Oral antidiabetic inhibitors of the sodium-glucose cotransporter (SGLT2i) reduce cardiovascular morbidity and mortality in DM2. The increase in arterial stiffness can participate in this morbidity and mortality. The aim of this study was to analyse the effect of the administration of dapagliflozin on arterial stiffness.
Patients and methodsProspective observational study that included 32 patients with DM2. Before starting dapagliflozin, and at 6 and 12 months, biochemical parameters in blood and urine were analysed. Before starting dapagliflozin and at 12 months the velocity of the carotid-femoral pulse (VPc-f) was determined by tonometry. Changes in the variables and their interrelation was analysed by repeated data ANOVA, Wilcoxon's test and multiple regression.
ResultsA significant decrease in the VPc-f was observed. There was no association between decreased VPc-f and changes in blood glucose, uric acid, blood pressure or weight.
ConclusionsDapagliflozin, in subjects with DM2, produces a medium to long-term decrease in arterial stiffness.
Los antidiabéticos orales inhibidores del cotransportador sodio-glucosa (iSGLT2) reducen la morbimortalidad cardiovascular en la DM2. El aumento de la rigidez arterial puede participar en esta morbimortalidad. El objetivo de este trabajo fue analizar el efecto de la administración de dapagliflozina en la rigidez arterial.
Pacientes y métodosEstudio observacional, prospectivo que incluyó 32 pacientes con DM2. Antes del inicio de dapagliflozina y a los 6 y 12 meses se analizaron parámetros bioquímicos en sangre y orina. Basalmente y a los 12 meses se determinó la velocidad de pulso carótida-femoral (VPc-f) mediante tonometría. El análisis de los cambios en las variables y su interrelación se hizo mediante ANOVA de datos repetidos, test de Wilconson y regresión múltiple.
ResultadosSe objetivo un descenso significativo de la VPc-f. No se evidenció asociación entre descenso de VPc-f y cambios de la glucemia, uricemia, presión arterial ni del peso.
ConclusionesDapagliflozina, en sujetos con DM2, produce a medio-largo plazo, una disminución de la rigidez arterial.
Type 2 diabetes mellitus (DM2) has a high incidence of cardiovascular (CV) morbidity and mortality.1 The increase in arterial stiffness (AS) observed in DM2 may contribute to this fact.2 The new anti-diabetic sodium glucose cotransporter type 2 (SGLT2i) inhibitor drugs reduce total mortality and CV morbidity and mortality in subjects with DM2.3 The CV benefits of the SGLT2i derive from the reduction of blood glucose, weight and blood pressure (BP), the increase in natriuresis, the preservation of renal function and possible direct CV effects.4 A decrease in AS promoted by the SGLT2i has also been suggested as an additional mechanism.4 However, there are no studies that analyse the long-term effect of SGLT2i on AS in patients with DM2. The purpose of this research was to study, prospectively, the long-term effect of an SGLT2i, dapagliflozin, on the carotid-femoral pulse wave velocity (c-fPWV), a standard measurement of AS in subjects with DM2.
Patients and methodsStudy design and populationObservational, prospective, longitudinal study. 32 subjects diagnosed with DM2 in whom treatment with dapagliflozin 10mg/day was indicated according to routine clinical practice were included. All patients gave informed consent to participate.
VariablesBlood samples were obtained before the start, at 6 months and at 12 months of treatment with dapagliflozin for routine biochemical and haematological determinations, and 24h urine and first morning urine for creatinine, glucose, sodium, uric acid and albumin determination. Glomerular filtration rate (GFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. Diabetic nephropathy was diagnosed due to a decreased GFR (<60ml/min/1.73m2 and/or albuminuria (albumin/creatinine in urine≥300mg/g) and exclusion of other causes of nephropathy.
BP measurement was carried out on each of the visits with an Omron M1 (Omron Healthcare Co. Ltd, Kyoto, Japan), following the recommendations of the European hypertension society.
Prior to dapagliflozin therapy and 12 months after treatment, c-fPWV was determined using the SphygmoCor Xcel (AtCor Medical Ply Ltd Suite 11, 1059-1063 Victoria Road West Ryde, NSW 2114 Australia). The carotid pulse wave was measured by applanation tonometry (high fidelity micromanometer [Millar Instrument]) and, simultaneously, the femoral pulse wave was measured with an inflated cuff over the femoral artery. The c-fPWV was calculated as the ratio between the corrected distance between the pulse wave measurement sites and the delay time between the carotid and femoral pulse waves. The average of 2 high quality measurements was considered as a valid result.
