Gemtuzumab ozogamicin (GO) is a monoclonal antibody with significant activity in CD33 + acute myeloid leukaemia (AML). At doses of 9 mg/m2, its benefit was limited by hepatotoxicity and sinusoidal obstruction syndrome (SOS). Fractionated doses improved toxicity without compromising efficacy. We evaluated the efficacy and the toxicity of low doses of GO.
MethodsTwenty-four patients with AML received 3 mg/m2 of GO as a part of the induction or reinduction therapy.
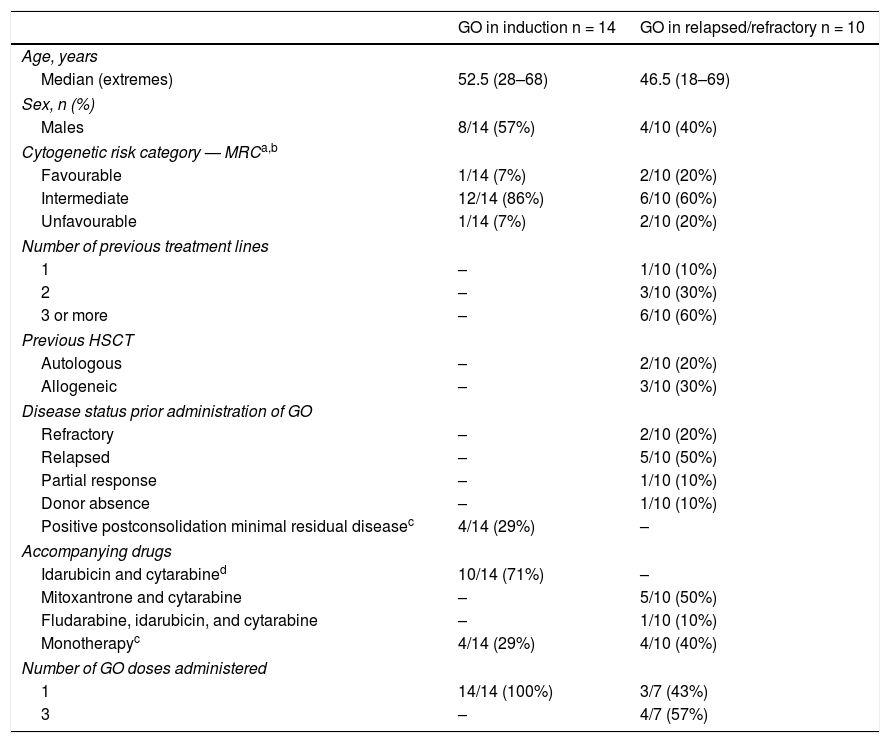
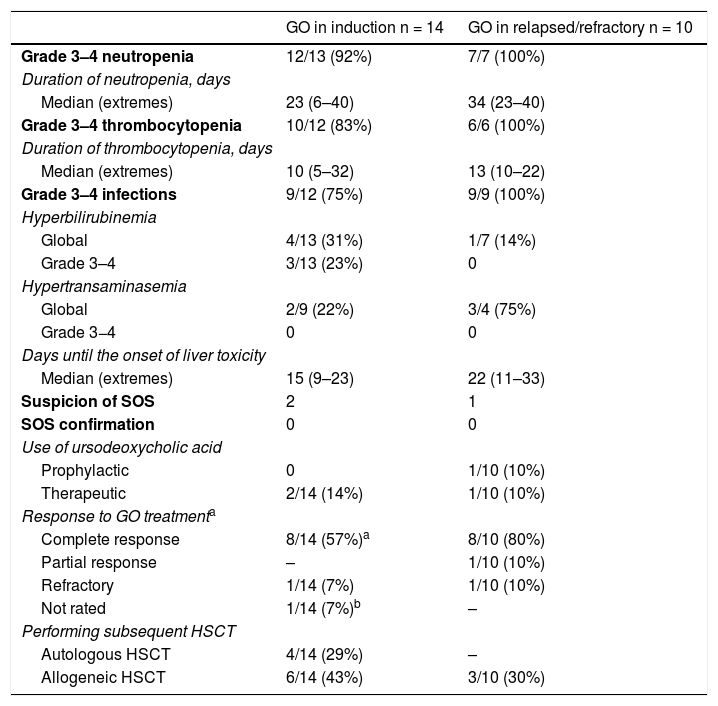
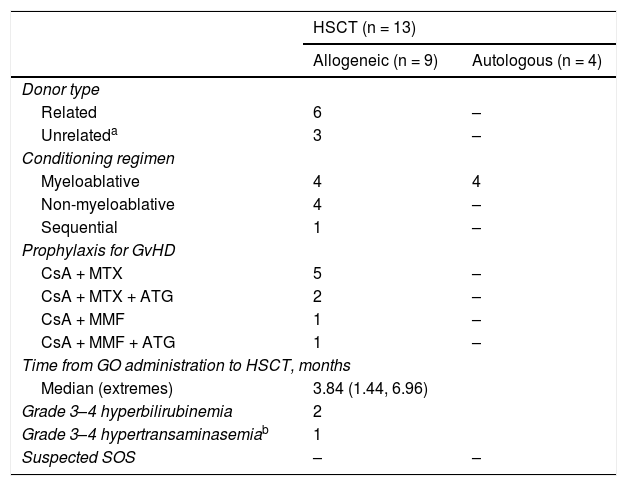
ResultsFourteen patients diagnosed with de novo AML and 10 patients with relapsed or refractory (R/R) AML received GO as a part of the induction or reinduction therapy. Three and no cases of hepatotoxicity were observed, respectively. Thirteen patients received a subsequent haematopoietic stem cell transplantation (HSCT) after GO therapy. Hepatotoxicity was observed in 2 patients and no SOS was observed in any patient.
ConclusionsThe administration of low dose GO is feasible and does not have impact on subsequent HSCT outcome. Although some degree of hepatotoxicity was observed, there were no cases of SOS, either before or after HSCT.
Gemtuzumab ozogamicina (GO) es un anticuerpo monoclonal activo frente la leucemia mieloide aguda (LMA) CD33+. A dosis de 9 mg/m2, su beneficio se vio limitado por la hepatotoxicidad y el síndrome de oclusión sinusoidal (SOS). Dosis fraccionadas (3 mg/m2) mejoraron la toxicidad sin comprometer la eficacia. En este estudio se evaluó la eficacia y la toxicidad de GO administrado a dosis bajas.
MétodosSe incluyeron 24 pacientes con LMA tratados con GO a dosis de 3 mg/m2 durante el tratamiento de inducción o reinducción.
ResultadosCatorce pacientes con LMA de novo y 10 con LMA en recaída o refractaria (R/R) recibieron GO como parte del tratamiento de inducción o reinducción. Se observó hepatotoxicidad grado 3–4 en 3 y ningún paciente, respectivamente. Trece pacientes recibieron posteriormente un trasplante de progenitores hematopoyéticos (TPH) observándose en ellos 2 casos de hepatotoxicidad y ningún caso de SOS.
ConclusionesLa administración de GO a dosis de 3 mg/m2 es segura y no compromete la realización de un ulterior TPH. Aunque la hepatotoxicidad fue frecuente, no se observó SOS antes o después del TPH.