of cell infection through the angiotensin 2 converter enzyme receptor and the limited antiviral innate immune response are the suspected causes for a more frequent cardiovascular damage in SARS-Cov2 infection. Knowledge of: the appearance during acute infection of other cardiac conditions beyond the classical myocarditis and pericarditis), the long-term cardiac manifestations (persistent Covid-19), and the increased incidence of myocarditis and pericarditis after vaccination; it is of special interest in order to offer our patients best practices based on current scientific evidence.
El SARS-Cov2 está causando actualmente una pandemia sostenida de Covid-19, con el riesgo de causar secuelas cardíacas a largo plazo en la población. El temor que el SARS-Cov2 cause un daño miocárdico mayor que otros virus convencionales se basa en su mecanismo de infección de células humanas a través del receptor de la enzima convertidora de la angiotensina 2 y las defensas antivirales innatas hasta ahora reducidas contra un nuevo virus. El conocimiento de: la aparición durante la infección aguda de otras afectaciones cardiacas además de las clásicas miocarditis y pericarditis, las manifestaciones cardiacas observadas a largo plazo (Covid-19 persistente) y, la incidencia incrementada de miocarditis y pericarditis tras la vacunación; resulta de especial interés a fin de ofrecer a nuestros pacientes la mejor atención posible basada en la evidencia científica actual.
Fiedler first used the term "acute interstitial myocarditis" in 1990 to describe 4 cases of fulminant heart failure in the absence of coronary, valvular or pericardial disease, in which, in isolation, the myocardium showed a characteristic inflammation due to invisible micro-organisms that years later were identified as viral particles1. In the early 16th century, Benivieni first described acute fibrinous pericarditis or cor villosum on the basis of detailed autopsies. It was Rondelet, in the 17th century, who described symptoms of fever and chest pain as characteristic of pericarditis2.
Myocarditis and pericarditis are the 2 most common cardiac manifestations seen after viral infection and result primarily from an inappropriate immune response, triggered by mechanisms involving T- and B-cells3.
The new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is currently causing a sustained COVID-19 pandemic with the risk of long-term cardiac sequelae in the population. The fear that SARS-CoV-2 causes greater myocardial damage than other conventional viruses is based on its mechanism of infection in human cells following binding of the SARS-CoV-2 spike protein (protein S) to the transmembrane angiotensin-converting enzyme 2 (ACE2) receptor of alveoli, myocardial tissue and blood vessels; additionally, innate antiviral defences are probably reduced against a new virus; the occurrence in patients with COVID-19 of cardiac involvement other than the classical myocarditis and pericarditis; and the occurrence of cardiac complications after vaccination against COVID-19, always fewer than those observed with the infection itself.
In this article we review heart disease and SARS-CoV-2 under 4 differential aspects: a) pathogenic mechanisms in the development of clinical features; b) cardiac disease observed during acute SARS-CoV-2 infection, especially in the pre-vaccination period; c) cardiac manifestations observed in the long-term, long COVID-19 and d) the increased incidence of myocarditis and pericarditis observed after COVID-19 vaccination.
