Several studies have reported the beneficial effect of glucocorticoids in the treatment of cytokine storm that occurs in patients with severe COVID-19. Various glucocorticoids regimens have been proposed.
MethodsRetrospective observational study that includes patients with severe SARS-CoV-2 pneumonia and compares admission to an Intensive Care Unit (ICU) or death during hospitalization in three groups of patients: no glucocorticoids treatment, use of glucocorticoids doses equivalent to less than 250 mg of prednisone daily and use of equivalent doses greater than or equal to 250 mg of prednisone daily. Multivariate analysis was performed using logistic regression, using the propensity index as a covariant.
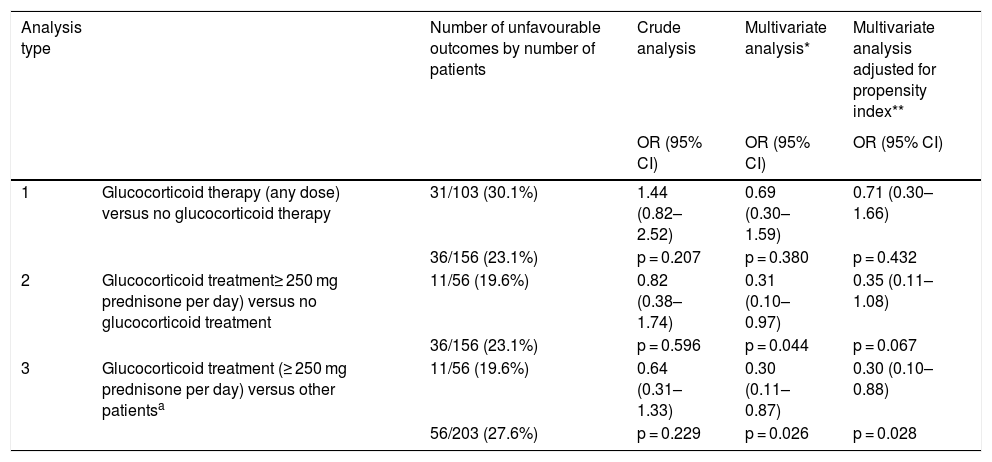
ResultsOf the 259 patients enrolled in the study, 67 (25.9%) had an unfavorable evolution, dying or requiring ICU admission. Comparative analyzes between different glucocorticoids treatments and the association with ICU admission or death were: glucocorticoids treatment (any dose) versus no glucocorticoids treatment (OR: 0.71 [0.30–1.66]), treatment with glucocorticoids (≥250 mg prednisone daily) versus no glucocorticoids treatment (OR: 0.35 [0.11–1.08]) and glucocorticoids treatment (≥250 mg prednisone daily) versus patients with glucocorticoids doses <250 mg prednisone daily or without glucocorticoids treatment (OR: 0.30 [0.10–0.88]).
ConclusionThe results of this study show that patients with severe SARS-CoV-2 pneumonia treated with glucocorticoids pulses with equivalent doses of prednisone greater than or equal to 250 mg have a more favorable evolution (less mortality and less admission to ICU).
Se han comunicado varios trabajos donde se ha demostrado un efecto beneficioso de los glucocorticoides como tratamiento de la tormenta de citocinas que se asocia a los cuadros graves por SARS-CoV-2, plateándose diferentes pautas de glucocorticoides.
MétodosEstudio observacional retrospectivo que incluye pacientes con neumonía grave por SARS-CoV-2 y compara el ingreso en una unidad de cuidados intensivos (UCI) o fallecimiento durante la hospitalización en 3 grupos de pacientes: sin tratamiento con glucocorticoides, uso de dosis diarias de glucocorticoides equivalentes menores a 250 mg de prednisona y dosis diarias equivalentes mayores o iguales a 250 mg de prednisona. Se realizó un análisis multivariante mediante regresión logística, utilizando el índice de propensión como covariante.
ResultadosDe los 259 pacientes incorporados al estudio 67 (25,9%) tuvieron una evolución desfavorable, falleciendo o precisando ingreso en UCI. Los análisis comparativos entre diferentes tratamientos con glucocorticoides, y la asociación con ingreso en UCI o fallecimiento fueron: tratamiento con glucocorticoides (cualquier dosis) versus sin tratamiento con glucocorticoides (OR: 0,71 [0,30–1,66]), tratamiento con glucocorticoides (≥250 mg de prednisona al día) versus sin tratamiento con glucocorticoides (OR: 0,35 [0,11–1,08]) y tratamiento con glucocorticoides (≥250 mg de prednisona al día) versus pacientes con dosis de glucocorticoides < 250 mg de prednisona o sin tratamiento con glucocorticoides (OR: 0,30 [0,10–0,88]).
ConclusiónLos resultados de este estudio muestran que los paciente con neumonía grave por SARS-CoV-2 tratados con pulsos con glucocorticoides con dosis equivalentes de prednisona mayor o igual de 250 mg tienen una evolución más favorable (menos mortalidad e ingreso en UCI).
About 80% of patients with coronavirus infection disease 2019 (COVID-19) have mild symptoms, but the rest may be more severely affected with the development of interstitial pneumonia that can be complicated by an acute respiratory distress syndrome, requiring admission to an intensive care unit (ICU) in up to 5% of the cases1,2.
Two overlapping phases in the pathogenesis of the disease of uncertain duration have been defined3,4. The first one, in which there is a prevalence of direct damage by the virus, and the second one resulting from the host's inflammatory response. Many patients with severe COVID-19 have an excessive inflammatory response caused by an uncontrolled release of pro-inflammatory cytokines, defined as cytokine storm, causing diffuse alveolar damage3–6.
