Hypertension is a prevalent condition among SARS-CoV-2 infected patients. Whether renin–angiotensin–aldosterone system (RAAS) inhibitors are beneficial or harmful is controversial.
MethodsWe have performed a national retrospective, nonexperimental comparative study from two tertiary hospitals to evaluate the impact of chronic use of RAAS inhibitors in hypertensive COVID-19 patients. A meta-analysis was performed to strengthen our findings.
ResultsOf 849 patients, 422 (49.7%) patients were hypertensive and 310 (73.5%) were taking RAAS inhibitors at baseline. Hypertensive patients were older, had more comorbidities, and a greater incidence of respiratory failure (−0.151 [95% CI −0.218, −0.084]). Overall mortality in hypertensive patients was 28.4%, but smaller among those with prescribed RAAS inhibitors before (−0.167 [95% CI −0.220, −0.114]) and during hospitalization (0.090 [−0.008,0.188]). Similar findings were observed after two propensity score matches that evaluated the benefit of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers among hypertensive patients. Multivariate logistic regression analysis of hypertensive patients found that age, diabetes mellitus, C-reactive protein, and renal failure were independently associated with all-cause mortality. On the contrary, ACEIs decreased the risk of death (OR 0.444 [95% CI 0.224–0.881]). Meta-analysis suggested a protective benefit of RAAS inhibitors (OR 0.6 [95% CI 0.42–0.8]) among hypertensive COVID-19.
ConclusionOur data suggest that RAAS inhibitors may play a protective role in hypertensive COVID-19 patients. This finding was supported by a meta-analysis of the current evidence. Maintaining these medications during hospital stay may not negatively affect COVID-19 outcomes.
La hipertensión es una condición prevalente entre los pacientes infectados por el SARS-CoV-2. Es controvertido si los inhibidores del sistema renina-angiotensina-aldosterona (SRAA) son beneficiosos o perjudiciales.
MétodosHemos desarrollado un estudio comparativo nacional retrospectivo y no experimental en 2 hospitales terciarios para evaluar el impacto del uso crónico de inhibidores del SRAA en pacientes hipertensos con COVID-19. Se realizó un metaanálisis para reforzar los hallazgos.
ResultadosDe 849 pacientes, 422 (49,7%) eran hipertensos y 310 (73,5%) tomaban inhibidores del SRAA al inicio del estudio. Los pacientes hipertensos eran mayores, tenían más comorbilidades y una mayor incidencia de insuficiencia respiratoria (−0,151; IC 95%: [−0,218; −0,084]). La mortalidad global en los pacientes hipertensos fue del 28,4%, pero fue menor entre los que tenían prescritos inhibidores del SRAA antes (−0,167; IC 95%: [−0,220; −0,114]) y durante la hospitalización (0,090; [−0,008; 0,188]). Se observaron hallazgos similares tras 2 emparejamientos de puntuación de propensión que evaluaron el beneficio de los inhibidores de la enzima convertidora de angiotensina y los bloqueadores de los receptores de angiotensina entre los pacientes hipertensos. El análisis de regresión logística multivariante de los pacientes hipertensos reveló que la edad, la diabetes mellitus, la proteína C reactiva y la insuficiencia renal se asociaban de forma independiente con la mortalidad por todas las causas. Por el contrario, los inhibidores de la enzima convertidora de angiotensina disminuyeron el riesgo de muerte (OR 0,444; IC 95%: 0,224-0,881). El metaanálisis indicó un beneficio protector de los inhibidores del SRAA (OR 0,6; IC 95%: 0,42-0,8) entre los hipertensos con COVID-19.
ConclusiónNuestros datos indican que los inhibidores del SRAA pueden desempeñar un papel protector en los pacientes hipertensos con COVID-19. Este hallazgo fue apoyado por un metaanálisis de la evidencia actual. Su mantenimiento durante la estancia hospitalaria puede no afectar negativamente a los resultados de la COVID-19.
In early December of 2019, several patients from Wuhan were found to have viral pneumonia caused by the acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Nobody could imagine then that this could lead to the coronavirus disease 2019 (COVID-19) outbreak with an estimated global mortality of 2.3% after 61,299,371 infected people worldwide.1
The virus enters the cell by binding its trimeric spike protein to the human receptor angiotensin-converting enzyme 2 (ACE2) and the activity of the serine protease TMPRSS2 for S protein priming.2,3 Expression of ACE2 is upregulated in cardiovascular disease,4 and in patients with diabetes mellitus and hypertension,5 which may favor the entrance of the SARS-CoV-2 into the cells and increase the virulence of the virus in the lung and heart. Moreover, renin–angiotensin–aldosterone system (RAAS) blockade increases the expression and activity of cardiac ACE2.6 On the other hand, recombinant human ACE2 has been shown to protect the ACE2-deficient mice model from lung failure in SARS induced by acid aspiration,7 and have been seen in severe cases of COVID-19.8
Importantly, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) block the RAAS by means of different mechanisms, so that their effects on ACE2 expression and activity should be different as well. Therefore, some authors warn about the harmful effect of RAAS blockers and advise its discontinuation to prevent poor outcome9 while others claim to maintain these drugs until more evidence was available.10
The aim of this national multicenter retrospective study was to (1) evaluate the impact of chronic RAAS inhibitors in hypertensive patients with COVID-19 and (2) estimate its average effect through a meta-analysis of the current evidence.
MethodsStudy design and data collectionThis is a retrospective nonexperimental comparative study from two tertiary Spanish centers (Hospital Clínico Universitario de Valladolid; Hospital Clínico Universitario de Santiago de Compostela) to evaluate the impact of chronic use of RAAS inhibitors in hypertensive COVID-19 patients. Definitive diagnosis of SARS-CoV-2 infection was confirmed through positive reverse transcriptase-polymerase chain reaction (RT-PCR). The inclusion criteria were patients>18 years old admitted between March 1st 2020 and April 30th 2020. The exclusion criteria were pregnant women and terminally ill patients.
The study was approved by our local ethics committee and consent was waived due to the retrospective nature of the study. Following the approval, a retrospective analysis of all the COVID-19 patients was performed dividing them into two groups according to their prior history of hypertension. Hypertensive patients were further classified according to their medical therapy before hospital admission into two groups: (1) renin–angiotensin–aldosterone system (RAAS) inhibitors and (2) those without RAAS inhibitors (see supplementary material, Figure-1). A total of 422 patients were included in the final analysis. The procedural strategy was established according to the protocols of each participating institution; however, the decision to maintain or withdraw RAAS inhibitors was determined according to the criteria of the treating medical team.
Study outcomes and main definitionsThe primary end-point was to evaluate all-cause mortality in hypertensive patients with and without RAAS inhibitors. Secondary outcomes were to assess the incidence of respiratory failure and any difference between ACEIs vs. ARB inhibitors.
Fever was considered when the temperature was over>37.5°C. Respiratory failure was defined as paO2<60mmHg, O2 saturation measured with pulse oximetry of <92% breathing room air, and need for invasive or non-invasive mechanical ventilation. Chronic renal failure was defined as a glomerular filtration rate <60mL/min or need for dialysis.
Statistical analysisCategorical variables are reported as absolute values and percentages. Continuous variables are reported as the mean±standard deviation or median and interquartile range. The normal distribution of quantitative variables was verified with the Kolmogorov–Smirnov test. Differences and their 95% confidence interval were calculated for all comparisons and considered significantly if 0 was not within the interval.
To identify factors that were predictive of death, a logistic regression model with the maximum likelihood method was constructed by using backward stepwise selection, which included the variables that were statistically significant in the bivariate analysis or clinically relevant. No more than 1 variable per 10 outcome events was entered in the logistic model to avoid overfitting. For the final model, we calculated odds ratios (OR) adjusted for each of the variables included, along with their 95% confidence interval (CI). Goodness of fit for each model was determined with the Hosmer–Lemeshow test and area under the curve.
