Evaluate the relationship between the presence of polymorphisms in genes involved in the pharmacodynamics of irinotecan (UGT1A, SLCO1B1, ABCB1 and ABCC2) and the safety of irinotecan in the treatment of metastatic colorectal cancer (mCRC).
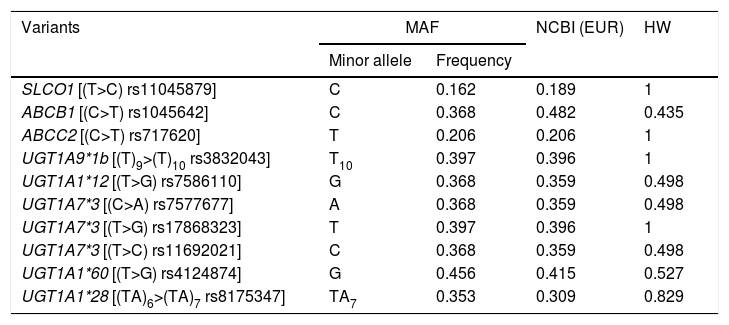
Patients and methodsProspective observational, single-centre study of 30 months duration, which included patients diagnosed with mCRC treated with FOLFIRI was carried out. Toxicity was evaluated in each treatment cycle according to the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0 NCI. Genomic DNA was obtained with a peripheral blood sample from an extraction method based on alkaline lysis. Genetic characterisation was performed using the LigthCycler®480 platform and allele-specific HybProbe® fluorescent probes. Analysed polymorphisms were: UGT1A1*28, UGT1A1*60, UGT1A7*1,*2,*3,*4, UGT1A7*12, UGT1A9*22, SLCO1B1 (rs11045879), ABCC2 (rs717620) and ABCB1 (rs1045642).
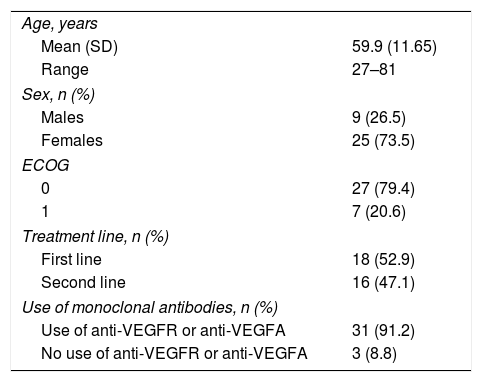
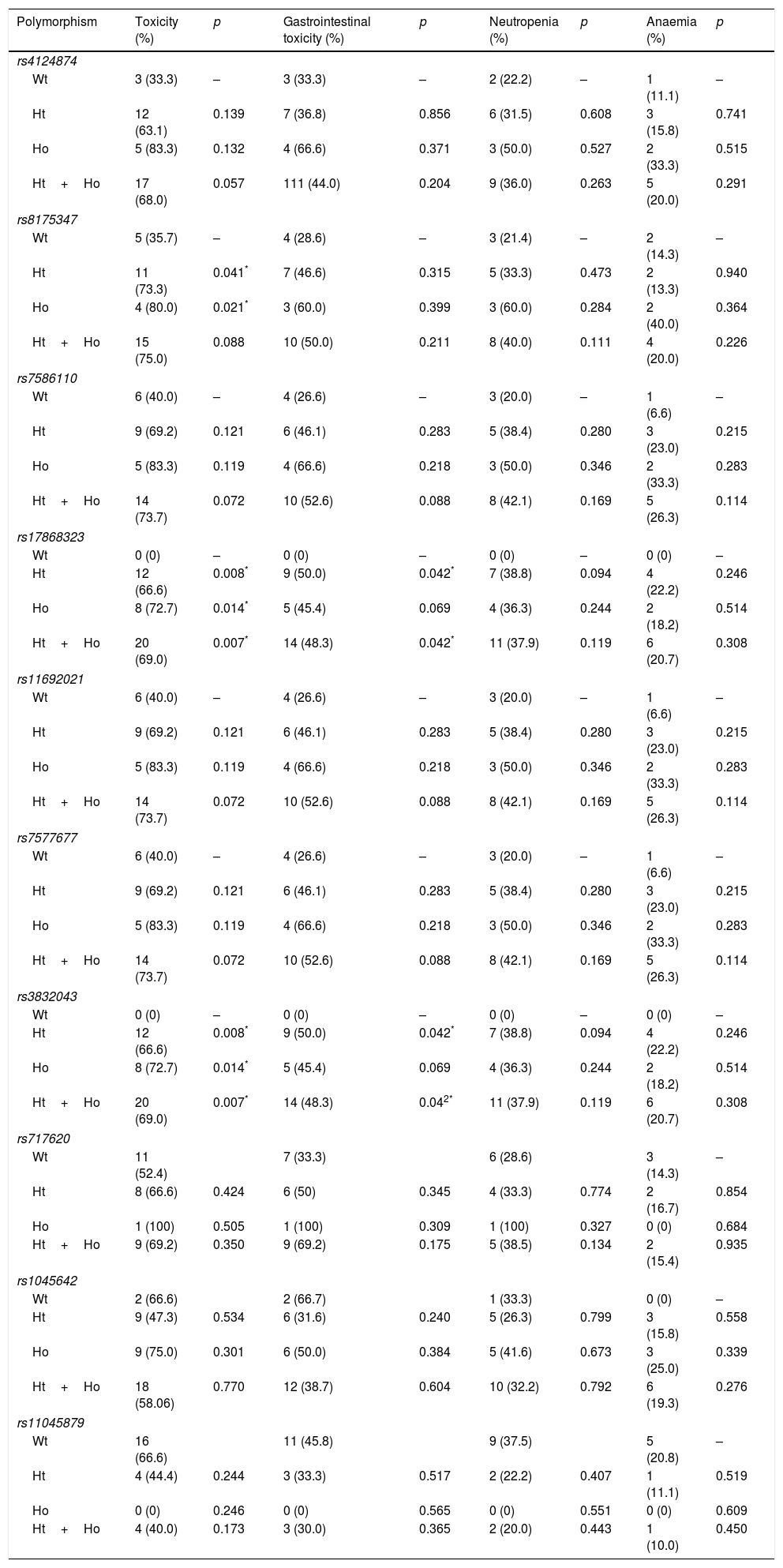
ResultsThirty-four patients were included (73.5% were male, mean age 59.9 years [27–81]) in the study. Polymorphisms rs8175347, rs17868323, rs3832043, rs11692021 and rs7577677 were associated with a higher incidence of adverse effects. Furthermore, it was observed that those patients with wild-type in UGT family genes analysed have lower rates of toxicity associated with irinotecan treatment than those with certain mutated allele (p=0.010).
ConclusionsThese results suggest that the presence of certain polymorphisms in the UGT1A family of genes is related to the development of toxicity during treatment with irinotecan.