Statistical analysisThe results are expressed as mean±standard deviation or as median and interquartile range, depending on the distribution assessed by the Shapiro–Wilk test. Categorical variables are expressed as frequencies. The analysis of the differences between the baseline values and those at 6 and 12 months was performed using the repeated measures ANOVA. The comparison between baseline c-fPWV values and at 12 months was performed with the Wilcoxon test. The study of the participation of variables potentially affecting the c-fPWV was carried out through multiple regression. The statistical analysis was performed with SPSS 22 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). Values of p<0.05 were considered significant.
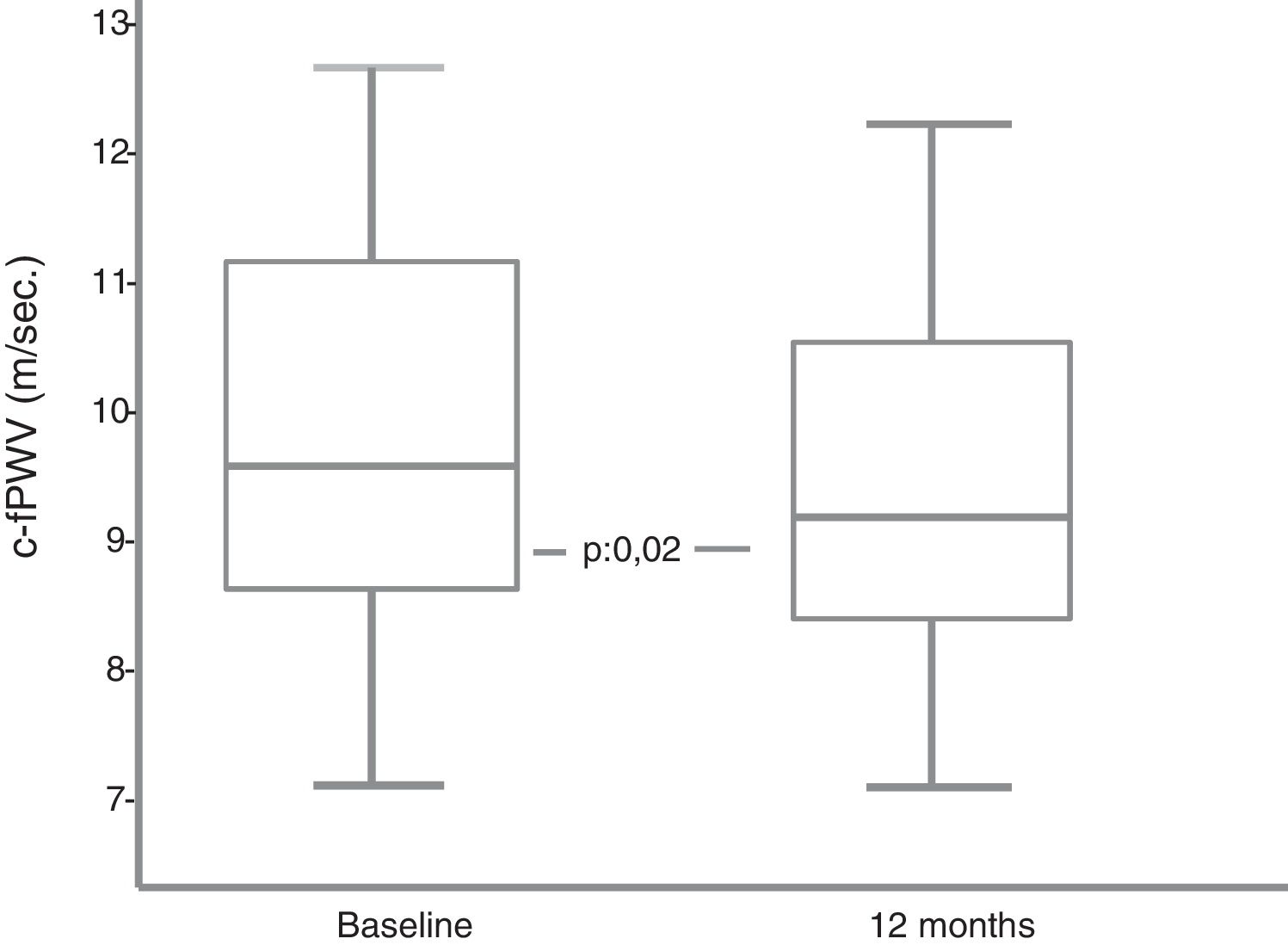
ResultsOf the 32 patients who started the study, 4 (2 men and 2 women) discontinued the study before 6 months (2 for genital mycosis, one for dyspepsia and one lost to follow-up). Compared to the rest of the patients who completed the follow-up, those 4 patients were older (66.5±10 vs. 53.9±8.7 years), their DM was longer (17.5±6 vs. 13±7.5 years), lower GFR (81.5±21 vs. 95.6±17ml/min/1.73m2), higher c-fPWV (11.7±2.3 vs. 9.8±1.4m/s) and similar HbA1c (8.7±1.2 vs. 8.6±1.3%).