To evaluate the impact of ACE and ARB inhibitors to find out whether COVID-19 hypertensive patients may benefit from an increased survival, a propensity score analysis was performed. The dependent variable was either ACEIs or ARBs and the independent variables chosen were: age, diabetes mellitus, chronic kidney disease, chronic obstructive pulmonary disease, ischemic heart disease, creatinine>1.5mg/dL and the hospital to match patients from the same center to inhibit potential differences in medical practices. Importantly, in the propensity analysis performed to test the benefit of ACEIs, patients taking ARBs were excluded from the matched cohort and vice versa. Pairs of patients were derived using greedy nearest neighbor method 1:1 with 1/5 of the standard deviation of the logit of the propensity score as a caliper. The MatchIt package (Ho et al., 2007) was used. All other analyses were conducted using the statistical software IBM SPSS Statistics, Version 25.0. Armonk, NY: IBM Corp.
In the meta-analysis, as a measure of the combined effect for the included studies, the OR was estimated, their 95 CI% and its statistical significance. The homogeneity between studies was contrasted by the QH statistic. Concerning the low sensitivity of this test, we consider p<0.10 values as significant. To overcome this limitation in some way, the I2 statistic was estimated as well, which measures the proportion of the total variation of the studies explained by the heterogeneity and its 95% CI. A random-effects model was used for those cases in which the I2 statistic was greater than 50% and a model of fixed effects for the opposite cases. Publication bias was assessed by Begg's tests and inspection of the funnel plot. Sensitivity analyses were used to test the stability of results. Analysis was performed with StataCorp. 2019 (Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.).
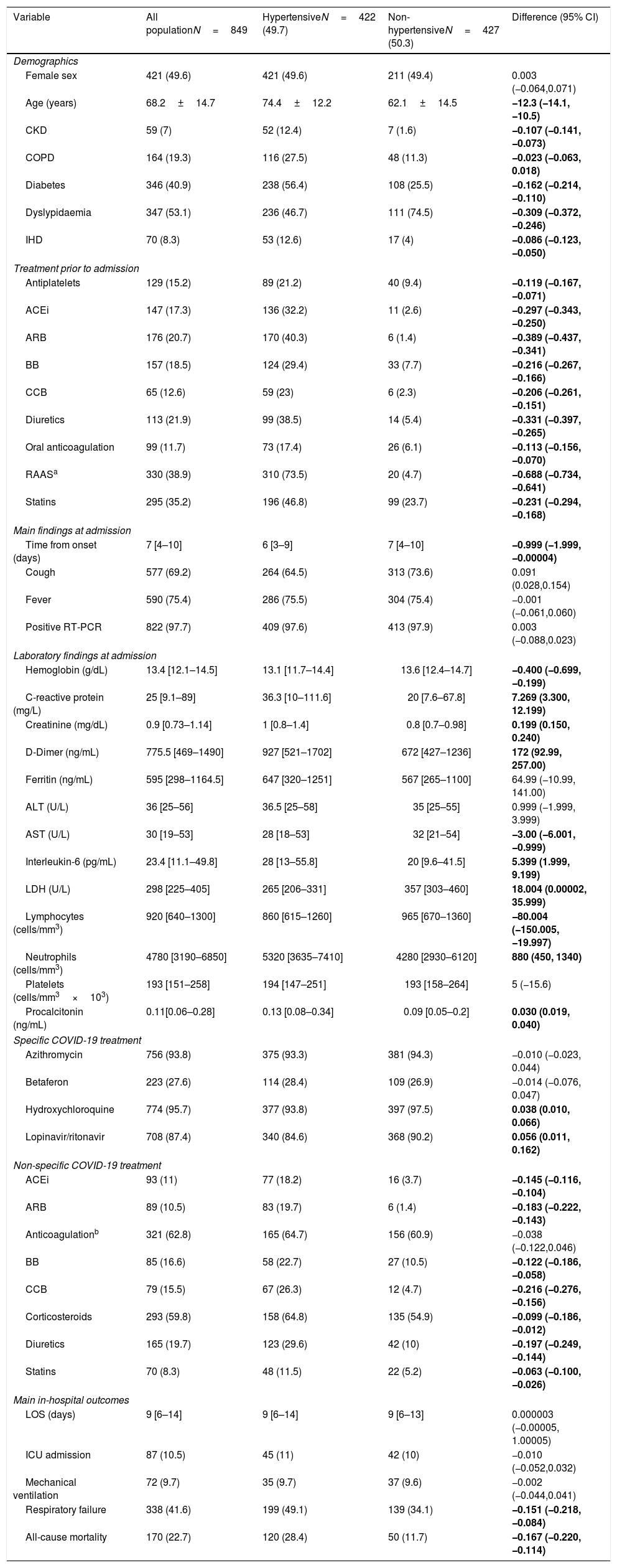
ResultsComparison of hypertensive vs. non-hypertensive COVID-19 patientsMain baseline characteristics and outcomes are summarized in Table 1. Our study group is made up of 849 patients with a definitive diagnosis of SARS-CoV-2 infections, a total of 422 (49.7%) patients were hypertensive and 427 (50.3%) non-hypertensive. Hypertensive patients were older (−12.3 [95% CI −14.1, −10.5]), showed a greater prevalence of several comorbidities, and were more commonly under chronic treatment. Specifically, a total of 330 patients were taking RAAS inhibitors (−0.688 [95% CI −0.734, −0.641]) when admitted to the hospital and they were more commonly prescribed among hypertensive patients.
Baseline characteristics and main features of hypertensive vs. non-hypertensive patients admitted due to coronavirus disease 2019.
| Variable | All populationN=849 | HypertensiveN=422 (49.7) | Non-hypertensiveN=427 (50.3) | Difference (95% CI) |
|---|---|---|---|---|
| Demographics | ||||
| Female sex | 421 (49.6) | 421 (49.6) | 211 (49.4) | 0.003 (−0.064,0.071) |
| Age (years) | 68.2±14.7 | 74.4±12.2 | 62.1±14.5 | −12.3 (−14.1, −10.5) |
| CKD | 59 (7) | 52 (12.4) | 7 (1.6) | −0.107 (−0.141, −0.073) |
| COPD | 164 (19.3) | 116 (27.5) | 48 (11.3) | −0.023 (−0.063, 0.018) |
| Diabetes | 346 (40.9) | 238 (56.4) | 108 (25.5) | −0.162 (−0.214, −0.110) |
| Dyslypidaemia | 347 (53.1) | 236 (46.7) | 111 (74.5) | −0.309 (−0.372, −0.246) |
| IHD | 70 (8.3) | 53 (12.6) | 17 (4) | −0.086 (−0.123, −0.050) |
| Treatment prior to admission | ||||
| Antiplatelets | 129 (15.2) | 89 (21.2) | 40 (9.4) | −0.119 (−0.167, −0.071) |
| ACEi | 147 (17.3) | 136 (32.2) | 11 (2.6) | −0.297 (−0.343, −0.250) |
| ARB | 176 (20.7) | 170 (40.3) | 6 (1.4) | −0.389 (−0.437, −0.341) |
| BB | 157 (18.5) | 124 (29.4) | 33 (7.7) | −0.216 (−0.267, −0.166) |
| CCB | 65 (12.6) | 59 (23) | 6 (2.3) | −0.206 (−0.261, −0.151) |
| Diuretics | 113 (21.9) | 99 (38.5) | 14 (5.4) | −0.331 (−0.397, −0.265) |
| Oral anticoagulation | 99 (11.7) | 73 (17.4) | 26 (6.1) | −0.113 (−0.156, −0.070) |
| RAASa | 330 (38.9) | 310 (73.5) | 20 (4.7) | −0.688 (−0.734, −0.641) |
| Statins | 295 (35.2) | 196 (46.8) | 99 (23.7) | −0.231 (−0.294, −0.168) |
| Main findings at admission | ||||
| Time from onset (days) | 7 [4–10] | 6 [3–9] | 7 [4–10] | −0.999 (−1.999, −0.00004) |
| Cough | 577 (69.2) | 264 (64.5) | 313 (73.6) | 0.091 (0.028,0.154) |
| Fever | 590 (75.4) | 286 (75.5) | 304 (75.4) | −0.001 (−0.061,0.060) |
| Positive RT-PCR | 822 (97.7) | 409 (97.6) | 413 (97.9) | 0.003 (−0.088,0.023) |
| Laboratory findings at admission | ||||
| Hemoglobin (g/dL) | 13.4 [12.1–14.5] | 13.1 [11.7–14.4] | 13.6 [12.4–14.7] | −0.400 (−0.699, −0.199) |