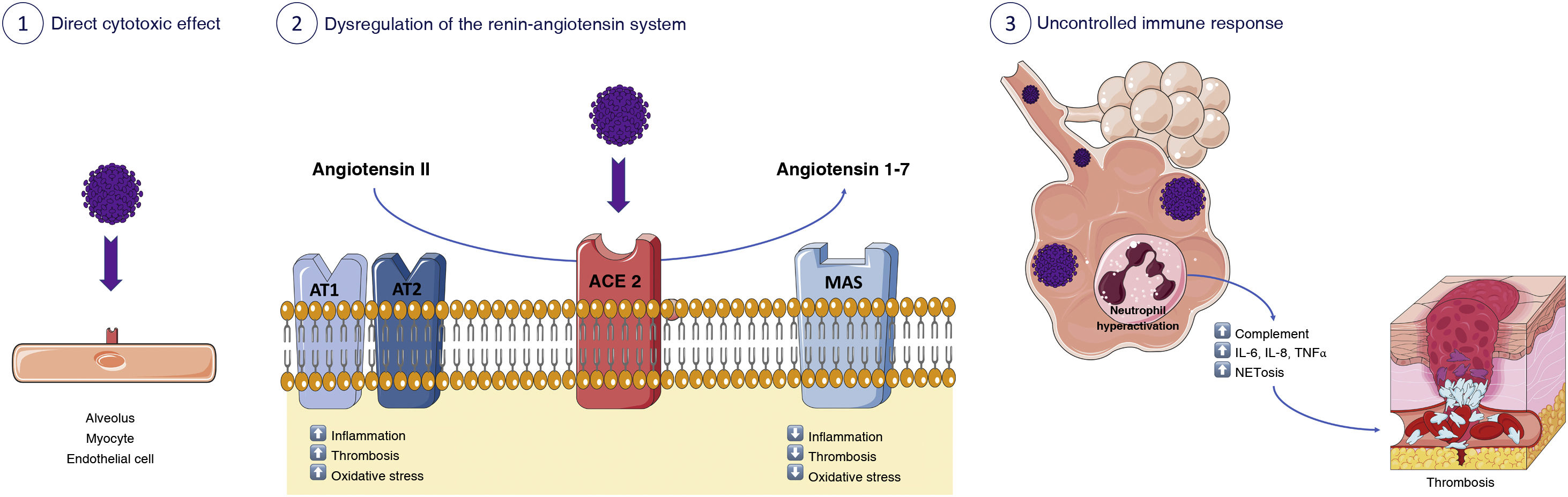
Pathogenic mechanisms involved in the development of heart disease after SARS-CoV-2 infectionThe cardiovascular system is significantly affected in COVID-19. From a pathogenic perspective, several different mechanisms have been identified, among them, 3 should be highlighted (Fig. 1). On the one hand, direct cell injury caused by SARS-CoV-2. Secondly, damage mediated by its antigens or by its structural components. Finally, non-specific myocardial injury related to the underlying inflammatory reaction and excessive immune response mediated by cytokines and other cellular messengers.
Pathogenic mechanisms involved in cardiac involvement after SARS-CoV-2 infection. 1) Direct cytotoxic effect of SARS-CoV-2 on alveoli, myocytes, and endothelial cells. 2) Dysregulation of the renin-angiotensin system with less inactivation of angiotensin 2 and less generation of angiotensin (1-7). 3) Immunothrombosis.
Cell damage is caused by the primary SARS-CoV-2 infection of myocytes, and also by direct injury to vascular endothelial cells, all of which express ACE2. Until now, conventional viruses exerted cell damage primarily on myocytes (for example, Coxsackie B) or on vascular endothelial cells (for example, herpes)3; but SARS-CoV-2 seems to cause both types of cell damage.
In addition, the interaction between ACE2 and the SARS-CoV-2 S protein induces a substantial loss of ACE2 receptor activity on the outer surface of the cell membrane4. This phenomenon leads to less angiotensin 2 inactivation and less angiotensin (1–7)5 generation. This imbalance would cause an excess of angiotensin 2 in patients with COVID-19, which triggers inflammation, thrombosis and other serious cardiovascular adverse reactions. The degree of ACE2 expression varies between individuals. Factors such as advanced age, high blood pressure, diabetes or previous cardiovascular diseases share a variable degree of ACE2 deficiency and have been linked to more severe forms of COVID-19. Therefore, it is very likely that it is not the excess but the deficiency of the ACE2 receptor activity that complicates the clinical features of COVID-196. On the other hand, ACE2 expression may be increased in patients taking ACE inhibitors or angiotensin-1 blockers, raising concerns about facilitating the entry of SARS-CoV-2 in patients at higher risk. However, the BRACE CORONA clinical trial has provided clinical data showing that there is no clinical benefit to routinely discontinuing these agents in patients hospitalised for COVID-19 with mild to moderate infection7.