In the absence of effective antiviral treatment, the only weapons currently available to us to control this inflammatory storm are supportive care and immunomodulatory treatment.
Glucocorticoid therapy has been a controversial issue in patients with SARS-CoV-2 infection. On the one hand, it is thought to inhibit tissue damage by reducing the inflammatory response, but on the other hand, it is feared that it may inhibit cell-mediated immunity, which could reduce viral clearance and worsen the course of the disease7.
Several studies, including one clinical trial, have reported a beneficial effect of glucocorticoids on the outcome of patients with COVID-198–14, although not all studies have found the same benefit15,16. The doses of glucocorticoids used have varied widely, with some authors favouring high-dose boluses (prednisone > 250 mg) because it has a greater effect on the so-called non-genomic pathway of action13,17.
The aim of the present study was to examine the progression of patients admitted with severe SARS-CoV-2 pneumonia in our hospital at the beginning of the pandemic, where various glucocorticoid treatment regimens were used, and to determine the association of glucocorticoid use with ICU admission or death.
MethodologyStudy design and patientsObservational and retrospective study that examines the efficacy of glucocorticoid treatment in patients between the ages of 18 and 75 years with severe SARS-CoV-2 pneumonia in a conventional hospital ward at the Infanta Sofía University Hospital (HUIS) in San Sebastián de los Reyes (Madrid), with admission date from 28th February 2020 to 9th April 2020.
The diagnosis of SARS-CoV-2 infection was confirmed by gene amplification through a polymerase chain reaction of a nasopharyngeal sample.
Severe pneumonia was defined by the presence of:
- -
Consolidations in more than 2 fields on chest X-ray upon arrival at the emergency department, or radiological worsening with the onset of new infiltrates in the first 48 h after hospital admission.
- -
Room air oxygen saturation <93% or an equivalent oxygen saturation/fraction of inspired oxygen ratio (SO2/FiO2) < 443 in the first 48 h after hospital admission.
Patients with a hospital stay of less than 48 h with previous immunosuppressant or glucocorticoid treatment, associated comorbidity, which had a more relevant role than SARS-CoV-2 infection and which actually motivated hospital admission, and patients with other diseases that could act as a confounding factor, such as chronic lymphatic leukaemia, in which lymphopenia was not assessable as a prognostic marker were excluded.
Exposure to glucocorticoids and definition of outcomeThe study compared patients who received standard treatment versus patients who received standard treatment plus glucocorticoids, and their association with the development of an unfavourable outcome.
An unfavourable outcome was defined as death or admission to the ICU during hospitalization. Hospital discharge without having been admitted to the ICU was defined as a favourable outcome.
The use of any dose of methylprednisolone or dexamethasone during admission was considered as glucocorticoid exposure. Two glucocorticoid exposure groups were defined. A group of patients who received maximum daily equivalent doses of prednisone under 250 mg and another group that received maximum daily equivalent doses of prednisone greater than or equal to 250 mg. Equivalent daily doses greater than or equal to 250 mg of prednisone were administered as intravenous boluses of dexamethasone 40 mg, or 250 or 500 mg of methylprednisolone for a period of 3–5 days.
The decision whether or not to initiate glucocorticoid treatment was made by the medical team treating each patient on a case-by-case basis. Patients were informed of their off-label use and gave their verbal consent, which was recorded in the electronic medical record.
Standard treatment included supportive measures with oxygen therapy and the combination of other drugs such as lopinavir-ritonavir, hydroxychloroquine, azithromycin, tocilizumab and anakinra, according to local, dynamic, guiding, and consensual hospital guidelines, which was updated according to the publications that became available during the inclusion period. Tocilizumab and anakinra were administered at the discretion of the treating medical team; interleukin 6 values had to be greater than 40 pg/mL in order to administer tocilizumab.
Working method and variablesPatients who met all the inclusion criteria and none of the exclusion criteria were selected from a database (HUIS-COVID) that was created to register the clinical data of patients hospitalized for COVID-19.
The variables analysed were: demographic characteristics (age, sex and race), previous medical history, medication prior to admission, date of onset of symptoms, date of hospital admission, date of the outcome, oxygen saturation and inspiratory fraction of oxygen ratio (SO2/FiO2) on arrival at the emergency department and at 48 h, laboratory data upon arrival at the emergency department and at 48 h (percentage of lymphocytes, C-reactive protein [CRP], lactate dehydrogenase [LDH] and D-dimer), radiological findings on arrival at the emergency department and at 48 h and treatments during admission.
Statistical analysisFor descriptive purposes, the qualitative variables were expressed as absolute frequency and percentage, the quantitative variables as medians and interquartile ranges (IQR).
First, a univariate analysis of the variables recorded in the different glucocorticoid exposure groups was performed. Qualitative variables were analysed using the Chi square test and quantitative variables using the U-Mann–Whitney test. Variables that showed statistically significant differences in univariate analysis (p < 0.05) were used through logistic regression to calculate the index of propensity to receive treatment with glucocorticoids (any dose) and equivalent doses of prednisone greater than or equal to 250 mg.
Subsequently, a univariate analysis was performed to describe the variables that were associated with the development of an unfavourable outcome. Finally, a multivariate analysis was performed using logistic regression to study the association of the use of glucocorticoids (any dose) and the use of equivalent doses of prednisone greater than or equal to 250 mg with the development of an unfavourable outcome. The multivariate analysis was adjusted to the variables that had shown an association in the univariate analysis. The same analysis was repeated incorporating the propensity indices with the use of glucocorticoids (any dose) or the exposure group with an equivalent dose of prednisone ≥ 250 mg. Statistical analysis was performed with the SPSS version 19.0 software.
