Pediatric hemato-oncological (HO) patients are highly susceptible to the occurrence of adverse events (AE), nevertheless few research has been done in this field. Our aim was to describe the incidence, type, severity and preventability of AE in these patients, including bone marrow transplant (BMT) patients, and to identify patient's risk factors for having an AE.
MethodsRetrospective cohort study. Children under 18yo hospitalized at the HO or BMT ward in 2016 were eligible for the study. Type of AE, severity and preventability were described as absolute and relative frequencies. Cumulative incidence of patients with at least one AE (CI_AE) and the rate of occurrence of all AE were calculated. Risk factors (sex, recovery probability, comorbidities and being a BMT patient) were analyzed using logistic regression.
Results114 patients were included, 58% were male, average age was 8.7yo and 25 were BMT patients. 44 had at least one AE, with CI_AE of 38.6% (95%CI 29.7–47.5). Overall rate of occurrence of AE was 2.5 cases per 100 patients-day (95%CI 2.15–2.98). For BMT and non-BMT patients they were 2.8 (95%CI 2.2–3.6) and 2.5 (95%CI 1.98–3.1) respectively. Healthcare related infection was the most frequent AE. Most AE were moderate and with high preventability. Being a BMT patient was the only independent factor associated with the occurrence of at least one AE (OR=11.5, p<0.001).
ConclusionsOur findings suggest that AE tend to be moderate and preventable in HO pediatric patients. BMT patients seem to be at greater risk of having an AE. Strategies focused on patient safety need to account for their specific characteristics.
Los pacientes hematooncológicos (HO) pediátricos son muy susceptibles de sufrir eventos adversos (EA), pero hay pocas investigaciones al respecto. El objetivo de este estudio fue describir la incidencia, tipo, severidad y posibilidad de prevención de EA en estos pacientes, incluyendo aquellos con trasplante de médula ósea (TMO) e identificar sus factores de riesgo para tener un EA.
MétodosCohorte histórica. Se incluyeron<18 hospitalizados en piso de HO o TMO en 2016. El tipo de EA, severidad y posibilidad de prevención se describieron con frecuencias absolutas y relativas. Se calculó la incidencia acumulada de pacientes con al menos un EA (EA-IA) y la tasa de ocurrencia de todos los EA. Los factores de riesgo (sexo, probabilidad de recuperación, comorbilidades y ser paciente de TMO) se analizaron mediante regresión logística.
ResultadosSe incluyeron 114 pacientes,58% de sexo masculino, edad media 8,7 años y 25 con TMO; 44 tuvieron al menos un EA (EA-IA: 38,6%, IC 95%: 29,7-47,5). La tasa global de ocurrencia de EA fue 2,5 casos/100 pacientes-día (IC 95%: 2,15-2,98). En los pacientes con y sin TMO fue 2,8 (IC 95%: 2,2-3,6) y 2,5 (IC 95%: 1,98-3,1), respectivamente. La infección relacionada con atención sanitaria fue el EA más frecuente. La mayoría de EA fueron moderados y altamente prevenibles. Ser paciente de TMO fue el único factor independiente asociado a tener por lo menos un EA (OR = 11,5, p<0,001).
ConclusionesNuestros resultados sugieren que los EA tienden a ser moderados y prevenibles en este grupo y los pacientes de TMO tienen un mayor riesgo de tener por lo menos un EA. Las estrategias enfocadas en mejorar la seguridad del paciente deben tener en cuenta las características específicas de esta población.