| C-reactive protein (mg/L) | 25 [9.1–89] | 36.3 [10–111.6] | 20 [7.6–67.8] | 7.269 (3.300, 12.199) |
| Creatinine (mg/dL) | 0.9 [0.73–1.14] | 1 [0.8–1.4] | 0.8 [0.7–0.98] | 0.199 (0.150, 0.240) |
| D-Dimer (ng/mL) | 775.5 [469–1490] | 927 [521–1702] | 672 [427–1236] | 172 (92.99, 257.00) |
| Ferritin (ng/mL) | 595 [298–1164.5] | 647 [320–1251] | 567 [265–1100] | 64.99 (−10.99, 141.00) |
| ALT (U/L) | 36 [25–56] | 36.5 [25–58] | 35 [25–55] | 0.999 (−1.999, 3.999) |
| AST (U/L) | 30 [19–53] | 28 [18–53] | 32 [21–54] | −3.00 (−6.001, −0.999) |
| Interleukin-6 (pg/mL) | 23.4 [11.1–49.8] | 28 [13–55.8] | 20 [9.6–41.5] | 5.399 (1.999, 9.199) |
| LDH (U/L) | 298 [225–405] | 265 [206–331] | 357 [303–460] | 18.004 (0.00002, 35.999) |
| Lymphocytes (cells/mm3) | 920 [640–1300] | 860 [615–1260] | 965 [670–1360] | −80.004 (−150.005, −19.997) |
| Neutrophils (cells/mm3) | 4780 [3190–6850] | 5320 [3635–7410] | 4280 [2930–6120] | 880 (450, 1340) |
| Platelets (cells/mm3×103) | 193 [151–258] | 194 [147–251] | 193 [158–264] | 5 (−15.6) |
| Procalcitonin (ng/mL) | 0.11[0.06–0.28] | 0.13 [0.08–0.34] | 0.09 [0.05–0.2] | 0.030 (0.019, 0.040) |
| Specific COVID-19 treatment | ||||
| Azithromycin | 756 (93.8) | 375 (93.3) | 381 (94.3) | −0.010 (−0.023, 0.044) |
| Betaferon | 223 (27.6) | 114 (28.4) | 109 (26.9) | −0.014 (−0.076, 0.047) |
| Hydroxychloroquine | 774 (95.7) | 377 (93.8) | 397 (97.5) | 0.038 (0.010, 0.066) |
| Lopinavir/ritonavir | 708 (87.4) | 340 (84.6) | 368 (90.2) | 0.056 (0.011, 0.162) |
| Non-specific COVID-19 treatment | ||||
| ACEi | 93 (11) | 77 (18.2) | 16 (3.7) | −0.145 (−0.116, −0.104) |
| ARB | 89 (10.5) | 83 (19.7) | 6 (1.4) | −0.183 (−0.222, −0.143) |
| Anticoagulationb | 321 (62.8) | 165 (64.7) | 156 (60.9) | −0.038 (−0.122,0.046) |
| BB | 85 (16.6) | 58 (22.7) | 27 (10.5) | −0.122 (−0.186, −0.058) |
| CCB | 79 (15.5) | 67 (26.3) | 12 (4.7) | −0.216 (−0.276, −0.156) |
| Corticosteroids | 293 (59.8) | 158 (64.8) | 135 (54.9) | −0.099 (−0.186, −0.012) |
| Diuretics | 165 (19.7) | 123 (29.6) | 42 (10) | −0.197 (−0.249, −0.144) |
| Statins | 70 (8.3) | 48 (11.5) | 22 (5.2) | −0.063 (−0.100, −0.026) |
| Main in-hospital outcomes | ||||
| LOS (days) | 9 [6–14] | 9 [6–14] | 9 [6–13] | 0.000003 (−0.00005, 1.00005) |
| ICU admission | 87 (10.5) | 45 (11) | 42 (10) | −0.010 (−0.052,0.032) |
| Mechanical ventilation | 72 (9.7) | 35 (9.7) | 37 (9.6) | −0.002 (−0.044,0.041) |
| Respiratory failure | 338 (41.6) | 199 (49.1) | 139 (34.1) | −0.151 (−0.218, −0.084) |
| All-cause mortality | 170 (22.7) | 120 (28.4) | 50 (11.7) | −0.167 (−0.220, −0.114) |
Abbreviations: ACEi: angiotensin-converting enzyme inhibitor; ALT: alanine aminotransferase; ARB: angiotensin receptor blocker; AST: aspartate aminotransferase; BB: beta-blockers; CCB: calcium channel blockers; CKD: chronic kidney disease; ICU: intensive care unit; IHD: ischemic heart disease; LDH: lactate dehydrogenase; LOS: length of stay; RAAS: renin–angiotensin–aldosterone system inhibitors; RT-PCR: reverse transcription-polymerase chain reaction;
Hypertensive patients were characterized at the time of admission because of higher level of inflammatory markers and parameters of organ damage. Lymphocyte count (−80.004 [95% CI −150.005, −19.997]) was also lower among hypertensive patients. Specific COVID-19 treatment was more commonly prescribed in non-hypertensive as opposed to: corticosteroids (−0.099 [95% CI −0.186, −0.012]), statins (−0.063 [95% CI −0.100, −0.026]), ACEIs (−0.145 [95% CI −0.116, −0.104]) and ARBs (−0.183 [95% CI −0.222, −0.143]). Compared to non-hypertensive patients, the incidence of respiratory failure (−0.151 [95% CI −0.218, −0.084]) and death rate (−0.167 [95% CI −0.220, −0.114]) was greater among hypertensive COVID-19 patients.
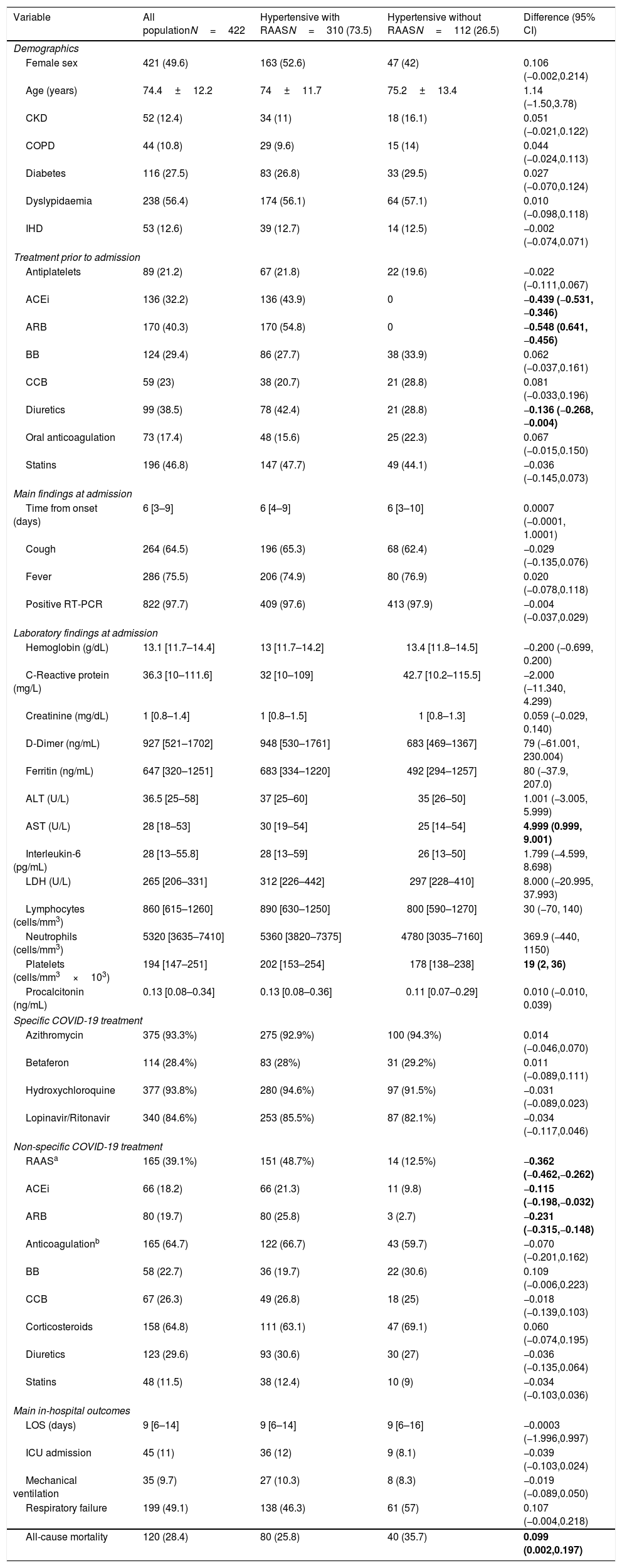
Comparison of hypertensive COVID-19 patients with and without RAAS inhibitorsThe main characteristics of hypertensive patients are summarized in Table 2. Of the hypertensive cohort (n=422), 73.5% of patients had chronic treatment with RAAS inhibitors and no differences were observed in basal clinical characteristics or main comorbidities. The proportion of patients receiving RAAS inhibitors during hospitalization was greater in previous RAAS users (−0.362 [95% CI −0.462, −0.262]) with comparable rates in respect to other treatments. Interestingly, in-hospital outcomes except for higher all-cause mortality in non-RAAS users (0.099 [95% CI 0.002, 0.197] were comparable). Such difference in respect to mortality, remained significant when we evaluated those with only RAAS vs. non-RAAS (0.220 [95% CI 0.095, 0.346]) before admission.
