Since enzyme replacement treatment (ERT) with idursulfase is available for Hunter syndrome (HS; mucopolysaccharidosis type II), for the first time, disease progression can be limited and organ damage reduced or prevented.
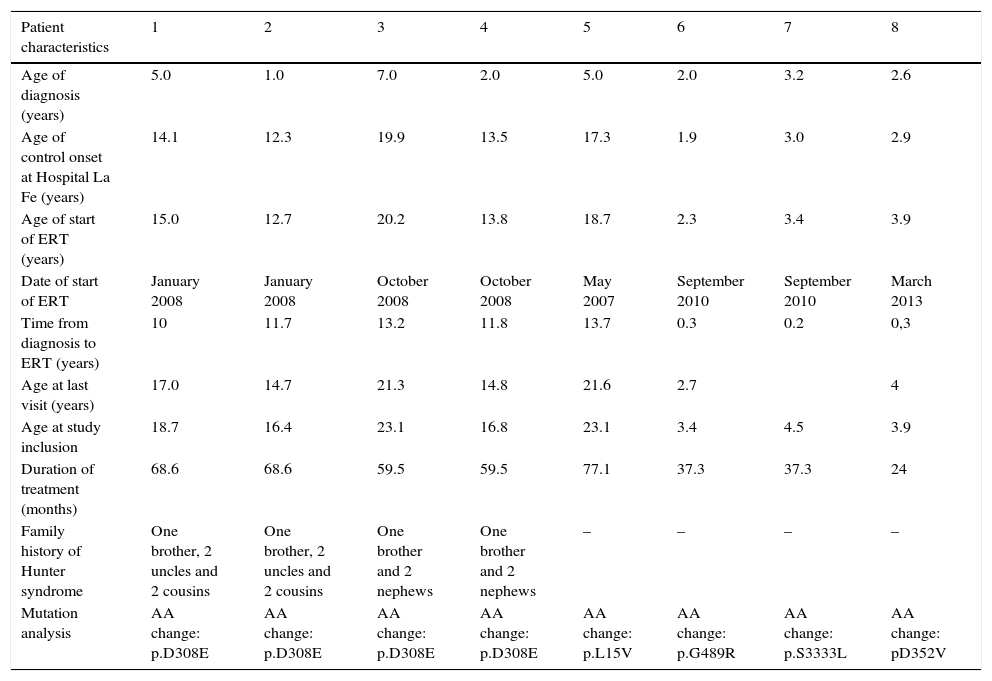
Patients and methodsWe described retrospectively the clinical evolution of eight HS males, treated with ERT and followed in routine clinical practice in Hospital Infantil La Fe (Valencia, Spain).
