To assess clinical outcomes according to the immunosuppressive treatment administered to patients with severe SARS-CoV-2 pneumonia and moderate inflammation.
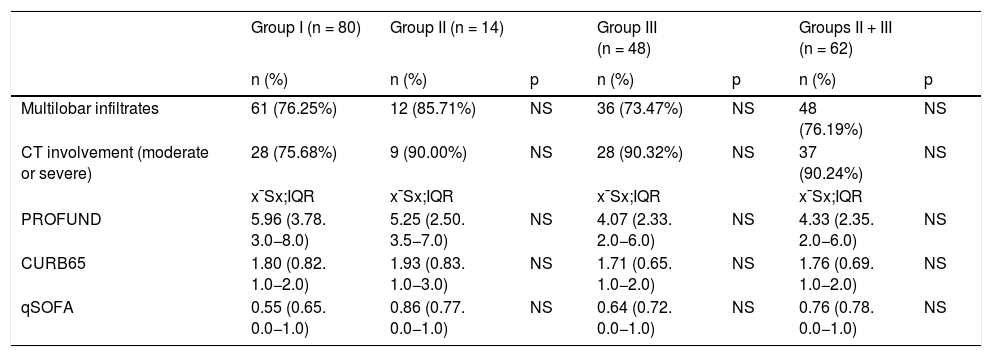
MethodsA retrospective observational cohort study involving 142 patients with severe COVID-19 pneumonia and moderate inflammation divided into three treatment groups (pulses of methylprednisolone alone [group I], tocilizumab alone [group II] and methylprednisolone plus tocilizumab [group III]). The aim was to assess intergroups differences in the clinical course with a 60-day follow-up and related analytical factors.
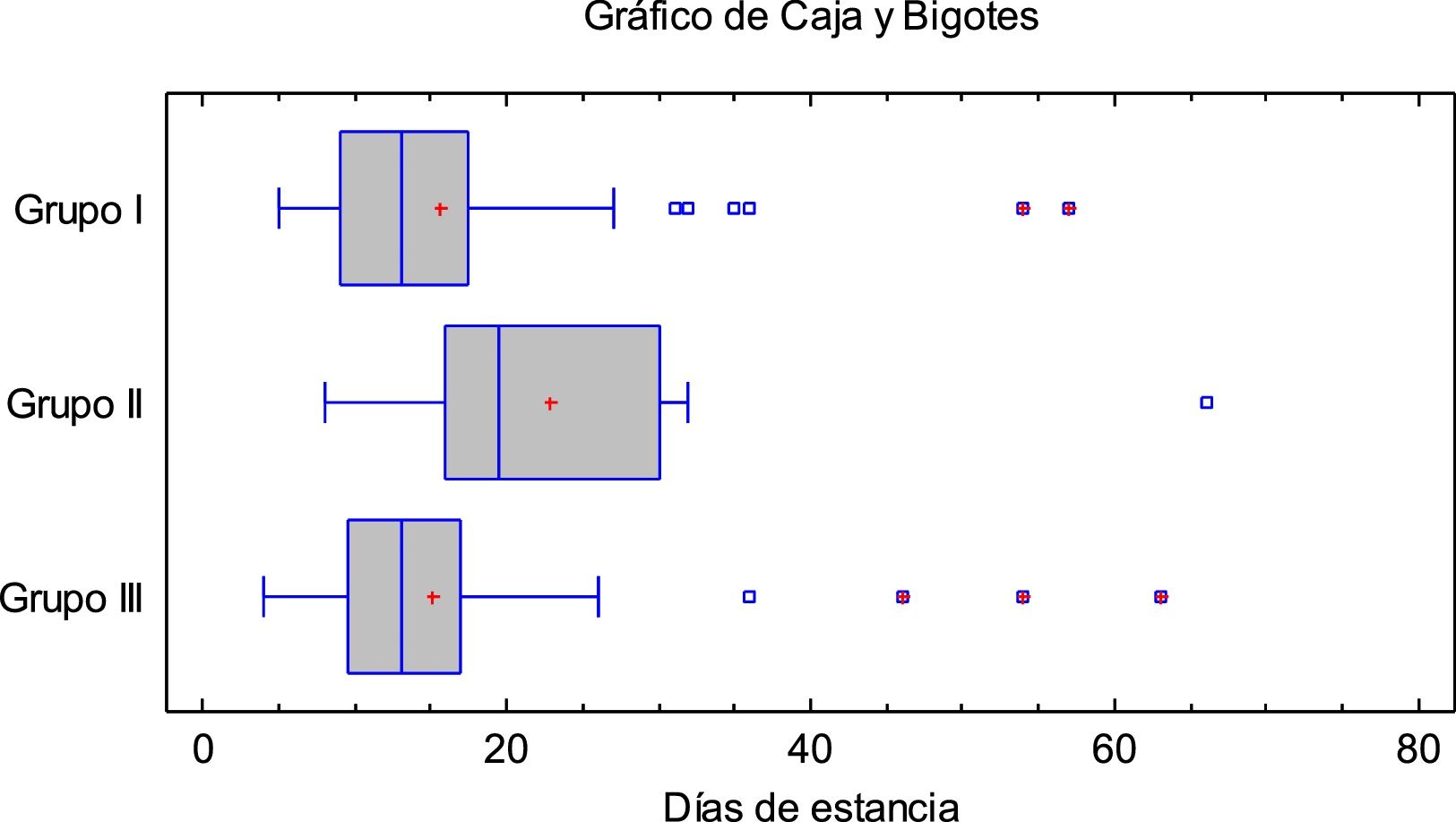
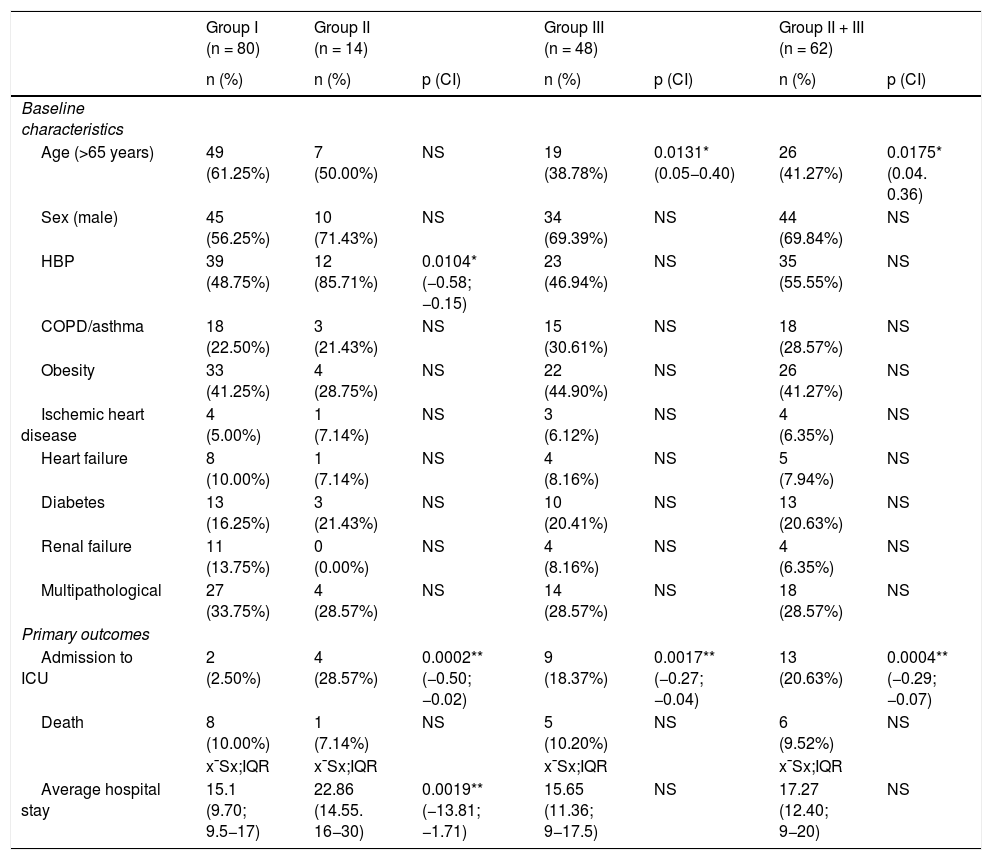
Results14 patients (9,8%) died: 8 (10%) in group I and 6 (9,5%) in groups II and III. 15 (10,6%) were admitted to ICU: 2 (2,5%) from group I, 4 (28,5%) from group II and 9 (18,4%) from group III. The mean hospital stay was longer in group II and clinical outcome was not associated with treatment.
ConclusionsTocilizumab seems to be not associated with better clinical outcomes and should be reserved for clinical trial scenario, since its widespread use may result in higher rate of ICU admission and longer mean hospital stay without differences in mortality rate and potentially adverse events.