Systemic immune response is probably the main risk factor associated with the documented cardiovascular complications in COVID-19. Innate immunity plays a key role as an early defence mechanism against SARS-CoV-2 infection. However, the uncontrolled innate immune response triggered by hyperactivated neutrophils will promote different coagulopathic pathways through excessive complement activation, cytokine storm and excessive production of neutrophil extracellular traps8, each of which can cause thrombosis by various mechanisms, tissue damage and other cardiovascular involvement5.
Cardiac manifestations during SARS-CoV-2 infectionEvidence of cardiac involvement is common among hospitalised COVID-19 patients9. A greater number of clinical manifestations and involvement has been observed within the spectrum compared to what is usually observed after a viral infection. Thus, the development of acute heart failure, including cardiogenic shock10,11, myocardial ischaemia or infarction12, left ventricular, right ventricular or biventricular dysfunction13,14, myocarditis15,16, stress cardiomyopathy17, arrhythmias18, venous thromboembolism19,20, arterial thrombosis20 and pericarditis16 has been described. The development of these complications, as well as increased mortality, is associated with the presence of pre-existing cardiovascular disease, such as coronary heart disease, heart failure or valvular heart disease, the presence of cardiovascular risk factors and other comorbidities, such as renal failure or a history of cancer21.
There are limited data on the incidence of heart failure in COVID-19. Between 3% and 50% of patients have been reported to develop it during acute infection10,11,22. Treatment used during COVID-19 (glucocorticoids, anti-inflammatory drugs) may lead to heart failure decompensation in patients with preserved ejection fraction. In addition, heart failure with reduced ejection fraction may be due to exacerbation of underlying or undiagnosed heart disease, or may be secondary to acute myocardial injury, such as myocardial infarction, myocarditis, myocardial stress, etc. Occasionally, although with low incidence, it can result in cardiogenic shock, which is usually mixed, together with septic shock, where early diagnosis is essential for prognosis.23 The right ventricle has been shown to play a pivotal role in the prognosis of SARS-CoV-2 infection because of its physiological relationship with the pulmonary circulation. Right ventricular dysfunction and dilatation may contribute to rapid haemodynamic deterioration, development of arrhythmias and sudden death. Thus, the presence of right ventricular dysfunction doubles the risk of mortality24.
Myocardial injury seen during SARS-CoV-2 infection may be due to myocardial infarction caused by plaque rupture (type 1 myocardial infarction), increased myocardial demand (type 2 myocardial infarction), or stress cardiomyopathy or myocarditis, all of which leads to increased troponin levels, commonly seen in COVID-1925–27. Differential diagnosis between myocardial infarction, myocarditis or stress cardiomyopathy will require conventional complementary tests, among which electrocardiography, echocardiography and cardiac magnetic resonance (CMR) or angiography will be of great importance. It is important to note that troponin levels are affected by multiple factors, such as infection itself, hypoxia and impaired renal function, so the possibility of false-positive myocardial injury in patients with COVID-19 must be considered25.
