We describe a novel delta-thalassemia mutation causing decreased hemoglobin (Hb) A2 levels associated with Hb Watts, variant Hb resulting from a trinucleotide deletion in Spain.
Patients and methodHb variant analysis was performed by cation-exchange high performance liquid chromatography (HPLC) and capillary zone electrophoresis. Polymerase chain reaction and DNA sequence analyses were used to identify mutations in the δ- and α-globin genes.
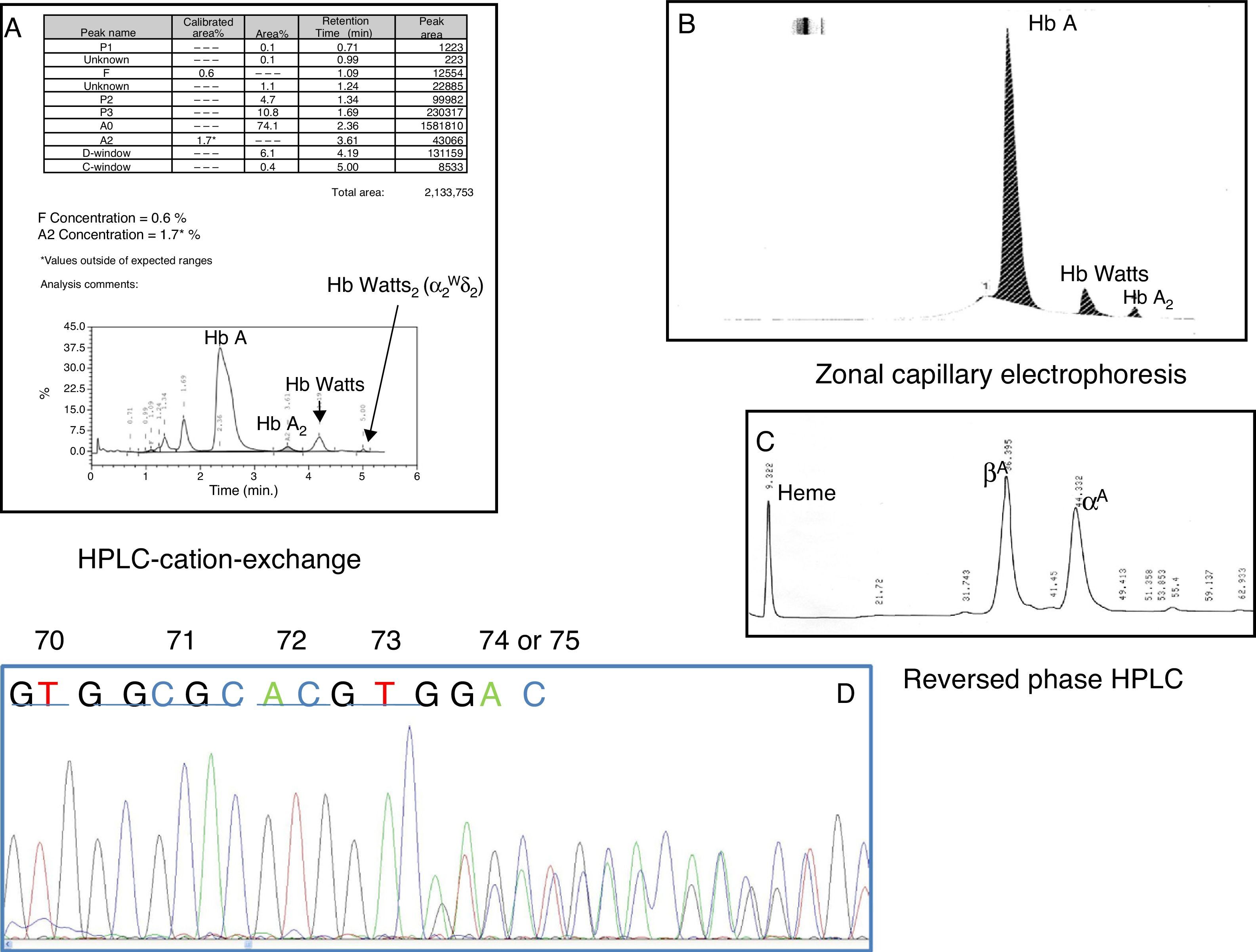
ResultsAbnormal Hb was observed on capillary zone electrophoresis in Z6 and by cation-exchange HPLC a slower peak than HbA was observed at an retention time of 4.19min. This variant Hb is called Hb Watts [α2 74(EF3)Asp->0 or α2 75(EF4)Asp->0; HBA2:c.226_228delGAC]. The decreased HbA2 percentage owes to an insertion of 27nt between nt 83 and 84 of IVS-I of the δ-globin gene.
