Romiplostim, a thrombopoietin-receptor agonist, is approved for second-line use in idiopathic thrombocytopenic purpura (ITP) patients where surgery is contraindicated. Anti-CD20 rituximab, an immunosuppressant, is currently used off-label. This analysis compared the cost per responder for romiplostim versus rituximab in Spain.
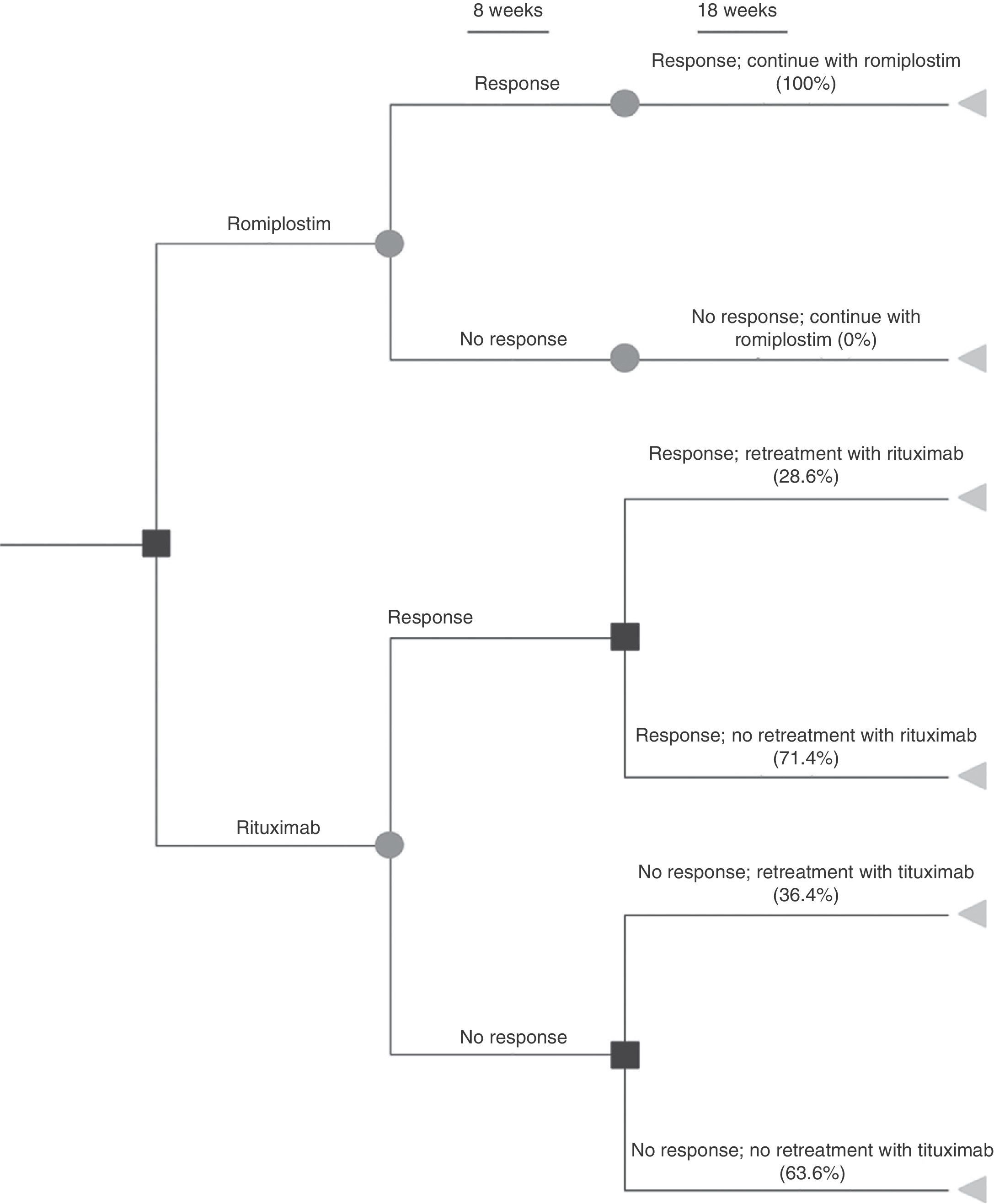
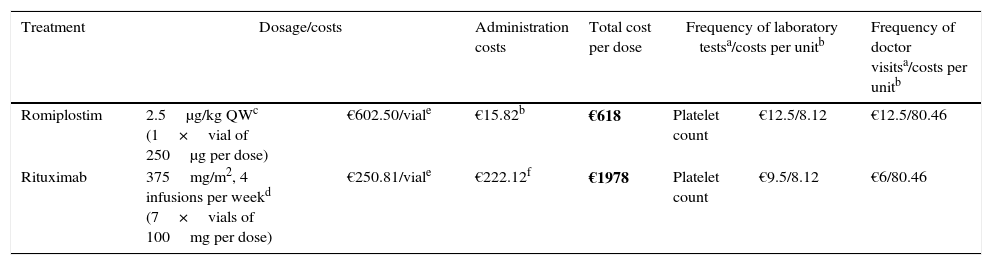
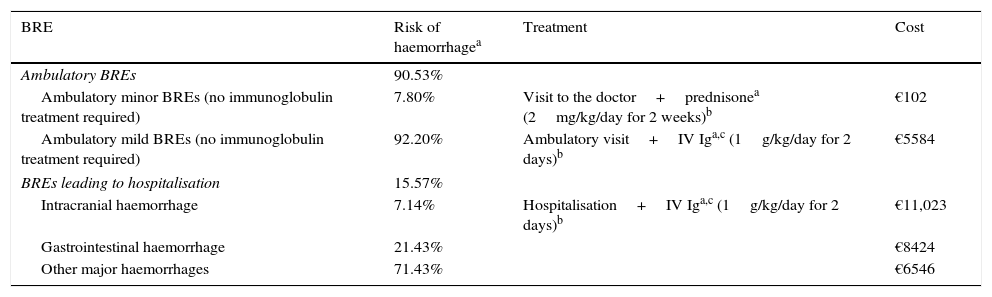
Materials and methodA decision analytic model was constructed to estimate the 6-month cost per responding patient (achieving a platelet count ≥50×109/L) according to the most robust published data. A systematic literature review was performed to extract response rates from phase 3 randomised controlled trials. Romiplostim patients received weekly injections; rituximab patients received 4 weekly intravenous infusions. Medical resource costs were obtained from Spanish reimbursement lists. Treatment non-responders incurred bleeding-related event (BRE) management costs as reported in clinical trials. Medical resource utilisation and clinical practice were based on Spanish treatment guidelines and validated by local clinical experts.