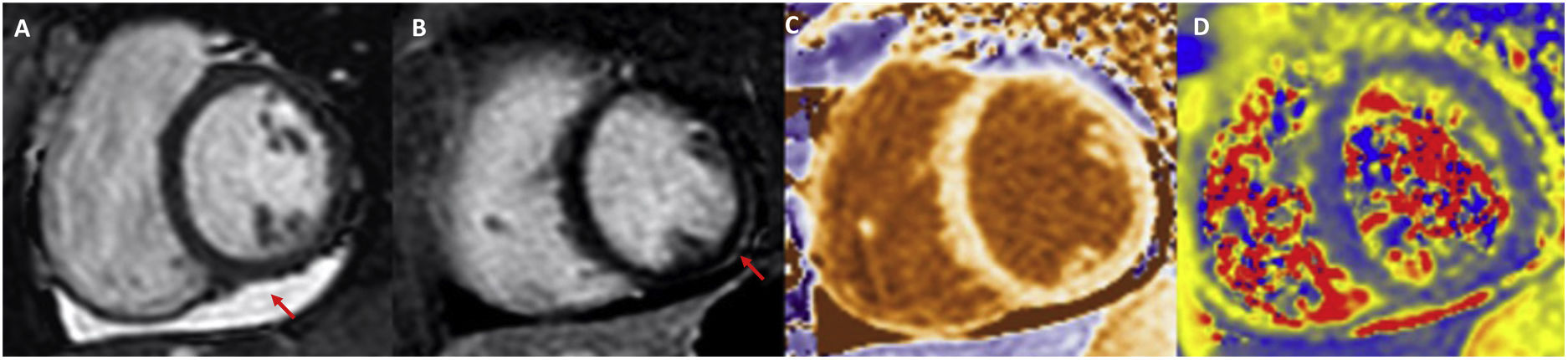
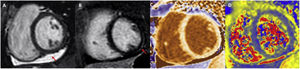
The true incidence of myocarditis is difficult to assess due to ambiguous definitions, assessment of unrepresentative populations and lack of systematic data collection. Studies in hospitalised patients, autopsy data and CMR indicate that the overall incidence is significant28. Fortunately, fulminant myocarditis appears to be quite rare29. As outlined above, the first diagnostic approach once suspicion has been established should be to perform CMR, as this is the most sensitive method to rule out ischaemia or pre-existing cardiomyopathy and, at the same time, to detect cardiac abnormalities as a consequence of SARS-CoV-2 infection, including myocardial inflammation, non-ischaemic epicardial scarring and pericardial effusion or enhancement (Fig. 2). Endomyocardial biopsy is reserved for cases with clinical deterioration refractory to treatment and with poor progression, in particular if there are conduction disturbances or ventricular arrhythmias, in which obstructive coronary artery disease has been previously ruled out. Treatment of myocarditis in stable patients is similar to that of patients without COVID-19. The use of low-dose beta-blockers together with an ACE inhibitor is recommended. If the patient has hemodynamic compromise, the use of intravenous glucocorticoids (already used in the treatment of COVID-19) should be considered.
Example of cardiac magnetic resonance in a patient with COVID-19 myocarditis. Pericardial effusion (A, arrow) is observed in CINE SSFP sequences, subepicardial delayed enhancement in the mid inferolateral segment (B, arrow) and increased T1 values in native mapping (C) and T2 values in mapping (D).
The development of pericarditis following acute SARS-CoV-2 infection is less common than myocardial involvement; however, it is associated with pericardial effusion in up to a third of cases16. Symptomatology and treatment are similar to that of pericarditis caused by other viruses, with NSAIDs such as ibuprofen administered for several weeks and colchicine for at least 3 months constitute the mainstay30. Exceptional cases of development of constrictive pericarditis have been reported, highlighting the importance of appropriate treatment according to clinical practice guidelines and at an early stage, especially in cases with associated pericardial effusion.
Long-term cardiac manifestations: Long COVIDA number of long-term cardiac manifestations have been reported following acute SARS-CoV-2 infection and are encompassed in the so-called long COVID ()31. The severity and complications observed in the acute phase of infection have meant that care and research efforts have focused on this initial stage of the infection. However, we may be just seeing the tip of an iceberg, and under each of the waves we have experienced, we may find thousands of initially mild, even asymptomatic cases, with persistent, diverse and non-specific manifestations, but limiting with respect to the patient's previous normal life. Long COVID, its present and future sequelae, is in our view the greatest current healthcare and scientific challenge of this pandemic.