ConclusionsWhen analyzing a chromatogram, the possibility of the existence of delta-thalassemia or an HbA2 variant should be considered, apart from alfa-, beta-thalassemia and structural hemoglobinopathies. To this end, each of the peaks and their percentages should be considered to allow for correct interpretation and to avoid misdiagnosis as much as possible.
Se describe una nueva mutación en el gen δ-globina (delta-talasemia), responsable de una disminución de los valores de hemoglobina (Hb) A2 y asociada a Hb Watts, variante de Hb debido a una deleción de trinucleótidos.
Pacientes y métodoEl análisis de Hb se llevó a cabo mediante high performance liquid chromatography (HPLC, «cromatografía líquida de alta resolución») de intercambio iónico y electroforesis capilar de zona. Se utilizaron técnicas de reacción en cadena de la polimerasa y secuenciación automática para identificar las mutaciones en los genes δ- y α-globina.
ResultadosLa Hb anómala se observó en la electroforesis capilar de zona en Z6 y por HPLC de intercambio iónico apareció un pico más lento que la HbA en un tiempo de retención de 4,19min. Esta variante de la Hb se llama Hb Watts [α2 74(EF3)Asp->0 o α2 75(EF4)Asp->0; HBA2:c.226_228delGAC]. El bajo porcentaje de HbA2 se debe a una inserción de 27nt entre los nucleótidos 83 y 84 de IVS-I del gen de δ-globina.
ConclusionesCuando se analiza un cromatograma se debe tener en cuenta la posibilidad de una delta-talasemia o una variante de HbA2, aparte de una alfa-talasemia, beta-talasemia y hemoglobinopatías estructurales. A tal fin, cada uno de los picos y sus porcentajes deben ser considerados para una correcta interpretación y evitar diagnósticos erróneos tanto como sea posible.
The main hemoglobins (Hb) in human adults are HbA and HbA2. They are both made of α globin chains linked to β chains (α2β2) or δ chains (α2δ2), respectively. The genes that control the synthesis of the δ and βchains are located on the short arm of chromosome 11, and are part of the globin cluster β, while α chain genes are located in chromosome 16.1 HbA2 does not have specific biochemical roles and, under normal conditions, its fraction can range from 2.0–2.22 to 3.2–3.4%3,4 of the total of Hb. Defects in gene δ-globin can modify the expression of HbA2 fraction, reducing or increasing its percentage. Scores below 2.0% are generally due to genetic defects that cause a reduction in the synthesis of the δ chain (delta-thalassaemia), synthesis of HbA2 variants, or when there is a homozygous alpha+-thalassaemia, a heterozygous alpha0-thalassaemia, Hb H disease and some cases of delta-beta0-thalassaemia. On the contrary, increased scores of HbA2 are associated, among others, with heterozygous beta-thalassaemia, a trait of sickle-shaped and Hb unstable cells.5
We categorised a new mutation in the δ-globin gene (delta-thalassaemia), responsible for the reduction of HbA2 levels and associated with Watts Hb, a variant of Hb due to a trinucleotide deletion.
Patient and methodThe proposita is a 58 year-old asymptomatic female from Jerez de la Frontera who underwent a diabetes check-up due to obesity. The analysis of the glycosylated Hb haemolysis using a high performance liquid chromatography technique (HPLC) (Variant™ II; Bio-Rad Laboratories, Hercules, CA, U.S.A.) showed an unusual peak in a retention time (RT) of 1.14min.
The Reference Centre for Thalassaemias and Other Hemoglobinopathies (San Carlos Clinical Hospital) received a sample of blood to obtain a molecular characterisation. Haematological data was obtained by means of an automated cell counter (Coulter® LH750 Analyser; Beckman Coulter, Brea, CA, U.S.A.). The levels of HbA2 and foetal Hb (Hb F) were obtained using HPLC-cation-exchange (HPLC-CE) (Variant™). Hbs were analysed by zonal capillary electrophoresis (ECdZ) (Sebia Capillarys® Flex; Sebia, Norcross, GA, U.S.A.) and HPLC-CE using the Bio-Rad's beta-thalassaemia short program (Bio-Rad, Hercules, CA, U.S.A.), which separates Hb variants using a saline gradient. The globin chains were analysed by reversed phase HPLC.6
After the isolation of genomic DNA with an automatic method (BioRobot® EZ1; Qiagen GmbH, Hilden, Germany), DNA was quantified with NanoDrop® 1000 (Thermo Scientific, Wilmington, DE, U.S.A.).