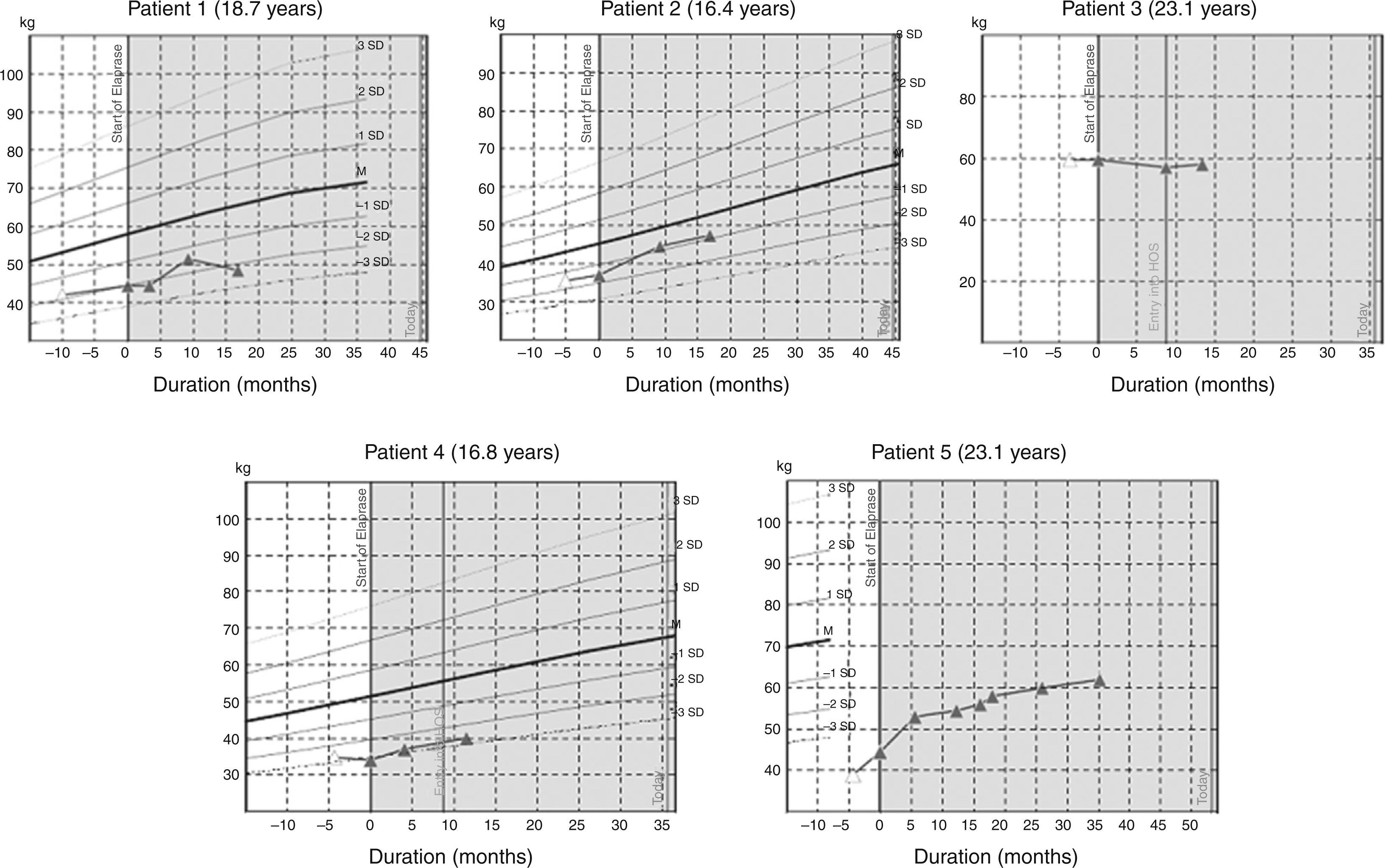
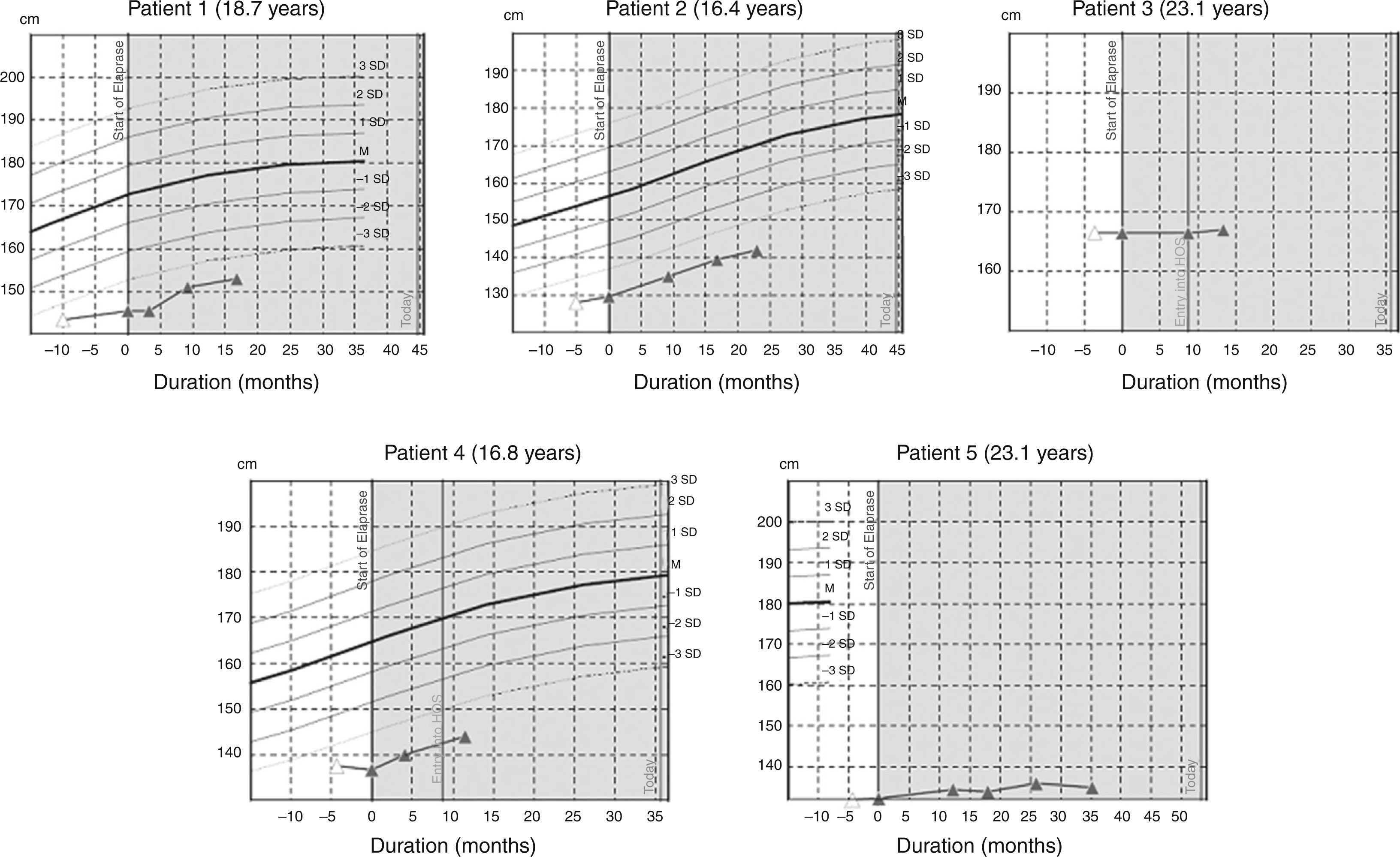
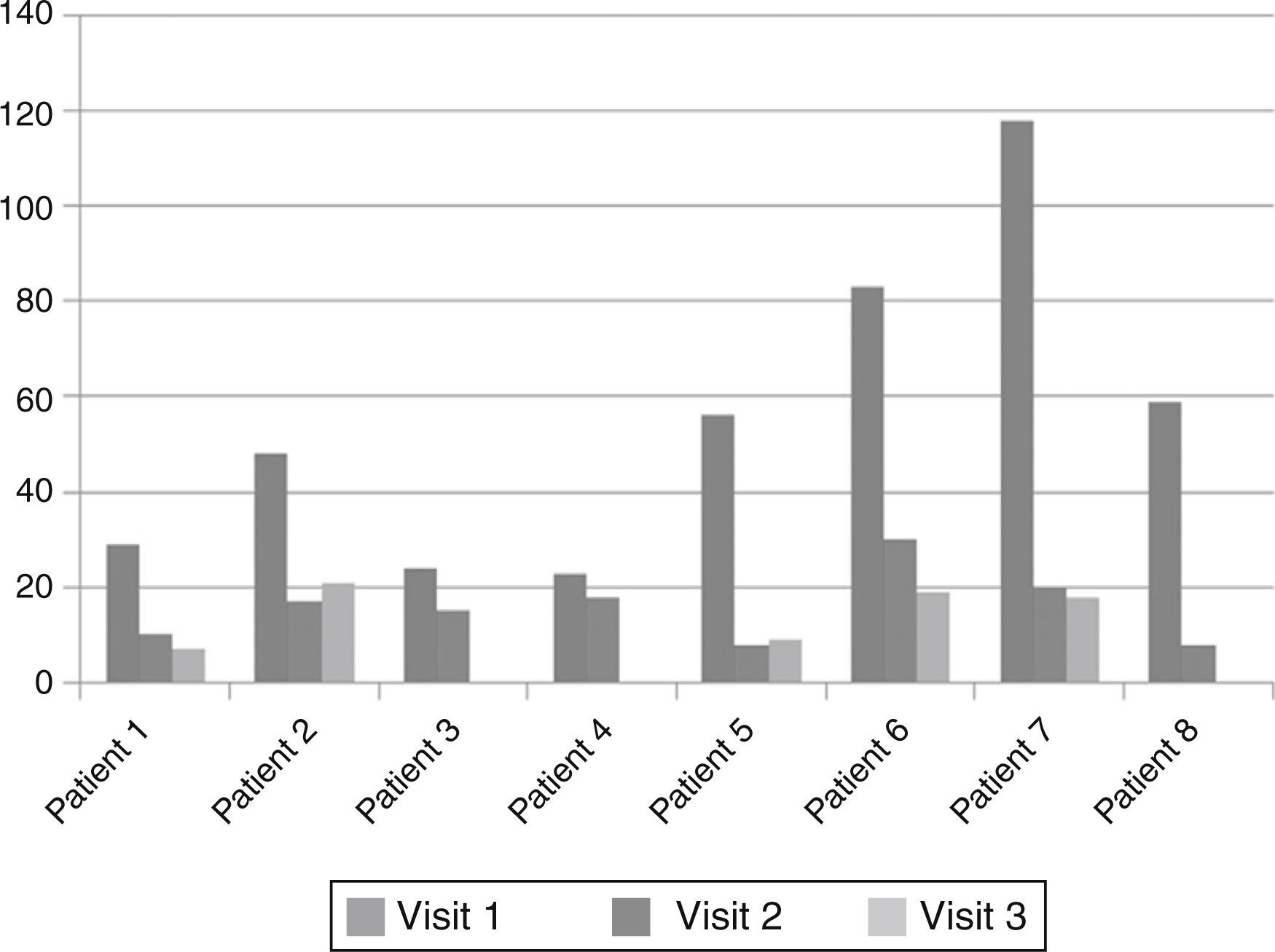
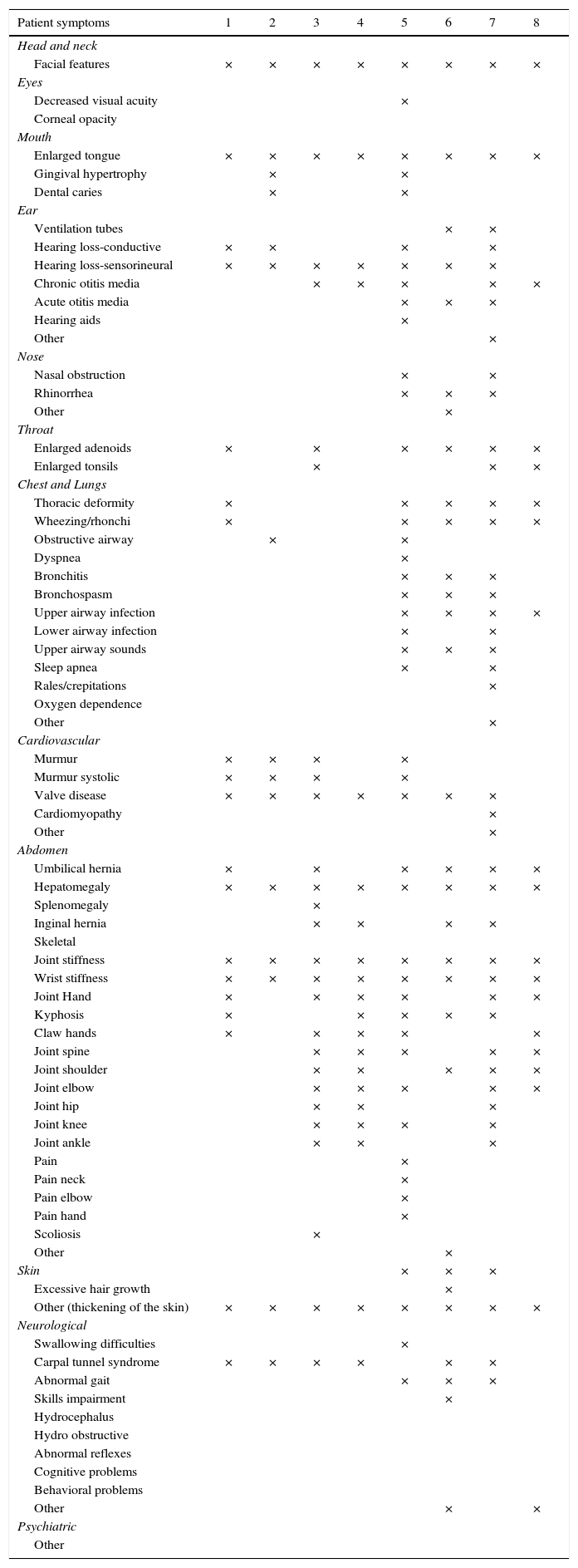
ResultsWe studied three children, three adolescents and two adults. Time from diagnosis to ERT ranged from 13.7 to 0.2 years, and duration of ERT ranged from 24 to 77.1 months. From the start of ERT, weight and height increased in children and adolescents and remained stable in adults. Glycosaminoglycans (GAG) decreased in all patients; in patient 5 (aged 23 years), we observed the highest reduction (86%) with recovery of carpal tunnel syndrome, splenomegaly and a decrease in nocturnal oxygen dependence.
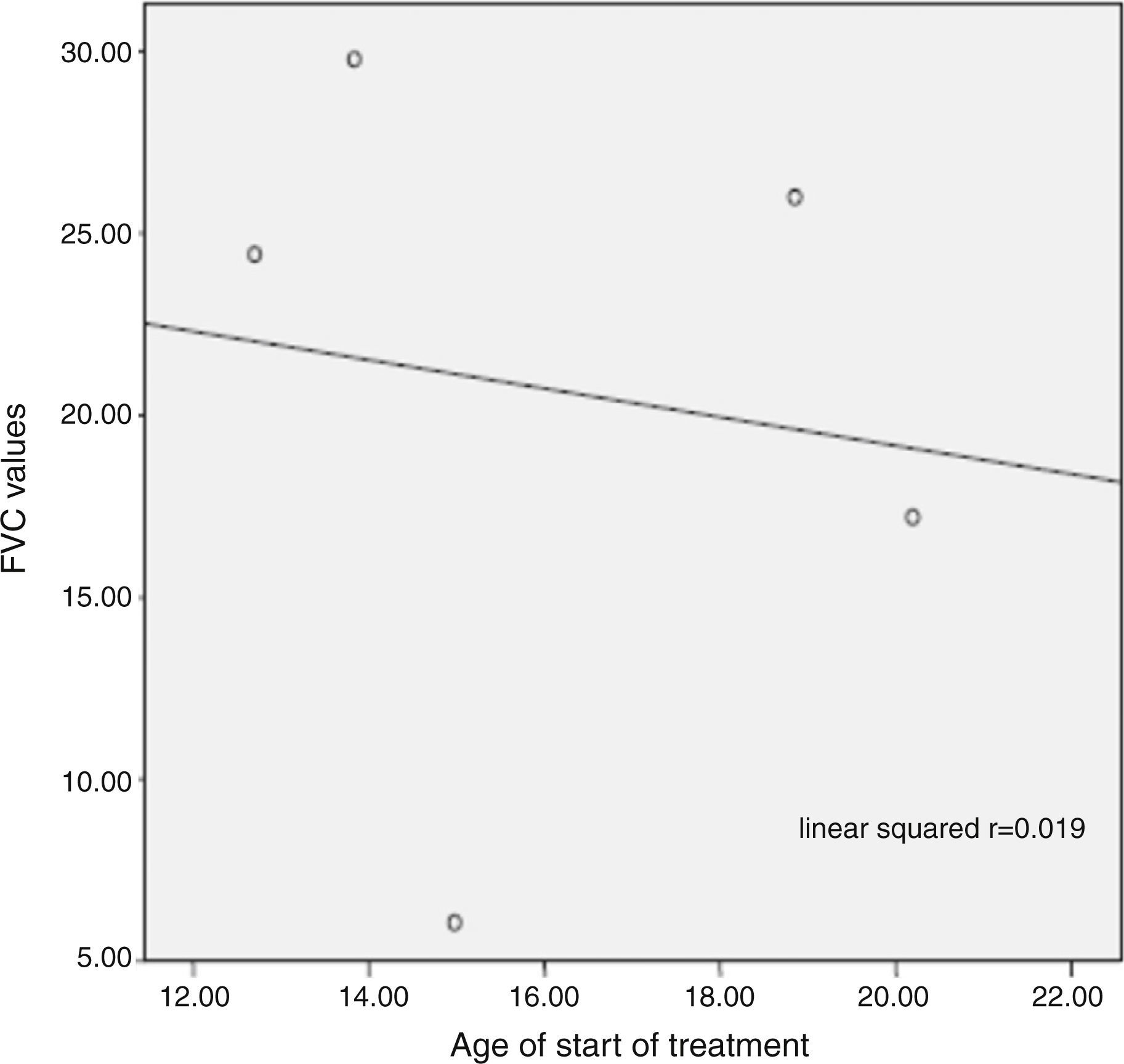
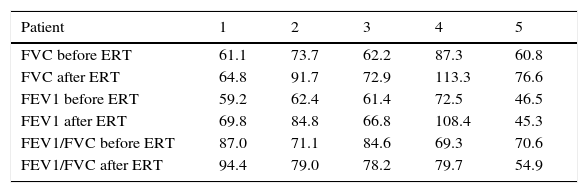
ConclusionOur results show that ERT improve respiratory impairment and organomegalies and decrease GAGs levels in all patients including children, adolescent and adults. While