Long COVID is defined as symptoms resulting from SARS-CoV-2 infection that persist beyond 12 weeks post-infection32,33. It is important, but not easy, to differentiate the term from others, such as sequelae or convalescence, both of which are consequences of severe organ involvement of the initial infection (e.g. in our setting, severe ventricular dysfunction following acute myocarditis is a post-covid sequelae that can cause persistent dyspnoea). Although they may appear to be similar concepts, they are very different patients and profiles. Sequelae occur in severe patients, with prolonged hospitalisation, all of them with confirmatory diagnostic tests (PCR), comorbidities, advanced age or male predominance. In contrast, long COVID is prevalent in a middle-aged (40–50 years), female population34,35, and occurs irrespective of the severity of the initial symptoms, with many patients even being found to be asymptomatic during the acute phase. In contrast to the sequelae of the initial infection, the identification of organ damage that justifies the clinical features is very difficult in long COVID, posing a major diagnostic and therapeutic challenge. Given the limited knowledge and evidence regarding this new entity, it is essential that we are strict, technical and methodical with the categorisation of these patients. This is evidenced in the recent ICD-11 publication, where a separate entity is already coded for long COVID (RA-02), which is necessary for homogeneous reporting of our findings or studies36.
The prevalence of persistent symptoms derived from SARS-CoV-2 infection is around 10%37. With more than 500 million positive cases worldwide, it is understandable how important long COVID can be to our society and healthcare system. In the cardiovascular field, the largest study published so far, focusing on post-acute symptoms (without differentiating between sequelae and persistent symptoms), has been conducted using the US Department of Veterans Affairs health database, with more than 150,000 patients with COVID-19 and 5 million historical and contemporary controls38. The study found an increased incidence of cardiovascular disease 30 days after SARS-CoV-2 infection (excess adverse cardiovascular events of 19 cases per 1000 person-years, HR = 1.63; 1.59–1.68), including cerebrovascular disease, arrhythmias, myocarditis and pericarditis, ischaemic heart disease, heart failure or thromboembolic disease. This higher incidence was independent of age, sex or the presence of other cardiovascular risk factors. In this study, subgroup analysis did show a clear correlation between the severity of infection during the acute phase and the incidence of cardiovascular complications, most of which were more common in patients admitted to hospital and especially to intensive care units. It seems clear, therefore, that most of the events recorded in this study are post-Covid sequelae and not long COVID.