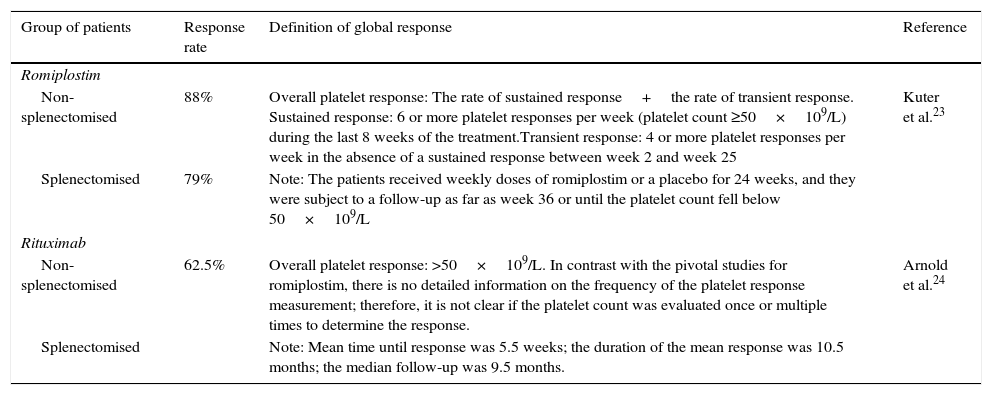
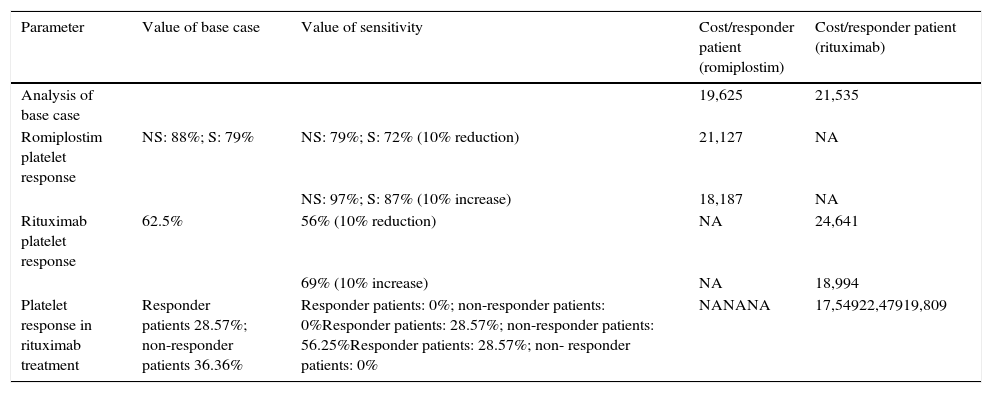
ResultsThe literature review identified phase 3 romiplostim trials with a response rate of 83%. Due to a lack of phase 3 controlled rituximab trials, a systematic review of studies was selected as the best source, reporting a response rate of 62.5%. The mean cost per patient for romiplostim was €16,289 and €13,459 for rituximab. Rituximab resulted in a 10% higher cost per responder (€21,535 versus €19,625 for romiplostim). Romiplostim use reduced drug administration, intravenous immunoglobulin, and bleeding-related costs compared to rituximab.
ConclusionsDue to its high level of efficacy leading to lower BRE costs, romiplostim represents an efficient use of resources for adult ITP patients in the Spanish Healthcare System.
Romiplostim, agonista del receptor de la trombopoyetina, está aprobado para el tratamiento de segunda línea en pacientes con trombocitopenia inmune primaria (PTI). El tratamiento con rituximab no es infrecuente, aunque esta indicación no esté recogida en la ficha técnica. Este análisis compara el coste por paciente respondedor a romiplostim frente a rituximab en España.
Materiales y métodoSe ha diseñado un modelo para estimar el coste de 6 meses de tratamiento por paciente que responde (recuento plaquetario ≥50×109/L). Este modelo toma las referencias conforme a los datos publicados más sólidos. Los pacientes tratados con romiplostim recibieron inyecciones semanales; los pacientes tratados con rituximab recibieron 4 infusiones intravenosas semanales. Los precios se obtuvieron de las listas de reembolso españolas. Los pacientes sin respuesta incurrieron en gastos por el tratamiento de episodios relacionados con sangrado (ERS), tal como se notificó en los ensayos clínicos. La utilización de recursos médicos y la práctica clínica se basaron en las guías de tratamiento españolas y fueron validadas por expertos locales.
ResultadosLas tasas de respuesta para romiplostim y rituximab fueron del 83 y 62,5%, y el coste medio por paciente fue de 16.289€ y13.459€, respectivamente. Con rituximab el coste por paciente respondedor fue un 10% superior (21.535€) comparado con romiplostim (19.625€). Romiplostim redujo el coste de administración de fármacos, el uso de inmunoglobulina intravenosa y los costes relacionados con ERS comparado con rituximab.
ConclusionesRomiplostim representaría una opción terapéutica eficiente en comparación con rituximab para el tratamiento de pacientes adultos con PTI crónica en el Sistema Nacional de Salud español.