Ethical aspectsThe hospital's research committee approved the conduct of the study. The study complies with the ethical guidelines and standards for research as reflected in the World Medical Association's Declaration of Helsinki and the Oviedo Convention on Human Rights and Biomedicine. All data was treated with the utmost confidentiality, in accordance with current legislation.
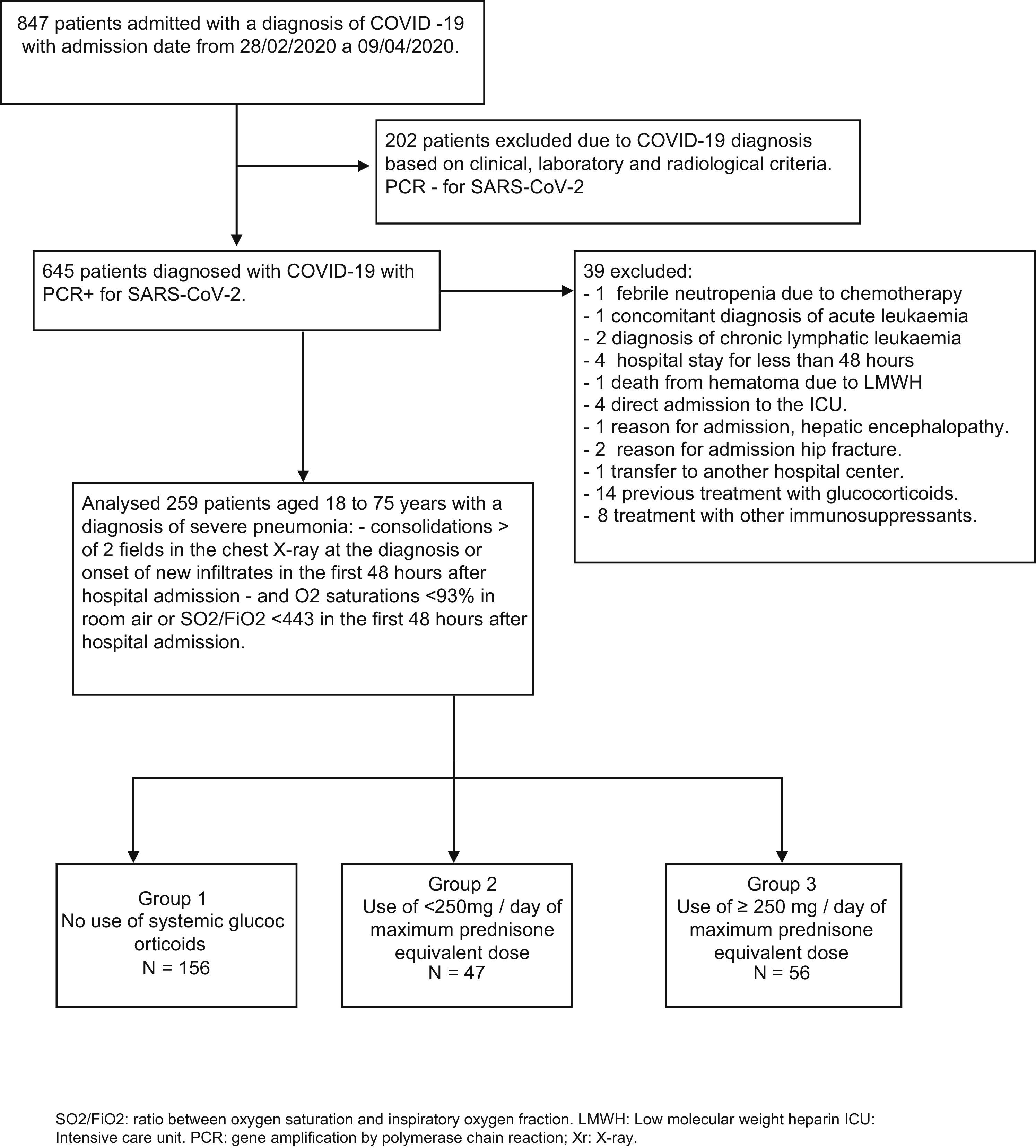
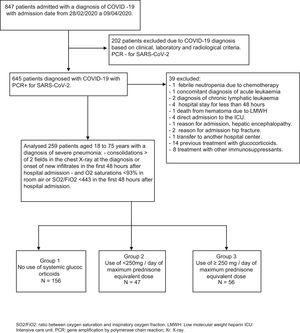
ResultsOf the 847 patients who were admitted to our hospital from 28th February to 9th April 2020 with a diagnosis of coronavirus infection (Fig. 1), 202 patients were initially excluded in whom the diagnosis was based on clinical, laboratory and radiological criteria.
Of the 645 patients in whom the diagnosis of SARS-CoV-2 was confirmed with a gene amplification study by polymerase chain reaction of a nasopharyngeal sample, 39 patients were excluded: one due to admission coinciding with febrile neutropenia due to chemotherapy, another for concomitant diagnosis of acute leukaemia, 2 with a diagnosis of chronic lymphatic leukaemia, 4 for hospital stays shorter than 48 h, one death from hematoma secondary to treatment with low molecular weight heparin (LMWH), 4 direct admissions to the ICU, one admission for hepatic encephalopathy as the main cause, 2 for admission of hip fracture, another for transfer to another center, 14 due to previous glucocorticoid use and 8 due to use of other immunosuppressive treatments.