The most common alpha-thalassaemia mutations were analysed through an Alpha-Globin StripAssay® multiplex polymerase chain reaction (ViennaLab Diagnostics GmbH, Vienna, Austria).
The α gene sequencing was carried out following the protocol published by De la Fuente-Gonzalo et al.7 The δ-globin gene was also analysed directly through sequencing; in this case it was necessary to amplify 2 segments, as previously described.8
All our haematologic indexes and clinical findings were collected after obtaining the informed consent of the proposita and the approval of the San Carlos Clinical Hospital Ethics Committee.
ResultsThe haematologic data (Hb 15.4g/dl, haematocrit 0.50l/l, red blood cells 5.59×1012/l, mean corpuscular volume 90.3fl, mean corpuscular Hb 27.5pg and erythrocyte amplitude index 12.6%), the peripheral blood smear and the iron metabolism were normal. During the HbA2 (1.7%) and Hb F (0.6%) quantification in the HPLC-CE, there was a slower RT peak compared to the HbA. The peak was of 4.19min (D-window) which corresponded to 6.1% of the total Hb, and a second RT peak of 5min of only 0.4% of the total Hb (Fig. 1A). By means of ECdZ, we could observe an abnormal Hb in Z6, the concentration of which was 6.9% (Fig. 1B). The analysis of the globin chains by reversed phase HPLC did not show any abnormal peaks (β and α). Given the percentage of abnormal Hb, this could be a variant of the α chain. To confirm this point, the DNA sequencing of the globin gene α2 showed 3 suppressed nucleotides (GAC) between codons 74 and 75, corresponding to aspartic acid; this variant of Hb is named Watts Hb [alpha2 74(EF3)Asp->0 or alpha2 75(EF4)Asp->0; HBA2:c.226_228delGAC] (Fig. 1C).
(A) Ion-exchange high performance liquid chromatography. (B) Zonal capillary electrophoresis. (C) Reversed phase high performance liquid chromatography. (D) Automated direct sequencing of exon 2 of α2 globin gene showing a GAC deletion between codons 74 and 75 corresponding to Watts Hb [α2 74 (EF3) Asp->0 or α2 75 (EF4) Asp->0; HBA2:c.226_228delGAC]. HPLC: high performance liquid chromatography.
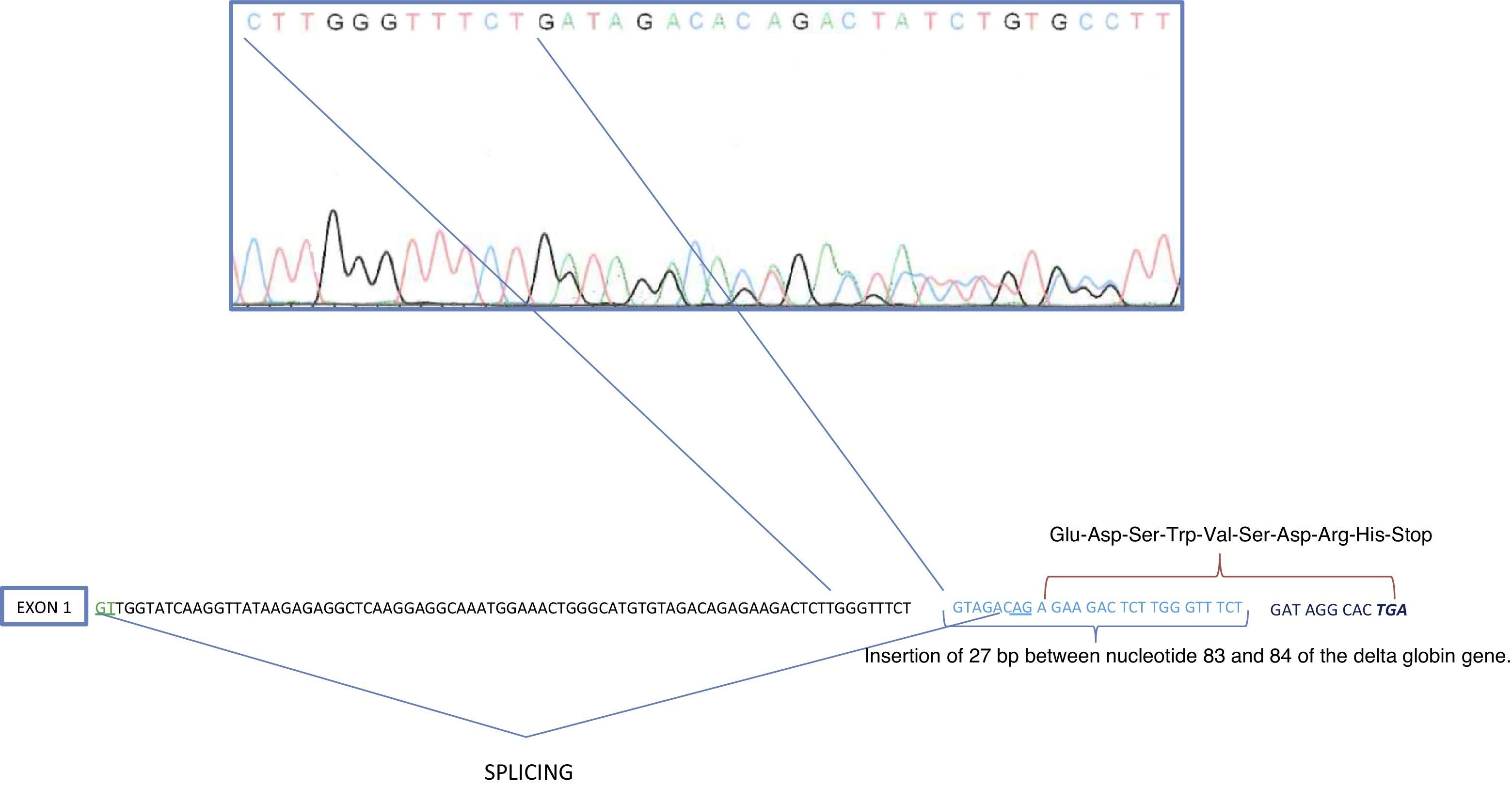
Due to the reduction in the HbA2 percentage and the small peak by HPLC-CE in an RT of 5min, we carried out a sequencing of the δ-globin gene, which showed an insertion of 27 base pairs (bp) between the nucleotides 83 and 84 of IVS-I (GTAGACAGAGAAGACTCTTGGGTTTCT). This anomaly of 27bp coincides with the sequence existing between IVS-I-nt-56 and IVS-I-nt-83 of the δ-globin gene (Fig. 2).