The age was 53.9±8.7 years, 61% male. DM duration was 13±7.5 years. 14% had a history of CV disease, 18% of retinopathy and 32% of diabetic nephropathy. 96% took oral hypoglycaemic agents (metformin 89%; sulfonylureas [SU] 14%; dipeptidyldipeptidase inhibitors [DPP4i]) 50%; glinides 10%; thiazolidinediones 3.6%). 14% received GLP-1 (glucagon-like peptide-1) receptor agonists treatment and 64% were treated with insulin glargine. During follow-up, the treatment with SU was suspended in 3 patients, treatment with GLP-1 agonists in one and the insulin was reduced/suspended in 2 cases. The initial weight was 93.7±17.6kg, observing a significant decrease at 6 and 12 months of dapagliflozin treatment (91.4±18kg, p=0.000 and 92.0±18.4kg, p<0.007, respectively). Systolic blood pressure (SBP) at 6 months (136.5 [19] mmHg) (median [interquartile range]) and at 12 months (136.5 [22] mmHg) was lower than SBP prior to the start of dapagliflozin (144.5 [29]) (p=0.000). A significant decrease in diastolic BP (DBP) was also observed at 6 and 12 months (78 [10] and 77 [12] mmHg vs. 83.5 [11] mmHg, respectively, p=0.001). No significant changes in heart rate were observed.
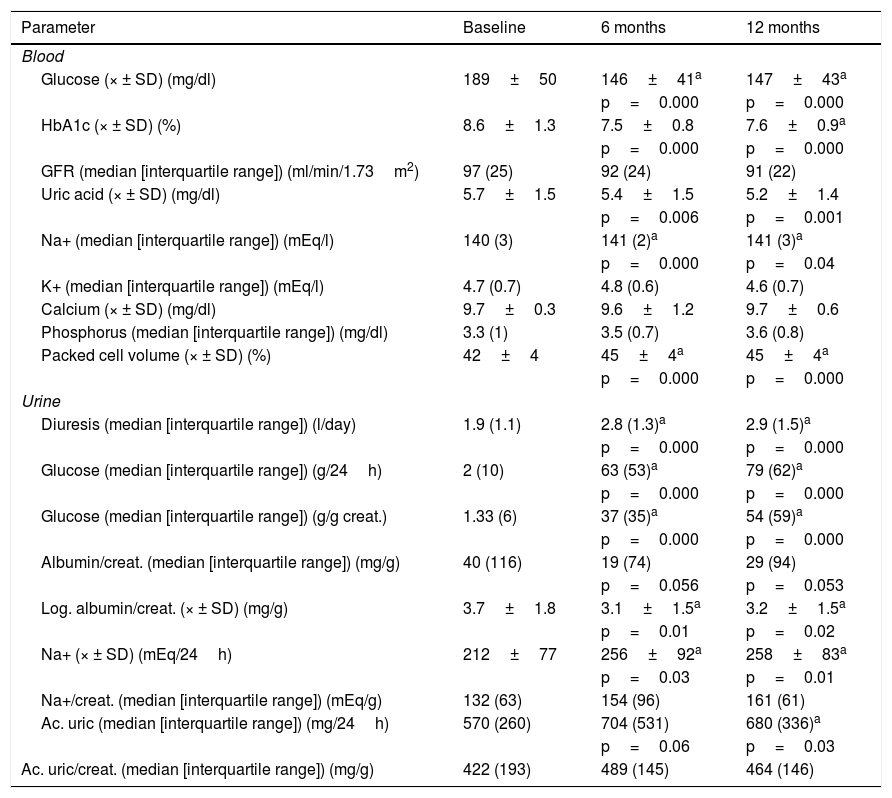
Changes of haematological and biochemical parameters in blood and urine are shown in Table 1.