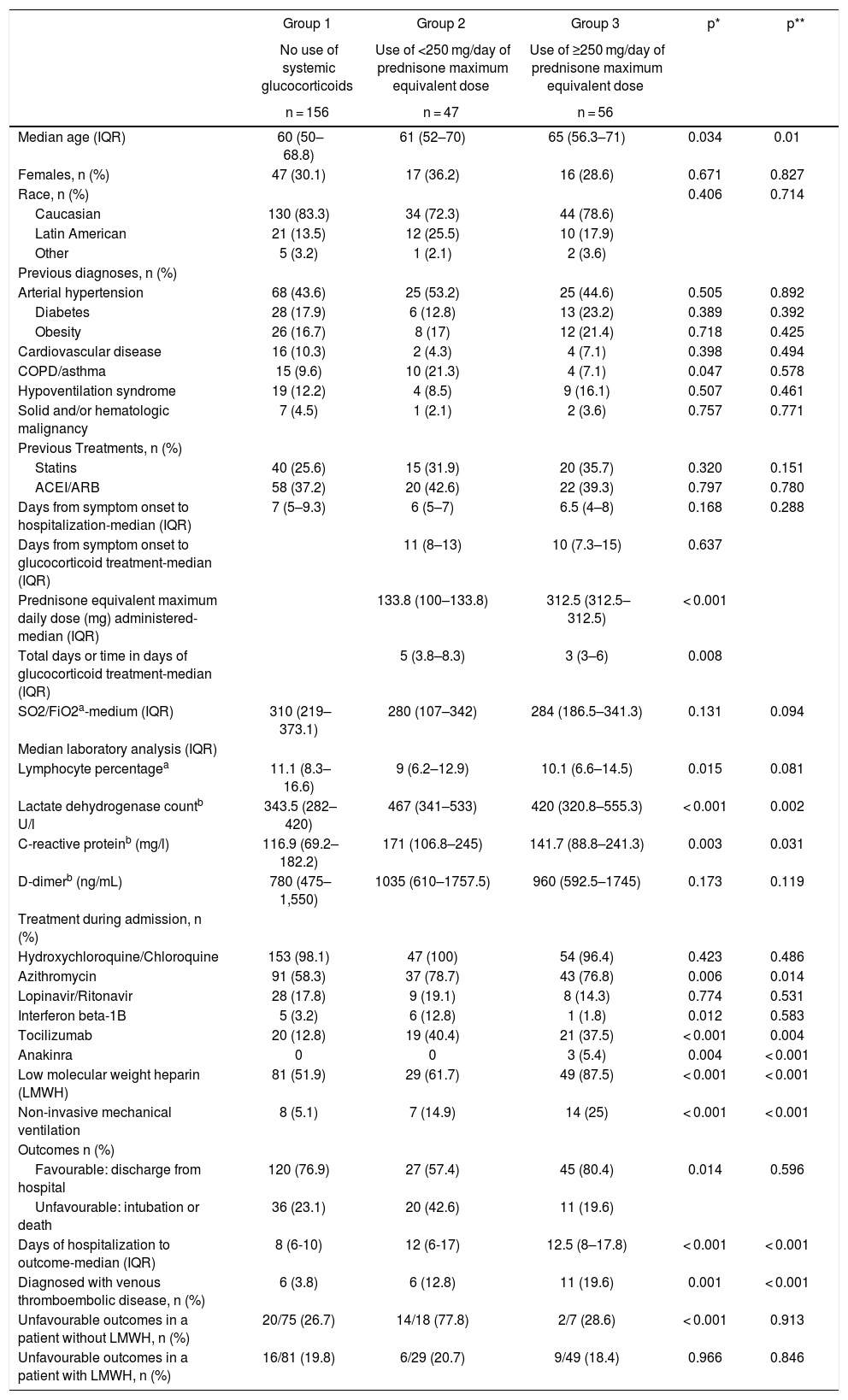
Finally, 259 patients aged 18–75 years with a diagnosis of severe SARS-CoV-2 pneumonia were included, of which 156 did not receive glucocorticoid treatment (group 1), 47 received maximum daily glucocorticoid equivalent doses of less than 250 mg of prednisone (group 2) and 56 received maximum daily glucocorticoid equivalent doses greater than or equal to 250 mg of prednisone (group 3). The characteristics of the patients stratified in different groups are detailed in Table 1. The patients who received glucocorticoids were older, with group 3 being the oldest. There were no differences regarding sex and race in the different groups. Comorbidity was similar, varying only the percentage of asthmatics, which was higher in group 2.
Patient characteristics.