Baseline characteristics and main features during admission of hypertensive hospitalized patients according to chronic anti-hypertensive baseline treatment.
| Variable | All populationN=422 | Hypertensive with RAASN=310 (73.5) | Hypertensive without RAASN=112 (26.5) | Difference (95% CI) |
|---|---|---|---|---|
| Demographics | ||||
| Female sex | 421 (49.6) | 163 (52.6) | 47 (42) | 0.106 (−0.002,0.214) |
| Age (years) | 74.4±12.2 | 74±11.7 | 75.2±13.4 | 1.14 (−1.50,3.78) |
| CKD | 52 (12.4) | 34 (11) | 18 (16.1) | 0.051 (−0.021,0.122) |
| COPD | 44 (10.8) | 29 (9.6) | 15 (14) | 0.044 (−0.024,0.113) |
| Diabetes | 116 (27.5) | 83 (26.8) | 33 (29.5) | 0.027 (−0.070,0.124) |
| Dyslypidaemia | 238 (56.4) | 174 (56.1) | 64 (57.1) | 0.010 (−0.098,0.118) |
| IHD | 53 (12.6) | 39 (12.7) | 14 (12.5) | −0.002 (−0.074,0.071) |
| Treatment prior to admission | ||||
| Antiplatelets | 89 (21.2) | 67 (21.8) | 22 (19.6) | −0.022 (−0.111,0.067) |
| ACEi | 136 (32.2) | 136 (43.9) | 0 | −0.439 (−0.531, −0.346) |
| ARB | 170 (40.3) | 170 (54.8) | 0 | −0.548 (0.641, −0.456) |
| BB | 124 (29.4) | 86 (27.7) | 38 (33.9) | 0.062 (−0.037,0.161) |
| CCB | 59 (23) | 38 (20.7) | 21 (28.8) | 0.081 (−0.033,0.196) |
| Diuretics | 99 (38.5) | 78 (42.4) | 21 (28.8) | −0.136 (−0.268, −0.004) |
| Oral anticoagulation | 73 (17.4) | 48 (15.6) | 25 (22.3) | 0.067 (−0.015,0.150) |
| Statins | 196 (46.8) | 147 (47.7) | 49 (44.1) | −0.036 (−0.145,0.073) |
| Main findings at admission | ||||
| Time from onset (days) | 6 [3–9] | 6 [4–9] | 6 [3–10] | 0.0007 (−0.0001, 1.0001) |
| Cough | 264 (64.5) | 196 (65.3) | 68 (62.4) | −0.029 (−0.135,0.076) |
| Fever | 286 (75.5) | 206 (74.9) | 80 (76.9) | 0.020 (−0.078,0.118) |
| Positive RT-PCR | 822 (97.7) | 409 (97.6) | 413 (97.9) | −0.004 (−0.037,0.029) |
| Laboratory findings at admission | ||||
| Hemoglobin (g/dL) | 13.1 [11.7–14.4] | 13 [11.7–14.2] | 13.4 [11.8–14.5] | −0.200 (−0.699, 0.200) |
| C-Reactive protein (mg/L) | 36.3 [10–111.6] | 32 [10–109] | 42.7 [10.2–115.5] | −2.000 (−11.340, 4.299) |
| Creatinine (mg/dL) | 1 [0.8–1.4] | 1 [0.8–1.5] | 1 [0.8–1.3] | 0.059 (−0.029, 0.140) |
| D-Dimer (ng/mL) | 927 [521–1702] | 948 [530–1761] | 683 [469–1367] | 79 (−61.001, 230.004) |
| Ferritin (ng/mL) | 647 [320–1251] | 683 [334–1220] | 492 [294–1257] | 80 (−37.9, 207.0) |
| ALT (U/L) | 36.5 [25–58] | 37 [25–60] | 35 [26–50] | 1.001 (−3.005, 5.999) |
| AST (U/L) | 28 [18–53] | 30 [19–54] | 25 [14–54] | 4.999 (0.999, 9.001) |
| Interleukin-6 (pg/mL) | 28 [13–55.8] | 28 [13–59] | 26 [13–50] | 1.799 (−4.599, 8.698) |
| LDH (U/L) | 265 [206–331] | 312 [226–442] | 297 [228–410] | 8.000 (−20.995, 37.993) |
| Lymphocytes (cells/mm3) | 860 [615–1260] | 890 [630–1250] | 800 [590–1270] | 30 (−70, 140) |
| Neutrophils (cells/mm3) | 5320 [3635–7410] | 5360 [3820–7375] | 4780 [3035–7160] | 369.9 (−440, 1150) |
| Platelets (cells/mm3×103) | 194 [147–251] | 202 [153–254] | 178 [138–238] | 19 (2, 36) |
| Procalcitonin (ng/mL) | 0.13 [0.08–0.34] | 0.13 [0.08–0.36] | 0.11 [0.07–0.29] | 0.010 (−0.010, 0.039) |
| Specific COVID-19 treatment | ||||
| Azithromycin | 375 (93.3%) | 275 (92.9%) | 100 (94.3%) | 0.014 (−0.046,0.070) |
| Betaferon | 114 (28.4%) | 83 (28%) | 31 (29.2%) | 0.011 (−0.089,0.111) |
| Hydroxychloroquine | 377 (93.8%) | 280 (94.6%) | 97 (91.5%) | −0.031 (−0.089,0.023) |
| Lopinavir/Ritonavir | 340 (84.6%) | 253 (85.5%) | 87 (82.1%) | −0.034 (−0.117,0.046) |
| Non-specific COVID-19 treatment | ||||
| RAASa | 165 (39.1%) | 151 (48.7%) | 14 (12.5%) | −0.362 (−0.462,−0.262) |
| ACEi | 66 (18.2) | 66 (21.3) | 11 (9.8) | −0.115 (−0.198,−0.032) |
| ARB | 80 (19.7) | 80 (25.8) | 3 (2.7) | −0.231 (−0.315,−0.148) |
| Anticoagulationb | 165 (64.7) | 122 (66.7) | 43 (59.7) | −0.070 (−0.201,0.162) |
| BB | 58 (22.7) | 36 (19.7) | 22 (30.6) | 0.109 (−0.006,0.223) |
| CCB | 67 (26.3) | 49 (26.8) | 18 (25) | −0.018 (−0.139,0.103) |