Top: Automated direct sequencing of δ-globin gene. Frameshift due to the insertion of 27 base pairs between nucleotides 83 and 84 of IVS-I. Bottom: Simulation of alternative place for the splicing caused by the frameshift and the sequence of the new amino acid residues (Glu-Asp-Ser-Trp-Val-Ser-Asp-Gln).
Both non-deletion alpha-thalassaemia and deletion alpha-thalassaemia were dismissed.
DiscussionThe alterations in the δglobin chain have no clinical significance and the only haematological parameter they seem to affect are HbA2 levels. If it is inherited together with a heterozygote beta-thalassaemia where the levels of HbA2 are usually increased, it would be the most common cause for HbA2 normalisation. In this study, there is a new delta0-thalassaemia associated with a structural hemoglobinopathy of chain α (Watts Hb), where the reduction of HbA2 levels repeats.
Watts Hb was first described in 1997 in a 37-year old Mexican woman, born in Watts (Los Angeles Country, CA, U.S.A.)9; there has not been any publication since then. Watts Hb is a variant of chain α globin and one of the few due to the bp deletion. Although the area where residues 74 and 75 of the α2 globin chain are located is not a critical area of the Hb molecule, the deletion of any of these residues seems to create a slight instability in the molecule, with no clinical signs or haematological alterations. It was detected during a diabetes check-up, but it does not affect the level of glycosylated Hb, which is why the diagnostic value of the test is not altered.
When there is a structural variant of Hb, whether it is a α1 or α2 chain, they usually exhibit slightly reduced levels of HbA2 (≈2.2%), because in most of them a corresponding variant of HbA210 is formed. However, in the case of this Watts Hb, the levels of HbA2 were lower than usual (1.7%), and since there was also an abnormal peak in the HPLC-CE at an RT of 5min, there probably is an anomaly in the δ globin chains.
The δ-globin gene sequencing revealed an insertion of 27bp between nucleotides 83 and 84 from IVS-I, which actually corresponded to a duplication of the sequence between nucleotides 56 and 83 of the same intron. This duplication has a new “AG” consensus sequence for the splicing of the first intron, and it is approximately 30bp ahead. There would be a frameshift simultaneously, so 28bp ahead of the alternative splicing, a stop codon would be formed (Fig. 2). This new read-through would codify a new protein of 40 amino acids, the first 31 would coincide with the δ globin chain and the rest would be established by the new sequence (Glu-Asp-Ser-Trp-Val-Ser-Asp-Gln). The resulting protein would be unstable and, therefore, it would be a delta0-thalassaemia, which would cause a reduction in HbA2 levels.