Values of haematological and biochemical parameters in baseline blood and urine, at 6 and 12 months.
| Parameter | Baseline | 6 months | 12 months |
|---|---|---|---|
| Blood | |||
| Glucose (× ± SD) (mg/dl) | 189±50 | 146±41a | 147±43a |
| p=0.000 | p=0.000 | ||
| HbA1c (× ± SD) (%) | 8.6±1.3 | 7.5±0.8 | 7.6±0.9a |
| p=0.000 | p=0.000 | ||
| GFR (median [interquartile range]) (ml/min/1.73m2) | 97 (25) | 92 (24) | 91 (22) |
| Uric acid (× ± SD) (mg/dl) | 5.7±1.5 | 5.4±1.5 | 5.2±1.4 |
| p=0.006 | p=0.001 | ||
| Na+ (median [interquartile range]) (mEq/l) | 140 (3) | 141 (2)a | 141 (3)a |
| p=0.000 | p=0.04 | ||
| K+ (median [interquartile range]) (mEq/l) | 4.7 (0.7) | 4.8 (0.6) | 4.6 (0.7) |
| Calcium (× ± SD) (mg/dl) | 9.7±0.3 | 9.6±1.2 | 9.7±0.6 |
| Phosphorus (median [interquartile range]) (mg/dl) | 3.3 (1) | 3.5 (0.7) | 3.6 (0.8) |
| Packed cell volume (× ± SD) (%) | 42±4 | 45±4a | 45±4a |
| p=0.000 | p=0.000 | ||
| Urine | |||
| Diuresis (median [interquartile range]) (l/day) | 1.9 (1.1) | 2.8 (1.3)a | 2.9 (1.5)a |
| p=0.000 | p=0.000 | ||
| Glucose (median [interquartile range]) (g/24h) | 2 (10) | 63 (53)a | 79 (62)a |
| p=0.000 | p=0.000 | ||
| Glucose (median [interquartile range]) (g/g creat.) | 1.33 (6) | 37 (35)a | 54 (59)a |
| p=0.000 | p=0.000 | ||
| Albumin/creat. (median [interquartile range]) (mg/g) | 40 (116) | 19 (74) | 29 (94) |
| p=0.056 | p=0.053 | ||
| Log. albumin/creat. (× ± SD) (mg/g) | 3.7±1.8 | 3.1±1.5a | 3.2±1.5a |
| p=0.01 | p=0.02 | ||
| Na+ (× ± SD) (mEq/24h) | 212±77 | 256±92a | 258±83a |
| p=0.03 | p=0.01 | ||
| Na+/creat. (median [interquartile range]) (mEq/g) | 132 (63) | 154 (96) | 161 (61) |
| Ac. uric (median [interquartile range]) (mg/24h) | 570 (260) | 704 (531) | 680 (336)a |
| p=0.06 | p=0.03 | ||
| Ac. uric/creat. (median [interquartile range]) (mg/g) | 422 (193) | 489 (145) | 464 (146) |
creat.: creatinine; eGFR: estimated glomerular filtration rate.
42.8% of the patients had high c-fPWV baseline values (≥10m/s). Among the 9 patients with diabetic nephropathy, 44% had high c-fPWV values. This percentage was reduced to 37% among those without nephropathy. The c-fPWV decreased significantly at 12 months of treatment (9.1 [8.4–10.1] vs. 9.65 [8.75–11.2] m/s) (p=0.02) (Fig. 1). The percentage variation was quantitatively but not significantly higher in patients with high baseline c-fPWV values (–7.8% [–3- –19%] vs. –2.8% [2- –9%]). Neither weight loss, BP, HbA1c, changes in GFR nor uricemia showed a significant participation in the changes of the c-fPWV in the multiple regression analysis.