| Corticosteroids | 158 (64.8) | 111 (63.1) | 47 (69.1) | 0.060 (−0.074,0.195) |
| Diuretics | 123 (29.6) | 93 (30.6) | 30 (27) | −0.036 (−0.135,0.064) |
| Statins | 48 (11.5) | 38 (12.4) | 10 (9) | −0.034 (−0.103,0.036) |
| Main in-hospital outcomes | ||||
| LOS (days) | 9 [6–14] | 9 [6–14] | 9 [6–16] | −0.0003 (−1.996,0.997) |
| ICU admission | 45 (11) | 36 (12) | 9 (8.1) | −0.039 (−0.103,0.024) |
| Mechanical ventilation | 35 (9.7) | 27 (10.3) | 8 (8.3) | −0.019 (−0.089,0.050) |
| Respiratory failure | 199 (49.1) | 138 (46.3) | 61 (57) | 0.107 (−0.004,0.218) |
| All-cause mortality | 120 (28.4) | 80 (25.8) | 40 (35.7) | 0.099 (0.002,0.197) |
| All populationN=219 | Hypertensive with only RAASN=156 (71%) | Hypertensive without RAASN=63 (29%) | Difference (95% CI), % | |
|---|---|---|---|---|
| LOS (days) | 9 [6–15] | 10 [7–15] | 8 [5–13] | 1.0005 (−0.0002,2.9995) |
| ICU admission | 28 (13.1) | 23 (15.2) | 5 (8.1) | −0.072 (−0.172,0.029) |
| Mechanical ventilation | 20 (10.6) | 15 (10.9) | 5 (9.8) | −0.011 (−0.112,0.089) |
| Respiratory failure | 100 (42.7) | 68 (45) | 32 (52.5) | 0.074 (−0.075,0.224) |
| All-cause mortality | 56 (25.6) | 30 (19.2) | 26 (41.3) | 0.220 (0.095,0.346) |
| All populationN=310 | Hypertensive with RAAS-oncN=151 (48.7%) | HypertensiveRAAS-offcN=159 (51.3%) | Difference (95% CI), % | |
|---|---|---|---|---|
| LOS (days) | 9 [6–14] | 9 [6–14] | 9 [6–15] | 0.0003 (−1.0003, 1.9996) |
| ICU admission | 36 (12) | 11 (7.3) | 25 (16.8) | 0.094 (0.021,0.168) |
| Mechanical ventilation | 27 (10.3) | 7 (5.3) | 20 (15.2) | 0.098 (0.025,0.171) |
| Respiratory failure | 100 (42.7) | 59 (40.7) | 79 (51.6) | 0.109 (−0.004,0.223) |
| All-cause mortality | 80 (25.8) | 32 (21.2) | 48 (30.2) | 0.090 (−0.008,0.188) |
Abbreviations: ACEi: Angiotensin-converting enzyme inhibitors; ALT: alanine aminotransferase; ARB: angiotensin receptor blocker; AST: aspartate aminotransferase; BB: Beta-blockers; CCB: Calcium channel blockers; CKD: Chronic kidney disease; ICU: Intensive care unit; IHD: Ischemic heart disease; LDH: Lactate dehydrogenase; LOS: Length of stay; RAAS: Rening angiotensin-aldosterone system inhibitors; RT-PCR: Reverse transcription-polymerase chain reaction;
Of note, the frequency of RAAS inhibitors usage during hospitalization in hypertensive COVID-19 patients with previous chronic treatment was 48.7%. During the observation period, those who kept RAAS inhibitors during hospitalization compared with their counterpart showed a smaller rate of intensive care unit admission (0.094 [95% CI 0.021, 0.168]) and need of mechanical ventilation (0.098 [95% CI 0.025, 0.171]). Furthermore, within the hypertensive patients with RAAS inhibitors during hospitalization, a lower rate of respiratory failure (0.109 [95% CI −0.004, 0.223]) and all-cause mortality (0.090 [95% CI −0.008, 0.188]) was observed. Finally, no differences were observed in respect to ACEIs vs. ARBs during hospital admission in respect to main outcomes (data not shown).
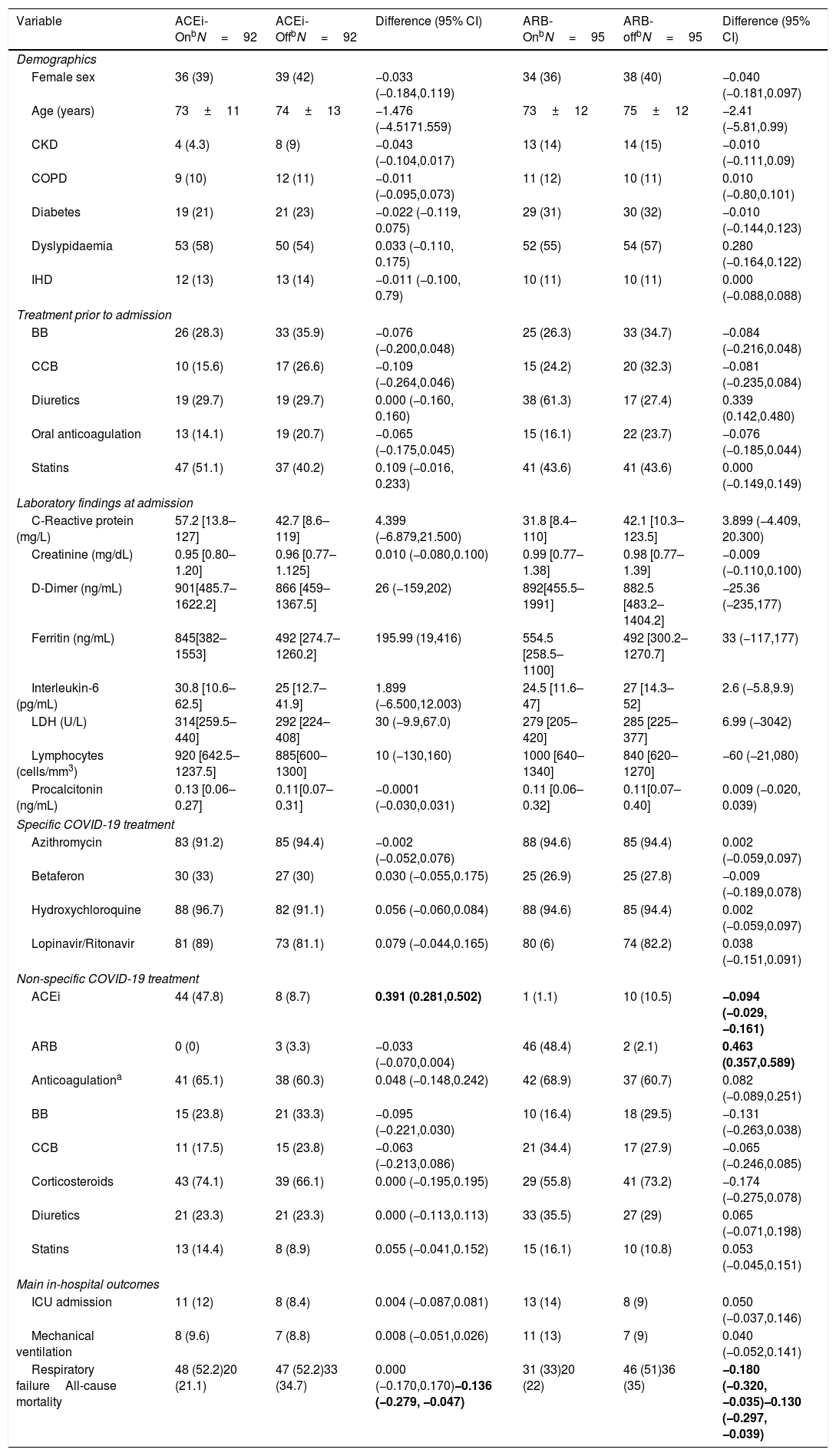
We performed a PSM to further evaluate the benefit of RAAS inhibitors, specifically whether chronic ACEIs or ARBs still show an association with lower all-cause mortality among hypertensive COVID-19 patients (see Table 3) after adjustment for potential cofounders. Compared with non-RAAS inhibitors, the incidence of all-cause mortality was lower irrespective of ACEIs (−0.136 [95% CI −0.279, −0.047]) or ARBs (−0.130 [95% CI −0.297, −0.039]) users in comparison to non-users. To further explore their potential benefit during in-hospital use, despite the small sample, we observed in the matched cohort that continuation of RAAS inhibitors were not associated with greater mortality the least
Baseline Characteristics and main features of the matched hypertensive cohort with chronic angiotensin converting enzyme inhibitors or angiotensin receptor blockers with COVID-19.