| Group 1 | Group 2 | Group 3 | p* | p** | |
|---|---|---|---|---|---|
| No use of systemic glucocorticoids | Use of <250 mg/day of prednisone maximum equivalent dose | Use of ≥250 mg/day of prednisone maximum equivalent dose | |||
| n = 156 | n = 47 | n = 56 | |||
| Median age (IQR) | 60 (50–68.8) | 61 (52–70) | 65 (56.3–71) | 0.034 | 0.01 |
| Females, n (%) | 47 (30.1) | 17 (36.2) | 16 (28.6) | 0.671 | 0.827 |
| Race, n (%) | 0.406 | 0.714 | |||
| Caucasian | 130 (83.3) | 34 (72.3) | 44 (78.6) | ||
| Latin American | 21 (13.5) | 12 (25.5) | 10 (17.9) | ||
| Other | 5 (3.2) | 1 (2.1) | 2 (3.6) | ||
| Previous diagnoses, n (%) | |||||
| Arterial hypertension | 68 (43.6) | 25 (53.2) | 25 (44.6) | 0.505 | 0.892 |
| Diabetes | 28 (17.9) | 6 (12.8) | 13 (23.2) | 0.389 | 0.392 |
| Obesity | 26 (16.7) | 8 (17) | 12 (21.4) | 0.718 | 0.425 |
| Cardiovascular disease | 16 (10.3) | 2 (4.3) | 4 (7.1) | 0.398 | 0.494 |
| COPD/asthma | 15 (9.6) | 10 (21.3) | 4 (7.1) | 0.047 | 0.578 |
| Hypoventilation syndrome | 19 (12.2) | 4 (8.5) | 9 (16.1) | 0.507 | 0.461 |
| Solid and/or hematologic malignancy | 7 (4.5) | 1 (2.1) | 2 (3.6) | 0.757 | 0.771 |
| Previous Treatments, n (%) | |||||
| Statins | 40 (25.6) | 15 (31.9) | 20 (35.7) | 0.320 | 0.151 |
| ACEI/ARB | 58 (37.2) | 20 (42.6) | 22 (39.3) | 0.797 | 0.780 |
| Days from symptom onset to hospitalization-median (IQR) | 7 (5–9.3) | 6 (5–7) | 6.5 (4–8) | 0.168 | 0.288 |
| Days from symptom onset to glucocorticoid treatment-median (IQR) | 11 (8–13) | 10 (7.3–15) | 0.637 | ||
| Prednisone equivalent maximum daily dose (mg) administered-median (IQR) | 133.8 (100–133.8) | 312.5 (312.5–312.5) | < 0.001 | ||
| Total days or time in days of glucocorticoid treatment-median (IQR) | 5 (3.8–8.3) | 3 (3–6) | 0.008 | ||
| SO2/FiO2a-medium (IQR) | 310 (219–373.1) | 280 (107–342) | 284 (186.5–341.3) | 0.131 | 0.094 |
| Median laboratory analysis (IQR) | |||||
| Lymphocyte percentagea | 11.1 (8.3–16.6) | 9 (6.2–12.9) | 10.1 (6.6–14.5) | 0.015 | 0.081 |
| Lactate dehydrogenase countb U/l | 343.5 (282–420) | 467 (341–533) | 420 (320.8–555.3) | < 0.001 | 0.002 |
| C-reactive proteinb (mg/l) | 116.9 (69.2–182.2) | 171 (106.8–245) | 141.7 (88.8–241.3) | 0.003 | 0.031 |
| D-dimerb (ng/mL) | 780 (475–1,550) | 1035 (610–1757.5) | 960 (592.5–1745) | 0.173 | 0.119 |
| Treatment during admission, n (%) | |||||
| Hydroxychloroquine/Chloroquine | 153 (98.1) | 47 (100) | 54 (96.4) | 0.423 | 0.486 |
| Azithromycin | 91 (58.3) | 37 (78.7) | 43 (76.8) | 0.006 | 0.014 |
| Lopinavir/Ritonavir | 28 (17.8) | 9 (19.1) | 8 (14.3) | 0.774 | 0.531 |
| Interferon beta-1B | 5 (3.2) | 6 (12.8) | 1 (1.8) | 0.012 | 0.583 |
| Tocilizumab | 20 (12.8) | 19 (40.4) | 21 (37.5) | < 0.001 | 0.004 |
| Anakinra | 0 | 0 | 3 (5.4) | 0.004 | < 0.001 |
| Low molecular weight heparin (LMWH) | 81 (51.9) | 29 (61.7) | 49 (87.5) | < 0.001 | < 0.001 |
| Non-invasive mechanical ventilation | 8 (5.1) | 7 (14.9) | 14 (25) | < 0.001 | < 0.001 |
| Outcomes n (%) | |||||
| Favourable: discharge from hospital | 120 (76.9) | 27 (57.4) | 45 (80.4) | 0.014 | 0.596 |
| Unfavourable: intubation or death | 36 (23.1) | 20 (42.6) | 11 (19.6) | ||
| Days of hospitalization to outcome-median (IQR) | 8 (6-10) | 12 (6-17) | 12.5 (8–17.8) | < 0.001 | < 0.001 |
| Diagnosed with venous thromboembolic disease, n (%) | 6 (3.8) | 6 (12.8) | 11 (19.6) | 0.001 | < 0.001 |
| Unfavourable outcomes in a patient without LMWH, n (%) | 20/75 (26.7) | 14/18 (77.8) | 2/7 (28.6) | < 0.001 | 0.913 |
| Unfavourable outcomes in a patient with LMWH, n (%) | 16/81 (19.8) | 6/29 (20.7) | 9/49 (18.4) | 0.966 | 0.846 |
IQR: interquartile range; cardiovascular disease: chronic atrial fibrillation and/or ischemic heart disease and/or heart failure; SO2/FiO2: ratio between oxygen saturation and inspiratory oxygen fraction.
Group 2 and 3 patients had lower SO2/FiO2 and lymphocyte percentages compared to group 1, with higher LDH, CRP and D-dimer values. There were no significant differences in the values of these variables between patients in groups 2 and 3. There were differences regarding the various treatments used during admission in each of the groups, being statistically significant in the treatments with LMWH, azithromycin, tocilizumab, interferon beta-1B and anakinra, and in the use of non-invasive mechanical ventilation (Table 1). Regarding glucocorticoid treatment, it was longer in group 2 patients. The median and IQR of the total treatment time with glucocorticoids in group 3 was 3 (3–6) days. A total of 21 patients (37.5%) in group 3 received other glucocorticoid regimens apart from glucocorticoid boluses. The median and IQR of equivalent dose of maximum daily prednisone administered in group 3 was 312.5 mg (312.5–312.5), vs 133.8 mg (100-133.8) from group 2 (Table 1).
