CT-P13 is a biosimilar drug of infliximab (IFX), effective in patients with inflammatory bowel disease (IBD). The monitoring of levels of IFX and anti-IFX antibodies is now considered part of the integral management.
ObjectiveTo compare the clinical response according to a strictly clinical (CLN) or proactive (PRO) approach based on the monitoring of levels in week 14, in clinical practice.
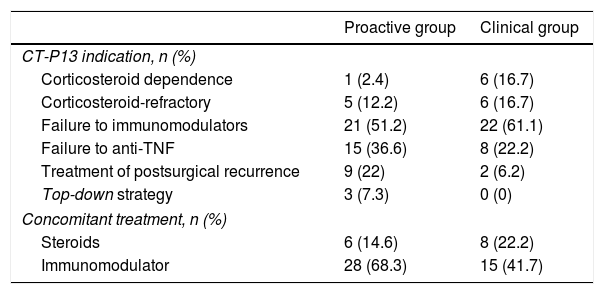
MethodsWe conducted a prospective study in IBD patients starting CT-P13. In the PRO group, levels of IFX and post-induction antibodies were systematically measured (week 14) and those with infraterapeutic levels (<3μg/ml) were intensified, irrespective of the clinical response.
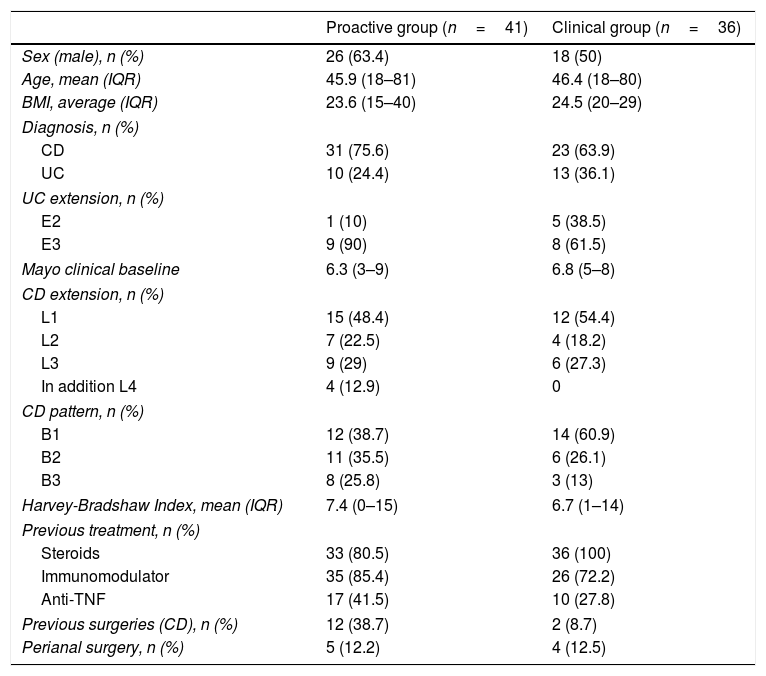
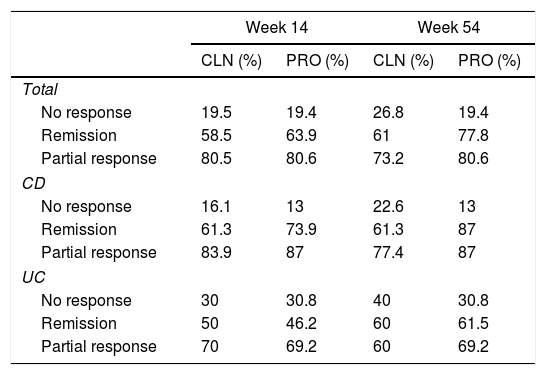
ResultsWe included 77 patients (23 ulcerative colitis and 54 Crohn's disease). Both PRO (n=41) and CLN (n=36) groups showed initial and long-term efficacy without significant differences. At week 14, 61% clinical remission (CR) (58.5% PRO, 63.9% CLN) and 80.5% at least partial response (PR) (80.5% PRO, 80.6% CLN). In week 54, 68.8% CR (61% PRO, 77.8% CLN) and 76.6% at least PR (73.2% PRO, 80.6% CLN). Of the patients in CR in week 14 (24 PRO, 23 CLN), 13 of the PRO group were intensified due to infra-therapeutic levels. In this subgroup no significant differences were observed in secondary loss of response (PRO 0%, CLN 8.7%).
ConclusionProactive management does not improve response or remission rates in the first year. The intensification of clinical remission patients with post-induction infratherapeutic levels does not seem to significantly prevent secondary loss of response in the first year.
CT-P13 es un fármaco biosimilar de infliximab (IFX), efectivo en pacientes con enfermedad inflamatoria intestinal (EII). La medición de niveles de IFX y anticuerpos anti-IFX forma parte del tratamiento integral de dicha enfermedad.
ObjetivoComparar la respuesta clínica en función de un abordaje estrictamente clínico (CLN) o proactivo (PRO) basado en la medición de niveles en la semana 14, en la práctica clínica.
MétodosEstudio prospectivo en pacientes que inician CT-P13 por EII. En el grupo PRO se midieron sistemáticamente los niveles de IFX y de anticuerpos postinducción (semana 14) y se intensificaron aquellos con niveles infraterapéuticos (<3μg/ml), independientemente de la respuesta clínica.
ResultadosSe incluyeron 77 pacientes (23 colitis ulcerosa y 54 enfermedad de Crohn). Ambos grupos, PRO (n=41) y CLN (n=36) presentaron una eficacia inicial y a largo plazo sin diferencias significativas. En la semana 14 hubo un 61% de remisión clínica (RC) (58,5% PRO, 63,9% CLN) y un 80,5% de al menos respuesta parcial (RP) (80,5% PRO, 80,6% CLN). En la semana 54 hubo un 68,8% de RC (61% PRO, 77,8% CLN) y un 76,6% de al menos RP (73,2% PRO, 80,6% CLN). De los pacientes en RC en la semana 14 (24 PRO, 23 CLN), 13 del grupo PRO fueron intensificados por niveles infraterapéuticos. En este subgrupo no se observaron diferencias significativas en la pérdida de respuesta secundaria (PRO 0%, CLN 8,7%).
ConclusiónUn manejo proactivo no mejoró las tasas de respuesta ni la remisión en el primer año. La intensificación de pacientes en RC y niveles infraterapéuticos postinducción no parece prevenir de forma significativa la pérdida de respuesta secundaria en el primer año.