| Variable | ACEi-OnbN=92 | ACEi-OffbN=92 | Difference (95% CI) | ARB-OnbN=95 | ARB-offbN=95 | Difference (95% CI) |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Female sex | 36 (39) | 39 (42) | −0.033 (−0.184,0.119) | 34 (36) | 38 (40) | −0.040 (−0.181,0.097) |
| Age (years) | 73±11 | 74±13 | −1.476 (−4.5171.559) | 73±12 | 75±12 | −2.41 (−5.81,0.99) |
| CKD | 4 (4.3) | 8 (9) | −0.043 (−0.104,0.017) | 13 (14) | 14 (15) | −0.010 (−0.111,0.09) |
| COPD | 9 (10) | 12 (11) | −0.011 (−0.095,0.073) | 11 (12) | 10 (11) | 0.010 (−0.80,0.101) |
| Diabetes | 19 (21) | 21 (23) | −0.022 (−0.119, 0.075) | 29 (31) | 30 (32) | −0.010 (−0.144,0.123) |
| Dyslypidaemia | 53 (58) | 50 (54) | 0.033 (−0.110, 0.175) | 52 (55) | 54 (57) | 0.280 (−0.164,0.122) |
| IHD | 12 (13) | 13 (14) | −0.011 (−0.100, 0.79) | 10 (11) | 10 (11) | 0.000 (−0.088,0.088) |
| Treatment prior to admission | ||||||
| BB | 26 (28.3) | 33 (35.9) | −0.076 (−0.200,0.048) | 25 (26.3) | 33 (34.7) | −0.084 (−0.216,0.048) |
| CCB | 10 (15.6) | 17 (26.6) | −0.109 (−0.264,0.046) | 15 (24.2) | 20 (32.3) | −0.081 (−0.235,0.084) |
| Diuretics | 19 (29.7) | 19 (29.7) | 0.000 (−0.160, 0.160) | 38 (61.3) | 17 (27.4) | 0.339 (0.142,0.480) |
| Oral anticoagulation | 13 (14.1) | 19 (20.7) | −0.065 (−0.175,0.045) | 15 (16.1) | 22 (23.7) | −0.076 (−0.185,0.044) |
| Statins | 47 (51.1) | 37 (40.2) | 0.109 (−0.016, 0.233) | 41 (43.6) | 41 (43.6) | 0.000 (−0.149,0.149) |
| Laboratory findings at admission | ||||||
| C-Reactive protein (mg/L) | 57.2 [13.8–127] | 42.7 [8.6–119] | 4.399 (−6.879,21.500) | 31.8 [8.4–110] | 42.1 [10.3–123.5] | 3.899 (−4.409, 20.300) |
| Creatinine (mg/dL) | 0.95 [0.80–1.20] | 0.96 [0.77–1.125] | 0.010 (−0.080,0.100) | 0.99 [0.77–1.38] | 0.98 [0.77–1.39] | −0.009 (−0.110,0.100) |
| D-Dimer (ng/mL) | 901[485.7–1622.2] | 866 [459–1367.5] | 26 (−159,202) | 892[455.5–1991] | 882.5 [483.2–1404.2] | −25.36 (−235,177) |
| Ferritin (ng/mL) | 845[382–1553] | 492 [274.7–1260.2] | 195.99 (19,416) | 554.5 [258.5–1100] | 492 [300.2–1270.7] | 33 (−117,177) |
| Interleukin-6 (pg/mL) | 30.8 [10.6–62.5] | 25 [12.7–41.9] | 1.899 (−6.500,12.003) | 24.5 [11.6–47] | 27 [14.3–52] | 2.6 (−5.8,9.9) |
| LDH (U/L) | 314[259.5–440] | 292 [224–408] | 30 (−9.9,67.0) | 279 [205–420] | 285 [225–377] | 6.99 (−3042) |
| Lymphocytes (cells/mm3) | 920 [642.5–1237.5] | 885[600–1300] | 10 (−130,160) | 1000 [640–1340] | 840 [620–1270] | −60 (−21,080) |
| Procalcitonin (ng/mL) | 0.13 [0.06–0.27] | 0.11[0.07–0.31] | −0.0001 (−0.030,0.031) | 0.11 [0.06–0.32] | 0.11[0.07–0.40] | 0.009 (−0.020, 0.039) |
| Specific COVID-19 treatment | ||||||
| Azithromycin | 83 (91.2) | 85 (94.4) | −0.002 (−0.052,0.076) | 88 (94.6) | 85 (94.4) | 0.002 (−0.059,0.097) |
| Betaferon | 30 (33) | 27 (30) | 0.030 (−0.055,0.175) | 25 (26.9) | 25 (27.8) | −0.009 (−0.189,0.078) |
| Hydroxychloroquine | 88 (96.7) | 82 (91.1) | 0.056 (−0.060,0.084) | 88 (94.6) | 85 (94.4) | 0.002 (−0.059,0.097) |
| Lopinavir/Ritonavir | 81 (89) | 73 (81.1) | 0.079 (−0.044,0.165) | 80 (6) | 74 (82.2) | 0.038 (−0.151,0.091) |
| Non-specific COVID-19 treatment | ||||||
| ACEi | 44 (47.8) | 8 (8.7) | 0.391 (0.281,0.502) | 1 (1.1) | 10 (10.5) | −0.094 (−0.029,−0.161) |
| ARB | 0 (0) | 3 (3.3) | −0.033 (−0.070,0.004) | 46 (48.4) | 2 (2.1) | 0.463 (0.357,0.589) |
| Anticoagulationa | 41 (65.1) | 38 (60.3) | 0.048 (−0.148,0.242) | 42 (68.9) | 37 (60.7) | 0.082 (−0.089,0.251) |
| BB | 15 (23.8) | 21 (33.3) | −0.095 (−0.221,0.030) | 10 (16.4) | 18 (29.5) | −0.131 (−0.263,0.038) |
| CCB | 11 (17.5) | 15 (23.8) | −0.063 (−0.213,0.086) | 21 (34.4) | 17 (27.9) | −0.065 (−0.246,0.085) |
| Corticosteroids | 43 (74.1) | 39 (66.1) | 0.000 (−0.195,0.195) | 29 (55.8) | 41 (73.2) | −0.174 (−0.275,0.078) |
| Diuretics | 21 (23.3) | 21 (23.3) | 0.000 (−0.113,0.113) | 33 (35.5) | 27 (29) | 0.065 (−0.071,0.198) |
| Statins | 13 (14.4) | 8 (8.9) | 0.055 (−0.041,0.152) | 15 (16.1) | 10 (10.8) | 0.053 (−0.045,0.151) |
| Main in-hospital outcomes | ||||||
| ICU admission | 11 (12) | 8 (8.4) | 0.004 (−0.087,0.081) | 13 (14) | 8 (9) | 0.050 (−0.037,0.146) |
| Mechanical ventilation | 8 (9.6) | 7 (8.8) | 0.008 (−0.051,0.026) | 11 (13) | 7 (9) | 0.040 (−0.052,0.141) |
| Respiratory failureAll-cause mortality | 48 (52.2)20 (21.1) | 47 (52.2)33 (34.7) | 0.000 (−0.170,0.170)−0.136 (−0.279, −0.047) | 31 (33)20 (22) | 46 (51)36 (35) | −0.180 (−0.320,−0.035)−0.130 (−0.297,−0.039) |
| RAAS-oncN=45 | RAAS-offcN=47 | Difference (95% CI) | RAAS-oncN=47 | RAAS-offcN=48 | Difference (95% CI) | |
|---|---|---|---|---|---|---|
| LOS (days) | 10 [6–17] | 9 [6–13] | 1.00 (−1.00, 4.00) | 7 [6–11] | 7 [4–14.5] | −6.11 (−3.00, 2.00) |
| ICU admission | 3 (6.8) | 5 (11.1) | −0.043 (−0.164,0.079) | 3 (6.4) | 10 (21.7) | −0.153 (−0.294,0.013) |
| Mechanical ventilation | 2 (5) | 3 (8.1) | −0.031 (−0.144,0.082) | 2 (5) | 9 (20.5) | −0.155 (−0.296,0.013) |
| Respiratory failure | 22 (53.7) | 23 (48.9) | 0.048 (−0.167,0.262) | 9 (19.6) | 22 (46.8) | −0.272 (−0.460,0.084) |
| All-cause mortality | 8 (17.8) | 12 (25.5) | −0.077 (−0.250,0.094) | 5 (10.6) | 15 (31.3) | −0.207 (−0.369,0.044) |
Abbreviations: ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BB: beta-blockers; CCB: calcium channel blockers; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; ICU: intensive care unit; IHD: ischemic heart disease; LDH: lactate dehydrogenase; LOS: length of stay.