Different studies using complementary diagnostic tests (mainly CMR and ergospirometry) have investigated the prevalence of cardiovascular abnormalities after infection, with mixed results. Short- and medium-term studies show a higher frequency of pathological findings on CMR in patients who had overcome infection (median 32–70 days), including enhancement or oedema, although in highly variable percentages (30–60 %)28. However, CMR studies at 6 months show contradictory findings. Thus, in a study of healthy athletes39, 19% of CMRs showed anomalies. On the contrary, in a study in healthcare personnel, no significant differences were found in the CMR findings compared to the control group40. The recognition that COVID-19 can have long-term consequences is prompting the different scientific societies to establish recommendations on the diagnosis and management of these sequelae41.
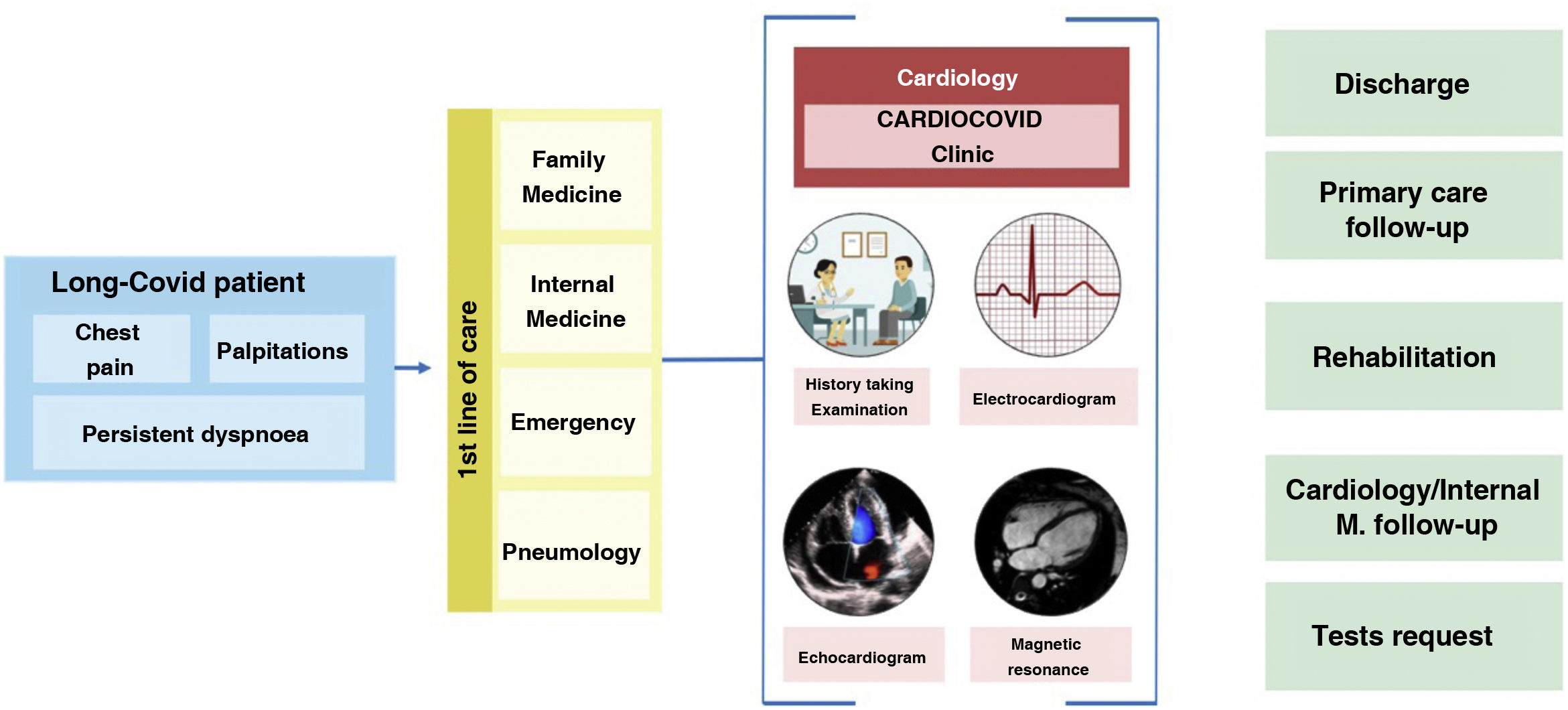
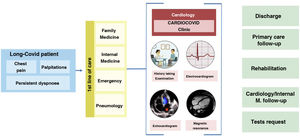
In our view, cardiovascular long COVID revolves around 3 symptoms that are very common and recurrent in our clinics: dyspnoea, chest pain and palpitations. The patient profile is middle-aged, female and with a history of infection more than 12 weeks before, or even, in many cases, undefined, because their initial presentation was mild or asymptomatic. The varied, intermittent and non-specific symptomatology greatly limits and worsens the quality of life, especially when compared with the state of health prior to the disease and, therefore, patients repeatedly visit different specialists (family practice, internists, emergency department, pneumology, etc.). Our experience shows that the vast majority of patients, at the time of referral, already have a proper medical record, in which the initial infection, acute symptoms, treatment received, and duration are of particular interest. An adequate history taking, and a system-based physical examination is essential to identify the organ involvement that explains the symptoms of the patients. In most patients, basic complementary tests such as blood tests, resting electrocardiogram or chest X-ray are necessary as part of the initial work-up. Despite all this, in a large percentage of patients with long COVID, these studies are negative, and we cannot find the cause that justifies the patient's heterogeneous clinical manifestations. For this reason, it is necessary to homogenise criteria and combine caseloads and, to this end, the creation of monographic clinics or units may be a good option. Fig. 3 summarises the initial standardisation of our practice.
Action protocol for patients with long COVID with cardiovascular symptoms. High-quality face-to-face consultation, where a detailed history taking, physical examination, electrocardiogram and echocardiography play a key role. More specific tests, such as cardiac MRI, 24 h monitoring or ergospirometry, will be indicated in a minority of patients after evaluation.