Analizar si existen diferencias en desenlaces clínicos según el tratamiento inmunosupresor recibido en pacientes con neumonía grave por SARS-CoV-2 e inflamación moderada.
MétodosEstudio de cohortes retrospectivo de 142 pacientes con neumonía grave COVID-19 e inflamación moderada. Se dividieron en tres grupos de tratamiento (pulsos de metilprednisolona solo [grupo I], tocilizumab solo [grupo II] y metilprednisolona más tocilizumab [grupo III]). Analizamos las diferencias intergrupos en el curso clínico con un seguimiento de 60 días y factores clínicos analíticos relacionados.
ResultadosFallecieron 14 pacientes (9,8%): 8 (10%) del grupo I y 6 (9,5%) de los grupos II y III. Quince (10,6%) ingresaron en UCI: 2 (2,5%) del grupo I, 4 (28,5%) del grupo II y 9 (18,4%) del grupo III. La estancia media hospitalaria fue mayor en los del grupo II. La evolución clínica no se asoció al tratamiento administrado.
ConclusionesEl uso de tocilizumab debería reservarse para escenarios de ensayos clínicos. Su utilización generalizada podría acompañarse de mayor estancia media hospitalaria e ingreso en UCI sin diferencias en la mortalidad con un potencial aumento de efectos adversos.