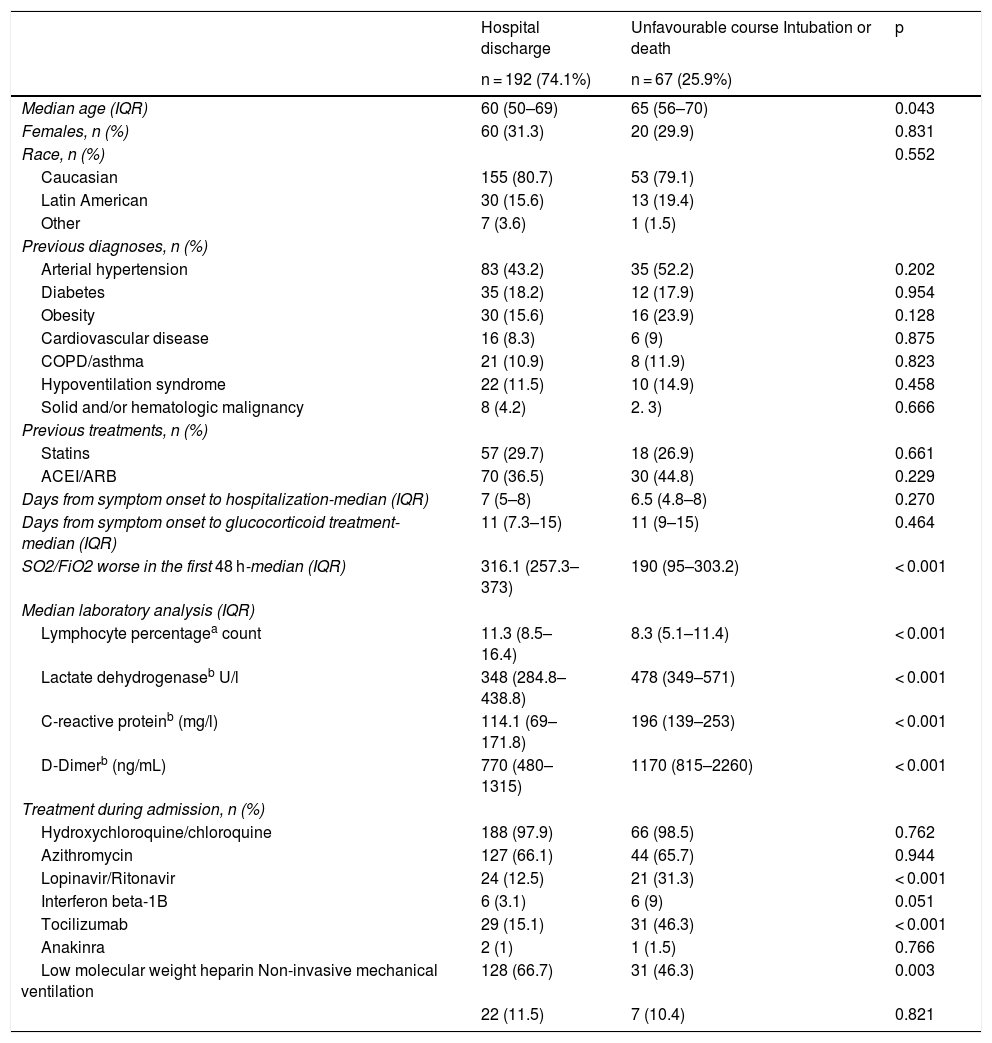
Of the 259 patients enrolled in the study, 67 (25.9%) had a course defined as unfavourable. They had lower SO2/FiO2 and lymphocyte percentages and higher D-dimer, LDH and CRP values (Table 2). In addition, these patients received more treatment with lopinavir-ritonavir, interferon beta 1-B, and tocilizumab than patients with a favourable outcome. On the other hand, patients with an unfavourable course were prescribed LMWH less often.
Factors associated with unfavourable course (admission to ICU or death).