Evaluar la relación entre la presencia de polimorfismos en los genes implicados en la farmacodinamia del irinotecán (UGT1A, SLCO1B1, ABCB1 y ABCC2) y la seguridad asociada al mismo en el tratamiento del cáncer colorrectal metastásico (CCRm)
Pacientes y métodosEstudio prospectivo observacional y unicéntrico de 30 meses de duración, en el que se incluyeron los pacientes diagnosticados de CCRm tratados con el esquema FOLFIRI. La toxicidad fue evaluada en cada ciclo de tratamiento según la Common Terminology Criteria for Adverse Events (CTCAE) v.4.0 NCI. La obtención del ADN genómico se realizó mediante una muestra de sangre periférica a partir de un método de extracción basado en lisis alcalina. La caracterización genética se realizó empleando la plataforma LigthCycler®480 y sondas fluorescentes HybProbe® específicas de alelo. Los polimorfismos analizados fueron: UGT1A1*28, UGT1A1*60, UGT1A7*1,*2,*3,*4, UGT1A7*12, UGT1A9*22, SLCO1B1 (rs11045879), ABCC2 (rs717620) y ABCB1 (rs1045642).
ResultadosFueron incluidos 34 pacientes (el 73,5% eran hombres, con una edad media de 59,9 años [27-81]). Los polimorfismos: rs8175347, rs17868323, rs3832043, rs11692021 y rs7577677 se relacionaron con una mayor incidencia de efectos adversos. Por otro lado, se observó que aquellos pacientes wild-type, en la serie de genes de la familia UGT analizada, presentan unas menores tasas de toxicidad asociada al tratamiento con irinotecán que aquellos que poseen alguno de los polimorfismos analizados (p=0,010).
ConclusionesEstos resultados sugieren que la presencia de determinados polimorfismos en la familia de genes UGT1A se encuentra relacionada con el desarrollo de toxicidad en el tratamiento con irinotecán en dosis para el esquema FOLFIRI.