In the approach to the assessment of dyspnoea, shortness of breath appears as one of the most common symptoms in patients with persistent disease. About 75% describe this condition during follow-up37,42. The non-specific nature of the symptoms (tiredness, fatigue, together with physical deconditioning) make this clinical condition a challenge when assessing these patients. A correct system-based history taking and a detailed physical examination will be the starting point of our study. The use of simple scales, such as the Borg or mMRC, will take minimal time and help to quantify the situation43,44. As a rule, the routine complementary tests performed in these patients are usually normal and show no evidence of organ involvement: lung X-rays and CT scans, echocardiography, and even NT-ProBNP levels do not usually provide pathological findings in persistent patients. More specific studies, such as ergospirometry, do reveal a maladaptation to exercise, with a decrease in peak oxygen consumption (VO2max) or first thresholds (VT1 threshold) also at the lower limits. In contrast, parameters assessing ventilatory function (respiratory reserve, baseline spirometry or oxygen saturation) are again rigorously normal, as are those focusing exclusively on cardiac pump function, such as the Oxygen Uptake Efficiency Slope (OUES) and Pulse O2. Consequently, it can be concluded that there is an involvement beyond isolated cardiac or pulmonary impairment, where the vascular, nervous and muscular system play a key role9,10. Hence the importance that adapted and supervised physical rehabilitation can have in the treatment and improvement of these patients45.
Chest painThe second most common cardiovascular symptom in long COVID is chest pain, with a prevalence close to 70% in numerous series of patients with persistent disease46. Described in some recent documents or communications as “retrosternal pain”, “lung burn” or “burning”, it is reminiscent of pleural-pericardial involvement. Burning pain not directly related to exertion, without coronary features, without irradiation or vagal reaction, but with clear worsening after physical activity. Sufferers describe it as intermittent, on and off, and limiting. An echocardiogram is advisable to rule out structural involvement and as an initial approach to the study of the pericardium, a highly innervated structure that is frequently affected in viral respiratory infections.
SARS-CoV-2-associated pericarditis is an entity that should always be considered, given the tropism of the virus for cardiac structures. Its involvement would explain many of the cases of chest pain (even persistent), discomfort, worsening after exercise and recurrence over time.
In addition, the significant endothelial involvement secondary to virus infection can lead to long-lasting vascular dysfunction, with a clinical and pathophysiological mechanism very similar to that of microvascular angina described in other clinical conditions. Recent studies and advanced diagnostic techniques such as stress CMR provide evidence of this condition in our environment35.
PalpitationsLastly, palpitations; although all types of arrhythmic events associated with SARS-CoV-2 have been described, from bradycardia to sudden death due to ventricular arrhythmic events9. At present, the condition most commonly associated with patients with persistent COVID is excessive and inappropriate tachycardia with minimal exertion or postural changes, very similar to the so-called postural tachycardia syndrome (POTS)47,48. Inappropriate tachycardia, greater than 30 beats per minute when standing upright, without accompanying orthostasis, according to its classic diagnostic criteria, would be the explanation for this symptomatology, which is so common in our clinics.
The involvement of the autonomic nervous system, already described in other entities, is clearly related to this abnormality, once again highlighting the close interaction between the nervous and vascular systems in the disease caused by the virus. Neurophysiological studies may be useful in order to identify this impairment, although simple standing exercises in the consultation room are usually sufficient for a first diagnostic approach.
As for the prognosis and treatment of POTS, beta-blockers, ivabradine and dietary hygiene measures (such as salt intake) have usually been used, with varying degrees of success.
Although we still do not know the pathogenic mechanisms involved in the cardiac manifestations of long COVID49, a sustained immune response over time could lead to fibrotic changes and displacement of desmosomal proteins that could explain these different cardiovascular manifestations.
Cardiac manifestations observed after COVID-19 vaccinationThe rapid introduction of COVID-19 vaccines, their different types (mRNA vaccines such as Pfizer-BioNTech or Moderna and vector vaccines such as AstraZeneca or Johnson & Johnson's Janssen) and the global magnitude of the pandemic are prompting a rigorous review of their safety, quality and efficacy.