A total of 110 mutations that affect the δ-globin gene have been described, 67 of which give rise to structural variants of HbA2 and the rest correspond to delta-thalassaemia.11 The majority of the genetic defects that cause delta-thalassaemias and alter the expression of the δ-globin gene are caused by the substitution of a single nucleotide (isolated mutation) and, to a lesser extent, by large gene deletions and crossovers. It is interesting that 65% of the lesions that cause some structural variant of the δ chain have their equivalent in the β gene, and 35% of the delta-thalassaemias are the same mutations that cause beta-thalassaemia. It is thought that these identical nucleotide substitutions have arisen due to independent mutations or as a result of gene conversion episodes.1 Most beta-thalassaemias have been detected due to their association with trans beta-thalassaemias, like the Corfu deletion (−7.2kb) linked to the CD39 (C>T)12 non-sense mutation, or in cis, like for instance the loss of an A in the CD59 of δ gene and the Knossos Hb [β27 (B9) Ala>Ser; HBB:c.82G>T].13 This new mutation is the first one that arises through the insertion of a sequence of 27bp.
Once we identified the cause for the HbA2 reduction, we only needed to analyse the peak in the C-window by HPLC-CE. We dismissed the possibility of a HbA’2 [δ16(A13) Gly>Arg; HBD:c.49G>C], since by HPLC this one elutes in the S-window,14–16 so it could correspond to a HbA2 formed by the αWatts chain variant. Said Hb was not detected by means of capillary electrophoresis, and the 1997 publication about Watts Hb did not mention any other HbA2 either. This could be due to its low percentage, which makes it difficult to distinguish in the Hb electrophoresis and, although it is easily detected by HPLC-CE, there still seems to be a lack of knowledge about the variants of HbA2.
We confirmed that the delta-thalassaemia has no clinical significance. Since it was inherited together with an α chain Hb variant, the haematological parameters were not altered. There was only a reduction in the HbA2 levels. In these cases, we have to take into account that the corresponding variant of HbA2 could be formed, although the percentage would be so small that it would not be detected by some of the electrophoretic techniques.16 The actual level of HbA2 would be comprised by the total of both concentrations.
In the cases in which a delta-thalassaemia in cis or in trans coexists with a beta-thalassaemia, the levels of HbA2 are reduced to normal levels; the typical haematological phenotype with thalassaemic features could change, increasing the risk of a wrong diagnosis.17,18
In conclusion, we must say that when analysing a chromatogram, not only should we weigh the possibility of an alpha-thalassaemia, a beta-thalassaemia and structural hemoglobinopathies, but also the possibility of a delta-thalassaemia or an HbA2 variant. Each peak and their percentages should be taken into account for a precise interpretation with the purpose of avoiding a wrong diagnosis as much as possible.
FundingThis study was funded by the Spanish Society of Haematology and Haemotherapy 2012 and by the Grant from FIS number PI12/01068.
AuthorshipAll the authors have had total access to the data, have participated in the analysis and/or have interpreted the results and have written the manuscript. All the authors have read and approved the final manuscript.
Conflict of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: González Borrachero ML, de la Fuente-Gonzalo F, González FA, Nieto JM, Villegas A, Ropero P. Delta0-talasemia por inserción de 27 pares de bases en el gen δ-globina con descenso de los valores de hemoglobina A2. Med Clin (Barc). 2015;144:312–316.





![(A) Ion-exchange high performance liquid chromatography. (B) Zonal capillary electrophoresis. (C) Reversed phase high performance liquid chromatography. (D) Automated direct sequencing of exon 2 of α2 globin gene showing a GAC deletion between codons 74 and 75 corresponding to Watts Hb [α2 74 (EF3) Asp->0 or α2 75 (EF4) Asp->0; HBA2:c.226_228delGAC]. HPLC: high performance liquid chromatography. (A) Ion-exchange high performance liquid chromatography. (B) Zonal capillary electrophoresis. (C) Reversed phase high performance liquid chromatography. (D) Automated direct sequencing of exon 2 of α2 globin gene showing a GAC deletion between codons 74 and 75 corresponding to Watts Hb [α2 74 (EF3) Asp->0 or α2 75 (EF4) Asp->0; HBA2:c.226_228delGAC]. HPLC: high performance liquid chromatography.](https://static.elsevier.es/multimedia/23870206/0000014400000007/v1_201512180144/S2387020615001680/v1_201512180144/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