| Hospital discharge | Unfavourable course Intubation or death | p | |
|---|---|---|---|
| n = 192 (74.1%) | n = 67 (25.9%) | ||
| Median age (IQR) | 60 (50–69) | 65 (56–70) | 0.043 |
| Females, n (%) | 60 (31.3) | 20 (29.9) | 0.831 |
| Race, n (%) | 0.552 | ||
| Caucasian | 155 (80.7) | 53 (79.1) | |
| Latin American | 30 (15.6) | 13 (19.4) | |
| Other | 7 (3.6) | 1 (1.5) | |
| Previous diagnoses, n (%) | |||
| Arterial hypertension | 83 (43.2) | 35 (52.2) | 0.202 |
| Diabetes | 35 (18.2) | 12 (17.9) | 0.954 |
| Obesity | 30 (15.6) | 16 (23.9) | 0.128 |
| Cardiovascular disease | 16 (8.3) | 6 (9) | 0.875 |
| COPD/asthma | 21 (10.9) | 8 (11.9) | 0.823 |
| Hypoventilation syndrome | 22 (11.5) | 10 (14.9) | 0.458 |
| Solid and/or hematologic malignancy | 8 (4.2) | 2. 3) | 0.666 |
| Previous treatments, n (%) | |||
| Statins | 57 (29.7) | 18 (26.9) | 0.661 |
| ACEI/ARB | 70 (36.5) | 30 (44.8) | 0.229 |
| Days from symptom onset to hospitalization-median (IQR) | 7 (5–8) | 6.5 (4.8–8) | 0.270 |
| Days from symptom onset to glucocorticoid treatment-median (IQR) | 11 (7.3–15) | 11 (9–15) | 0.464 |
| SO2/FiO2 worse in the first 48 h-median (IQR) | 316.1 (257.3–373) | 190 (95–303.2) | < 0.001 |
| Median laboratory analysis (IQR) | |||
| Lymphocyte percentagea count | 11.3 (8.5–16.4) | 8.3 (5.1–11.4) | < 0.001 |
| Lactate dehydrogenaseb U/l | 348 (284.8–438.8) | 478 (349–571) | < 0.001 |
| C-reactive proteinb (mg/l) | 114.1 (69–171.8) | 196 (139–253) | < 0.001 |
| D-Dimerb (ng/mL) | 770 (480–1315) | 1170 (815–2260) | < 0.001 |
| Treatment during admission, n (%) | |||
| Hydroxychloroquine/chloroquine | 188 (97.9) | 66 (98.5) | 0.762 |
| Azithromycin | 127 (66.1) | 44 (65.7) | 0.944 |
| Lopinavir/Ritonavir | 24 (12.5) | 21 (31.3) | < 0.001 |
| Interferon beta-1B | 6 (3.1) | 6 (9) | 0.051 |
| Tocilizumab | 29 (15.1) | 31 (46.3) | < 0.001 |
| Anakinra | 2 (1) | 1 (1.5) | 0.766 |
| Low molecular weight heparin Non-invasive mechanical ventilation | 128 (66.7) | 31 (46.3) | 0.003 |
| 22 (11.5) | 7 (10.4) | 0.821 |
Cardiovascular disease: chronic atrial fibrillation and/or ischemic heart disease and/or heart failure; IQR: interquartile range; SO2/FiO2: ratio between oxygen saturation and inspiratory oxygen fraction.
Regarding the use of glucocorticoids and the association with an unfavourable course, the crude analysis did not find significant differences between the use of high doses of glucocorticoids and not receiving glucocorticoids, although there were significant differences with respect to the group of patients treated with lower glucocorticoids doses, the latter group showing an unfavourable course of up to 42.6% (Table 1).
The multivariate analysis (Table 3) demonstrated a beneficial effect of the use of high doses of glucocorticoids (group 3) compared to patients not treated with glucocorticoids and patients treated with lower doses of corticosteroids (group 2). This association was statistically significant, although statistical significance was lost when only compared with the group that had not received corticosteroid treatment and adjusted with the propensity index as a covariate.
Comparative analyses between different treatments with or without glucocorticoids and the association with unfavourable outcomes (admission to the ICU or death).
| Analysis type | Number of unfavourable outcomes by number of patients | Crude analysis | Multivariate analysis* | Multivariate analysis adjusted for propensity index** | |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| 1 | Glucocorticoid therapy (any dose) versus no glucocorticoid therapy | 31/103 (30.1%) | 1.44 (0.82–2.52) | 0.69 (0.30–1.59) | 0.71 (0.30–1.66) |
| 36/156 (23.1%) | p = 0.207 | p = 0.380 | p = 0.432 | ||
| 2 | Glucocorticoid treatment≥ 250 mg prednisone per day) versus no glucocorticoid treatment | 11/56 (19.6%) | 0.82 (0.38–1.74) | 0.31 (0.10–0.97) | 0.35 (0.11–1.08) |
| 36/156 (23.1%) | p = 0.596 | p = 0.044 | p = 0.067 | ||
| 3 | Glucocorticoid treatment (≥ 250 mg prednisone per day) versus other patientsa | 11/56 (19.6%) | 0.64 (0.31–1.33) | 0.30 (0.11–0.87) | 0.30 (0.10–0.88) |
| 56/203 (27.6%) | p = 0.229 | p = 0.026 | p = 0.028 |
Adjusted for age, ratio between oxygen saturation and inspiratory oxygen fraction (SO2/FiO2) worse in the first 48 h, percentage of lymphocytes lower in the first 48 h, highest CRP in the first 48 h, highest LDH in first 48 h, lopinavir/ritonavir treatment, tocilizumab treatment and low molecular weight heparin treatment.
Multivariate analysis adjusted to the same variables described above plus the propensity index of each glucocorticoid regimen specified in each type of analysis. The analysis to calculate the propensity index included the variables: age, lowest SO2/FiO2 in the first 48 h, lower percentage of lymphocytes in the first 48 h, highest CRP values in the first 48 h, highest LDH values in the first 48 h, azithromycin treatment, interferon beta 1B treatment (except in Test 2), tocilizumab treatment, anakinra treatment, low molecular weight heparin treatment, use of non-invasive ventilation and history of asthma (except in analysis 2).
Six patients were not included in the multivariate analysis because they did not have LDH values. There were 6 patients who had not received glucocorticoids and who had a favourable course. The D-dimer variable was also not included, as it had not been collected in 14 patients. There was a correlation between D-dimer values and the percentage of lymphocytes (Pearson's correlation index of –0.130, with a p = 0.042) and CRP values (Pearson's correlation index of 0.200, with a value of p = 0.002).