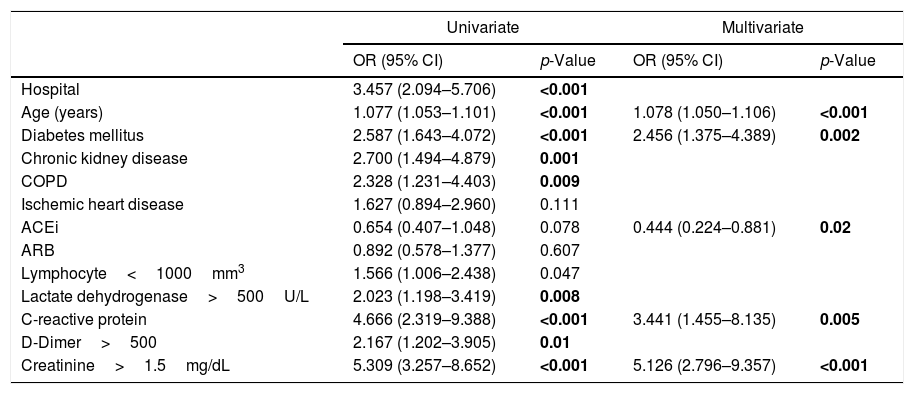
After a univariate analysis of the hypertensive COVID-19 patients, a logistic regression model was performed (seeTable 4) including the following variables: age, diabetes mellitus, chronic kidney disease, ischemic heart disease, ACEIs at admission, ARBs at admission, lymphocytes<1000/mm3, lactate dehydrogenase>250U/L, D-dimer>500μm/L, creatinine>1.5mg/dL, C-reactive protein>10mg/L and hospital. The final model identified that among hypertensive patients age, diabetes mellitus, C-reactive protein, and creatinine were associated with a greater risk of all-cause mortality. On the contrary, ACEIs at admission independently protected from mortality (OR 0.444 [95% CI 0.224–0.881], p=0.02).
Predictors of all-cause mortality in the study hypertensive population.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Hospital | 3.457 (2.094–5.706) | <0.001 | ||
| Age (years) | 1.077 (1.053–1.101) | <0.001 | 1.078 (1.050–1.106) | <0.001 |
| Diabetes mellitus | 2.587 (1.643–4.072) | <0.001 | 2.456 (1.375–4.389) | 0.002 |
| Chronic kidney disease | 2.700 (1.494–4.879) | 0.001 | ||
| COPD | 2.328 (1.231–4.403) | 0.009 | ||
| Ischemic heart disease | 1.627 (0.894–2.960) | 0.111 | ||
| ACEi | 0.654 (0.407–1.048) | 0.078 | 0.444 (0.224–0.881) | 0.02 |
| ARB | 0.892 (0.578–1.377) | 0.607 | ||
| Lymphocyte<1000mm3 | 1.566 (1.006–2.438) | 0.047 | ||
| Lactate dehydrogenase>500U/L | 2.023 (1.198–3.419) | 0.008 | ||
| C-reactive protein | 4.666 (2.319–9.388) | <0.001 | 3.441 (1.455–8.135) | 0.005 |
| D-Dimer>500 | 2.167 (1.202–3.905) | 0.01 | ||
| Creatinine>1.5mg/dL | 5.309 (3.257–8.652) | <0.001 | 5.126 (2.796–9.357) | <0.001 |
Abbreviations: ACEi: Angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blocker; COPD: Chronic obstructive pulmonary disease.
Hosmer–Lemeshow, p=0.530 AUC 0.836 (0.793, 0.878).
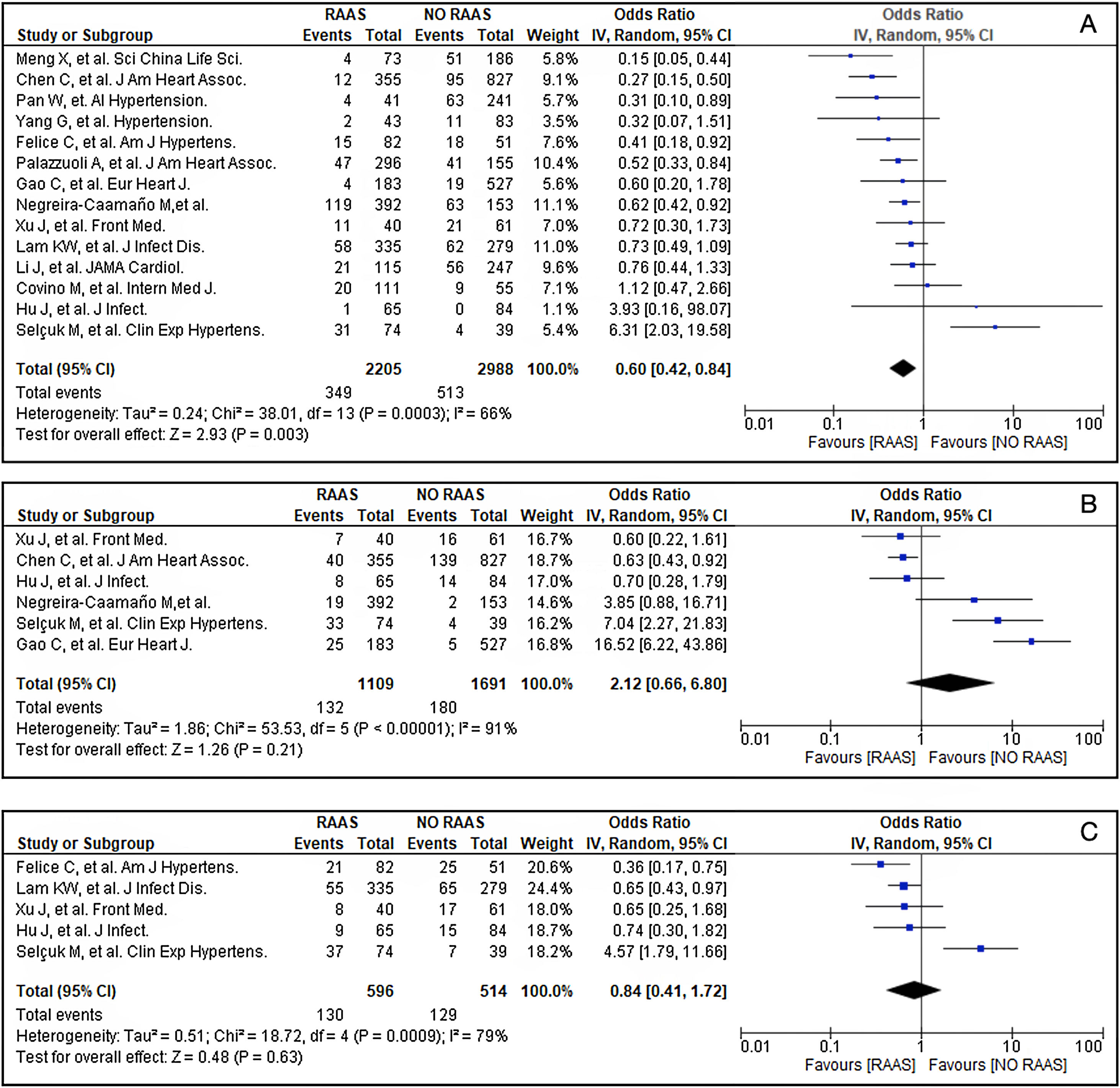
A review of the literature was conducted by two researchers (AA and PC) in PubMed and from the reference list of the retrieved studies to evaluate the impact of chronic use of RAAS inhibitors among hypertensive COVID-19 admitted patients. Eligible studies were retrospective peer-reviewed published in English that evaluated the impact of RAAS inhibitors in all-cause mortality of hypertensive COVID-19 patients between January/November 2020 (see supplementary material, Table S1).11–24 Exclusion criteria were: (1) non-peer-reviewed papers from preprint servers, (2) abstracts, and (3) samples<100 patients. The following terms were searched: “COVID-19” or “SARS-CoV-2” and “hypertension” and “renin–angiotensin–aldosterone system” or “angiotensin receptor blocker” or “angiotensin-converting enzyme inhibitor”. The study quality of the selected studies was assessed by using Newcastle–Ottawa Scale (NOS; Table S1). Overall, prior use of RAAS inhibitors among admitted hypertensive COVID-19 patients was associated with lower all-cause mortality (OR 0.6 [95% CI 0.42–0.8]; p<0.003) (Fig. 1A). We did not observe a protective effect of RAAS inhibitors in respect to mechanical ventilation (OR 2.12 [95% CI 0.66–6.80]; p=0.21) (Fig. 1B) or intensive care unit admission (OR 0.84 [95% CI 0.41–1.72]; p=0.63) (Fig. 1C).