Until the COVID-19 pandemic, the incidence of myocarditis or pericarditis following vaccination against viral agents had not been well researched50. The most representative data come from smallpox vaccination (considered eradicated by WHO in 1980 and therefore with an absence of innate antiviral defences in vaccinated individuals, as in many cases of COVID-19 vaccination), with rates of myocarditis and pericarditis between 8 and 55 cases per 100,000 vaccinations51,52. In the general population, the rate of myocarditis ranges between 10 and 20 cases per 100,000 individuals/year53.
The evident temporal relationship between myocarditis and pericarditis after vaccination against COVID-19 has led to the belief that the vaccine may act as a trigger for these cardiological complications54. So far, in most of the reported cases, patients received COVID-19 mRNA vaccines (Pfizer-BioNTech or Moderna); the majority were young males; the median time to onset of symptoms after vaccine administration was 3.8 ± 4.5 days; 3 out of 4 patients experienced symptoms after the second dose, with chest pain (89%) and fever (33%) as the most common presenting symptoms. Troponin elevation, electrocardiographic changes with diffuse ST elevation, and changes consistent with myocarditis on cardiac magnetic resonance were constant findings of the diagnostic techniques used55.
According to the US Centers for Disease Control and Prevention, the estimated rate of myocarditis and pericarditis is 12.6 cases per million vaccine recipients with the second dose of COVID-19 mRNA vaccine among persons aged 12–39 years54. Data from the UK Medicines and Healthcare products Regulatory Agency are even more explicit and show a low incidence of myocarditis and pericarditis following vaccination. It is higher with mRNA vaccines than with vector vaccines56. The overall rate of myocarditis observed after vaccination (first, second dose, etc.) with Pfizer-BioNTech is 10 cases per million doses administered and the rate of pericarditis is 7 cases per million doses. For the Moderna vaccine, it is 18 cases of myocarditis per million doses and 10 cases of pericarditis per million doses. For AstraZeneca, the rate is lower than previous mRNA vaccines, with 5 cases of myocarditis per million doses and 5 cases of pericarditis per million doses administered. These data are similar to those of a large European epidemiological study that has estimated the risk of myocarditis after vaccination with the Pfizer-BioNTech and Moderna vaccines in a cohort of 23.1 million Nordic citizens. In that cohort, young men had an excess of myocarditis in 40–70 cases per million vaccinated after the second dose of the Pfizer-BioNTech vaccine and 90–280 cases in the Moderna vaccine57.
Although the mechanisms for the development of myocarditis/pericarditis are unclear, molecular mimicry between SARS-CoV-2 protein S and autoantigens triggers pre-existing dysregulated immune pathways in certain individuals. Increased autoantibodies against certain autoantigens and increased frequency of natural killer cells have been reported. The reasons for the male prevalence of myocarditis cases are unknown, but possible explanations relate to sex hormone differences in immune response and myocarditis, and also to under-diagnosis of heart disease in women.
The development of myocarditis or pericarditis after one dose of a COVID-19 vaccine advises against a subsequent dose until additional safety data are available. Depending on the risk of the individual, additional doses of non-mRNA vaccines (Astra Zeneca or Janssen) could be considered56.
Despite rare cases of myocarditis and pericarditis, the risk/benefit assessment of COVID-19 vaccination shows a favourable balance, with a higher number of cases of myocarditis/pericarditis after SARS-CoV-2 infection than after vaccination, for all age and sex groups; therefore, vaccination against COVID-19 is recommended for all those aged 12 years and older29,58,59.
FundingThe Cardiology Service of the University Hospital of Salamanca is a member of the CIBERCV group (CB16/11/00374) and receives annual grants to finance its research structure.
David González-Calle is under a Río Hortega contract at the Instituto de Salud Carlos III at the University Hospital of Salamanca.
Conflict of interestsThe authors declare no conflicts of interest.