DiscussionThe results of this study suggest that patients with severe SARS-CoV-2 pneumonia treated with glucocorticoid pulses, with prednisone equivalent doses greater than or equal to 250 mg, have a more favourable course than patients treated with lower doses of glucocorticoids, or than those who did not receive glucocorticoid treatment (Table 3). This favourable course was not observed in the crude analysis but was observed when adjusted for other variables such as those associated with more severity such as age, SO2/FiO2, lymphocyte percentage, D-dimer, LDH and CRP. In the same vein as the results of our study are those reported by Calleja et al.13, who analysed the course of patients treated with high doses of glucocorticoids (>250 mg of methylprednisolone) associated or not with tocilizumab, finding a trend towards a decrease in deaths and intubation when both drugs were combined.
We have not found this favourable course with lower glucocorticoid doses, as has been shown in other studies. The results of the RECOVERY clinical trial have recently been reported14, which compared treatment with dexamethasone doses of 6 mg per day, for 10 days, to the standard treatment, showing a decrease in mortality at 28 days (21.5% vs. 25%). This was more evident in patients undergoing invasive mechanical ventilation (29% vs. 40.7%).
A possible explanation for the lack of benefit derived from the use of glucocorticoids with lower doses in our study could be due to the characteristics of these patients, as this group had a lower percentage of LMWH use (Table 1), especially considering that up to almost 78% of the patients without LMWH in this group had an unfavourable course (Table 1). This fact could mean an important role for undiagnosed or untreated thromboembolic disease as a cause of unfavourable course in this group 18–22. In this sense, it is worth wondering whether using lower doses of glucocorticoids could have a lower anti-inflammatory effect and therefore, further favour the development of thromboembolic disease, especially in this group of patients who had worse severity markers (SO2/FiO2 and percentage of lower lymphocytes and higher D-dimer, CRP and LDH values). One hypothesis is that prednisone doses lower than 250 mg would not fully activate the non-genomic17 pathway of glucocorticoid action, acting only on the genomic pathway, and may diminish its effect on the cytokine storm. On the other hand, it should be taken into account that the non-genomic mechanism of action is faster and could have other effects, such as inhibition of platelet aggregation23, which could influence the course of patients with severe SARS-CoV-2 pneumonia.
Group 1 (patients not treated with glucocorticoids) had the lowest percentage of treatment with LMWH, although these patients were less severe (higher SO2/FiO2 and percentage of lymphocytes and lower D-dimer, CRP and LDH values). The absence of LMWH treatment is probably less relevant in this group, since 26.7% of the patients without LMWH treatment in this group had an unfavourable course, compared to 77.8% of group 2 and 28, 6% of group 3 (Table 1). The fact that the first patients treated for COVID-19 in our hospital were included in the study could explain the high percentage of patients without LMWH treatment, since LMWH treatment in these patients was not standardised at that time.
On the other hand, it should be noted that the patients treated with glucocorticoids in our study had a longer hospital stay than the patients not treated with them, a result that differs from other publications, which demonstrated benefits with these drugs. We interpret this as a marker of higher severity in these patients, as well as the lower SO2/FiO2 value and percentage of lymphocytes or higher values of D-dimer, LDH and CRP, and even the higher use of non-invasive mechanical ventilation in these patients. One hypothesis would be that, as the use of glucocorticoids was not standardised, the most severe patients received glucocorticoid treatment even later than many authors recommend. Fadel et al. demonstrated a reduction in hospital stay in patients treated with glucocorticoids when they were administered early8.
The limitations of our work, apart from being a single-center study, are those inherent in retrospective cohort studies, when comparing groups that are not totally homogeneous. To try to minimize these biases, the model was adjusted to the indices of propensity to receive glucocorticoid treatment. As previously mentioned, patients treated with glucocorticoids had higher severity markers. The adjustment was carried out using the propensity index as a covariate in the logistic regression model for the development of unfavourable outcomes. We did not use other methods such as matching or stratification for the small sample size of our study. However, the key limitation of the propensity index adjustment is that it only adjusts for recorded variables. In this sense, there are variables not recorded in our data that could influence the time of prescribing glucocorticoids, such as the course of dyspnoea during admission or of SO2/FiO2 or chest X-ray 48 h after admission, or other variables that are difficult to record, such as the subjective assessment of the doctor.
This study points to a beneficial effect of glucocorticoids at equivalent doses of prednisone > 250 mg in patients with severe SARS-CoV-2 pneumonia, but more clinical trials are needed to study the effect of glucocorticoids in these patients, determining both the ideal glucocorticoid dose and the most appropriate starting time. At the time of writing, many clinical trials are underway to assess the effect of other immunomodulators in patients with severe COVID-19, but it should not be forgotten that these drugs are more expensive than glucocorticoids and may even face supply problems in the event of increased demand in further waves of the current pandemic.
Conflict of interestsThe authors declare that they have no conflict of interest directly or indirectly related to the contents of the manuscript.
D. Arranz, R. Beltrán, R. Cadenas, C. Casanova, J.A. de Boer, R. del Valle, G. García, B. Iglesias, A. Lung, O. Madridano, M. Martín, M. Merino, M. Moral, S. Muñoz, M. Neira, B. Núñez, I. Perales, A. Pérez, M. Reche, T. Reinoso, J.M. Rizo, C. Sánchez, M. Sereno and T. Valbuena.
The rest of the members of the HUIS-COVID-19 Working Group (including the main authors of the manuscript) are listed in Appendix A at the end of the article.
Please cite this article as: Pascual Pareja JF, García-Caballero R, Soler Rangel L, Vázquez-Ronda MA, Roa Franco S, Navarro Jiménez G, et al. Efectividad de los glucocorticoides en pacientes hospitalizados por neumonía grave por SARS-CoV-2. Med Clin (Barc). 2021;156:221–228.