DiscussionAs in other studies, our data confirm that dapagliflozin produces a sustained decrease in blood glucose, weight, BP and uricemia.4
Our main finding is that, for the first time, dapagliflozin demonstrates a medium-long term AS decrease in subjects with DM2 that may have CV and renal benefits by decreasing central BP, attenuating the damage induced by pulsatility. Structural echocardiography changes or cardiac function were not evaluated. We do observe a decrease in albuminuria in which a decrease in c-fPWV could participate together with renal hemodynamic changes, and the decrease in weight and BP induced by dapagliflozin.
The underlying mechanisms of the decrease in c-fPWV are not clear. AS is influenced by neurohumoral and structural factors. In subjects with DM2 the administration of dapagliflozin, acutely, reduces the c-fPWV, regardless of changes in BP.5 The persistent decrease in c-fPWV after 12 months suggests that there may be a structural component in the reduction of AS.
Chronic kidney disease with reduced GFR is associated with an increase in AS.6 In our study, subjects in whom DM coexisted with kidney disease had a higher baseline c-fPWV; however, after treatment with dapagliflozin, we only observed a slight non-significant decrease in GFR, so this variable does not seem to contribute to the observed changes in AS.
Hyperglycaemia induces changes in collagen that increase AS.7 A better glycaemic control is associated with an attenuation/prevention of increased AS in DM2. A significant decrease in HbA1c is observed in our study, but we did not objectify a significant relationship between glycaemic and c-fPWV changes. On the other hand, the decrease in BP, weight, insulin dose and administration of some antihypertensive drugs are associated with the decrease in c-fPWV.8 In our study, insulin was reduced/suspended in only 2 cases, changes in other hypoglycaemic agents with possible vascular effects were minimal, antihypertensive treatment was not modified and, in the regression study, neither weight nor BP changes were shown as significant explanatory variables for a decreased c-fPWV, probably due to the limited number of cases. Neither the significant decrease in uricemia, attributable to an increase in urinary uric acid excretion, showed a significant relationship with the decrease in c-fPWV.
An increase in the total Na+ content is found in DM2. There is a link between the increase in Na+ content and structural and functional changes of large arteries, independent of BP, with increased AS.9 SGLT2i, by inhibiting the sodium-glucose cotransporter and the Na+/H+ exchanger in the proximal tubule, induces natriuresis. The few studies that have quantified the natriuretic effect of SGLT2i have observed a transient increase in urinary sodium excretion. We observed a slight decrease in serum Na+, which we attribute to a decrease in blood glucose and a persistent increase in natriuresis, which, however, did not reach statistical significance when it was related to urinary creatinine excretion. In the absence of a strict sodium intake control, the evaluation of natriuresis is difficult. We believe, however, that our data suggest a probable persistent increase in dapagliflozin-induced natriuresis which, on the other hand, has been shown to reduce the tissue content of Na+ in subjects with DM2.10 The increase in natriuresis, together with the reduction of the Na+ content of the smooth muscle fibre of the myocardium and the vascular wall by inhibition of the Na+/H+ exchanger can contribute to the reduction of AS.
ConclusionsThe administration of dapagliflozin produces in subjects with DM2 a medium-long term decrease in AS, in which multiple mechanisms can be involved: decrease of blood glucose, BP, weight, uric acid and Na+ body content, among others.
The longitudinal nature of the study with medium-long term data, without relevant changes in concomitant treatment, reinforces our results. However, our study has limitations: its observational nature, the lack of control of sodium intake and the reduced number of patients. Since the 4 patients who did not complete the follow-up had higher c-fPWV values and the fact that in our study the decrease in c-fPWV seems to be of greater magnitude in cases in which it is abnormally high, the inclusion of these patients could have reinforced our findings. Long-term randomized studies in DM2 with CV event analysis are necessary to confirm the beneficial effect of SGLT2i on AS and its participation in CV benefits, and its comparison with new hypoglycaemic agents with possible vascular effects.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Hidalgo Santiago JC, Maraver Delgado J, Cayón Blanco M, López Saez JB, Gómez-Fernández P. Efecto de dapagliflozina sobre la rigidez arterial en pacientes con diabetes mellitus tipo 2. Med Clin (Barc). 2020;154:171–174.