cardiac manifestations and facial features stabilized, responses in other parameters were heterogeneous.
Desde que la Terapia de reemplazo enzimático (TRE) con Idursulfasa está disponible para el Síndrome de Hunter (SH; mucopolisacaridosis tipo II) la progresión de la enfermedad puede limitarse y posiblemente reducir y prevenir el daño orgánico.
Pacientes y métodosDescribimos retrospectivamente la evolución de 8 pacientes con SH, tratados con TRE y revisados según práctica clínica habitual en el Hospital Infantil La Fe (Valencia, España).
ResultadosEstudiamos 3 niños, 3 adolescentes y 2 adultos El tiempo desde el diagnóstico hasta inicio de TRE fue de 0,2 a 13,7 añosy la duración de la TRE de 24 a 77,1 meses. Tras iniciar la TRE, el peso y la talla de los niños y adolescentes se incrementaron permaneciendo estable en los adultos. Los glucosaminoglicanos (GAG) disminuyeron en todos los pacientes; la mayor reducción (86%) se observó en un adulto que mejoró el túnel carpiano, disminuyó la esplenomegalia y la dependencia nocturna de oxígeno.
ConclusiónNuestros resultados muestran que la TRE mejora la función respiratoria, las organomegalias y reducen los niveles e GAGs urinarios en todos los pacientes incluyendo niños, adolescentes y adultos. Las manifestaciones cardiacas y facials permanecieron estables. Los resultados en otros parámetros fueron heterogeneos.