Forrest plot of showing the Odds Ratio (OR) of the main outcomes: (A) All-cause mortality; (B) Mechanical ventilation; and (C) Intensive care unit admission. *Vertical line represents “no difference” point between hypertensive COVID-19 patients with chronic RAAS vs. Non-RAAS inhibitors treatment; Horizontal lines 95% confidence interval (CI). Squares represent the odds ratio for each study (the size of each square denotes the proportion of information given by each study); Diamonds represent pooled odds ratios from all studies.
Ever since the beginning of the current global outbreak, the potential beneficial or harmful effects of RAAS inhibitors have been a subject of ongoing discussions. Our main findings are: (1) in hypertensive COVID-19 patients prior treatment with RAAS inhibitors were associated with a lower risk of all-cause mortality; (2) this protective benefit was also observed after PSM in both ACEIs and ARBs users; (3) a meta-analysis showed that previous RAAS inhibitors use was associated with mortality risk decrement in COVID-19 with preexisting hypertension; (4) continuation of RAAS inhibitors during hospitalization in hypertensive COVID-19 patients are not associated with adverse outcomes.
Previous studies have assessed the effect of RAAS blockade on COVID-19 hospitalized patients with overall positive results.25 For instance, Reynolds et al.26 obtained data from 12,594 patients’ electronic health records and failed to show an association between ACEIs and ARBs with a positive test result or severe disease by performing a propensity and a Bayesian analysis. Interestingly, two retrospective studies showed a neutral effect on mortality of previous treatment with RAAS inhibitors,27,28 these differences could be explained by the fact that they evaluate a low-risk population. On the contrary, high-risk COVID-19 patients are characterized by heart disease29 and a great burden of several cardiovascular risk factors; In particular, hypertension is associated with disease severity and mortality.30,SR1
We have observed in our cohort a high prevalence of hypertensive patients (49.7%), of whom 73.5% were under chronic RAAS treatment. ACE2 favors SARS-CoV-2 entry into the cells,SR2 RAAS inhibitors have shown in experimental models to increase ACE2 expression6,SR3,SR4 and AG-II plasma levels were correlated with total viral load and severity of lung injury in COVID-19 patients.SR5 An interesting clinical study performed on 1128 patients suggests that RAAS blockade is beneficial in the context of previous hypertension and COVID-19.29 Nevertheless, the inclusion criteria were very stringent: only patients who received RAAS blockade during hospital stay were considered so that nothing is known about patients who were on those medications before admission, and only 31 patients took ACE inhibitors. More importantly, patients older than 74 years have been excluded from the study; older patients are particularly affected by COVID-19, with 35,5% older than 74 years in our series, and bear poor prognosis. Taken into account this information, we decided to explore the impact of chronic use of RAAS inhibitors in hypertensive COVID-19 separately.
Our work suggests that maintenance RAAS blockade exceeds by far the negative effects shown in experimental studies. According to our findings, hypertensive patients from our sample had higher crude mortality in comparison to non-hypertensive patients, but an adjusted regression logistic analysis and both propensity score analysis showed a positive association between previous treatment with RAAS inhibitors in hypertensive COVID-19 patients. Our results support the well-known protective effects of blocking the RAAS in hypertension, diabetes mellitus, chronic kidney disease, and ischemic heart disease that may offset any putative assistance to the SARS-CoV-2 entrance into the cells.
To further address the impact of chronic use of RAAS inhibitors in hypertensive COVID-19 patients, we performed a meta-analysis.11–24 In this meta-analysis, previous treatment with RAAS inhibitors was associated with a lower risk of all-cause mortality among hospitalized hypertensive COVID-19 patients. Moreover, we found a neutral effect on intensive care unit admission and the need of mechanical ventilation. Several are the potential explanations, particularly if we take into account the high observed heterogenicity, but a meta-regression analysis ruled out a potential contributing role of the studied sample size. Thereby, other major explanations could be a “healthy user-sick stopper”SR6 or delayed hospitalization among other unmeasured cofounders.
Overall, additional studies are still needed to elucidate all the potential scenarios as many studies have not been designed to detect a potential causal association or identify differences between ACEi and ARB. In this sense, some studies are testing whether losartan improves outcomes in COVID-19. Previous studies and our findings suggest that RAAS blockade is not harmful before and during the hospital stay in hypertensive patients, but the majority of ongoing trials include patients without chronic RAAS blockers intake who have respiratory failure (NCT 04312009, NCT 04340557, NCT 04335123), a completely different scenario. Based on the current evidence, losartan could be of benefit in ambulatory COVID-19 patients as it is being tested in another trial (NCT 04311177) although that is again a different subset of patients. In this regard, the investigators of the RASTAVI trial (NCT03201185), which is currently randomizing patients with severe aortic stenosis who have an indication for a percutaneous aortic prosthesis to ramipril or not, showed that randomization to ramipril had no impact on the incidence or severity of COVID-19.SR7
The retrospective nature of our work bears the inherent limitations of this kind of investigation and should be considered as a hypothesis generator. First, the collection of data relies on documents not always updated; data on the specific drug and dosage have been checked when possible and always with the electronic records. Second, the results may not be generalizable as we did not evaluate outpatients and the meta-analysis should be interpreted cautiously due to the high heterogenicity, likely explained by the variability of the studied samples and especially with the disease severity or other unmeasured confounding factors (such as drug doses, time of inclusion and study design). The comparison between patients who maintain and discontinue RAAS blockers during hospital stay is biased since it can be assumed that these medications are halted in those who need mechanical ventilation or are in a worse clinical condition (“indication bias”). We cannot be certain of the impact of maintaining or withdrawing RAAS inhibitors in them.
To summarize, our results suggest a positive association between ACEIs and ARBs intake and survival in COVID-19 patients with preexisting hypertension who need hospitalization. Our findings are supported by a meta-analysis. Moreover, maintaining these medications during hospital stays may be associated with better outcomes. Future studies are warranted, as only a prospective randomized study in hypertensive patients free of the infection testing RAAS blockade against placebo would give us evidence-based answers.
Financial sourcesThis work has received financial support from the Gerencia Regional de Salud de Castilla y León (GRS COVID 113/A/20).
Conflicts of interestThe authors declare that they have no conflicts of interest.