Improper clinical use of tumor markers (TM) may cause unnecessary additional studies to confirm or refute a positive result. After observing 2 adverse events due to a wrong use of TM, a protocol for improving their use was implemented. The objective of this study was to determine the impact of the implementation of the protocol.
Material and methodThis was a pre-postintervention study, where analytical requests of carcinoembryonic antigen, CA15.3, CA19.9 and CA125 were analyzed during one year in patients not undergoing checking of neoplasia. A protocol was implemented and physicians were trained as recommended by the European Group on Tumor Markers, limiting its use to monitor the disease and its treatment. The study period was 2010–2014.
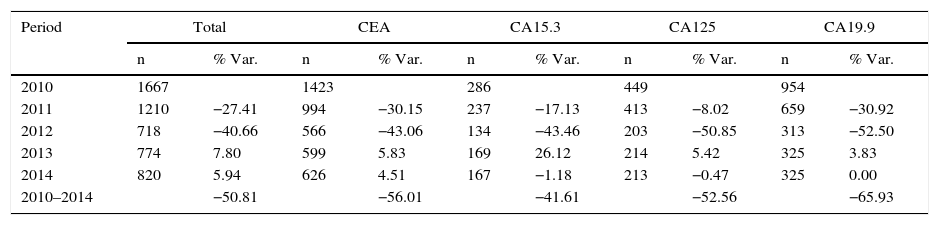
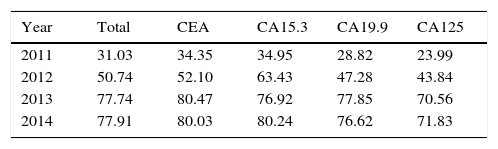
ResultsThe total number of requests dropped 50.81% and the percentage of adequacy of TM increased, each year, from 31.03 to 77.91%.
ConclusionsThe implementation of a protocol for the proper use of TM contributes to a safer use, avoiding incorrect studies and unnecessary and harmful tests for the patient.
Los marcadores tumorales (MT) son pruebas accesibles para la actividad clínica. Su uso inadecuado puede provocar pruebas complementarias innecesarias para confirmar o refutar un resultado positivo. Tras 2 acontecimientos adversos por un uso incorrecto de MT, se implementó un protocolo para el uso adecuado y seguro de estos. El objetivo de este trabajo fue determinar el impacto de la implementación de dicho protocolo.
Material y métodoEstudio pre-postintervención. Se analizó el uso, durante un año, de peticiones de MT (antígeno carcinoembrionario, CA15.3, CA19.9, CA125) de pacientes no sometidos a revisión oncológica. Se implementó un protocolo, formándose a los facultativos según las recomendaciones del Grupo Europeo de Marcadores Tumorales, limitando su uso al seguimiento de la enfermedad y monitorización de tratamientos. Período estudiado: 2010-2014.
ResultadosEl número total de peticiones descendió un 50,81%, y el porcentaje de adecuación de los MT aumentó anualmente desde un 31,03 hasta un 77,91%.
ConclusionesLa implantación de un protocolo del uso adecuado de MT contribuye a un uso seguro, evitando estudios no indicados y evitando pruebas complementarias innecesarias y lesivas para el paciente.