Patient safety is now recognized globally as a healthcare priority and efforts have been made worldwide in order to improve identification and prevention of adverse events.1 Large reviews of medical records estimate that around 4% and 17% of hospital admissions are associated with an adverse event (AE) and a significant proportion of them are preventable.2
Knowing the frequency, severity and preventability of adverse events is key in designing strategies for the improvement of patient safety. In this regard, the ENEAS was a national study, conducted in 2008 in Spain that estimated an incidence of AE of 9.3%, 16% of which were severe and 43% were considered preventable.3 Some research has been done specifically in children, although not as extensively as in adults. A study done among pediatric patients in Canadian hospitals in 2012 showed an overall incidence of AE of 9.2%, of which 44.7% was preventable.4 In developing countries, a study done in a pediatric Ethiopian service showed an incidence of 9.2 per 100 admissions of adverse drug events (ADEs),5 while in Argentina an article reported 11% of AE per 100 admissions in the Department of Pediatrics at a tertiary care hospital.6
Theoretically, the complexity of pediatric patients with hemato-oncological (HO) conditions puts them at an increased risk for occurrence of AE. This can be related to immunosuppression secondary to treatment, length of stay and illness severity,4 nevertheless few research has been done specifically in this field so far. In Australia, a study performed over a 6-month period in a tertiary pediatric hospital to identify ADEs, showed that most of them (31%) were related to chemotherapy.7 However, we did not find studies addressing in particular the characterization of AE in pediatric HO patients.
The aim of this study was to describe the incidence, type, severity and preventability of AE in pediatric HO patients, both BMT and non-BMT, and to identify patient's risk factors for having an AE in the HO ward and the BMT Unit at Clínica Imbanaco – Grupo QuirónSalud, which is a high complexity institution in Cali, Colombia.
Material and methodsStudy design, participants and data collectionWe conducted an historical cohort study. All children under eighteen years of age hospitalized in the HO ward and the BMT Unit in 2016 were eligible. Assuming a sampling error of 10%, a confidence of 95% and under the assumption that the prevalence of AE was 50%, a sample size of 96 individuals was estimated. Clinical records from admission to discharge were reviewed by a team of three physicians and two nurse practitioners. All registries were reviewed in a weekly meeting by the whole research team. Inconsistencies were reviewed by the principal investigator; review of the clinical chart was performed again if necessary. The study was approved by the Research Ethics Committee of the institution.
VariablesAdverse events. Adverse event was defined as any unexpected event related to healthcare and not to the underlying illness, that caused damage, disability, increase of length of stay or death.8 Dangerous events that could be stopped and did not reach the patient were classified as incidents and these were not included in the analysis.
Description of AE. Types of AE were adapted from the ENEAS study and included the following categories: healthcare related infections, medication related effects, healthcare complications, procedures complications.9 Severity was assigned to AE according to the Common Criteria for Adverse Events, version 4.03.10 Preventability was measured with a six-points scale used by several authors.9,11,12 This scale goes from 1 (virtually no evidence for preventability) up to 6 (virtually certain evidence for preventability). The scale is further categorized in three levels: high preventability (4–6 points), low preventability (2–3) and no preventability (1). The scales were applied by the principal investigator who is a pediatric hemato-oncologist with expertise in Epidemiology and Patient Safety.
Risk factors. In the analysis of risk factors for having at least one AE, the variables selected were sex, recovery probability, comorbidities and being a BMT patient. Recovery probability was given by the likelihood of the patient recovering his basal status not conditioned by the adverse event, and was classified as very likely, likely, less likely and unlikely. This was determined by an experienced pediatric hematologist-oncologist according to the underlying disease. A similar method was used in the ENEAS study.9 Comorbidities burden was established by the Charlson Index.13 Being a BMT patient was defined as being at any phase of a BMT procedure.
Statistical analysisProportions, means, medians, standard deviation (SD) and interquartile range (IQR) were used to describe the variables. Type of AE, severity and preventability were described in frequency tables among BMT and non-BMT patients with the correspondent comparison statistical test.
Cumulative incidence of the patients who had at least one AE was calculated. The rate of occurrence of all AE was calculated assuming each of them as independent events. Both measurements and their confidence intervals were calculated for both patients and for BMT and non-BMT patients.
Risk factors were analyzed with a logistic regression model. Bivariate logistic analysis was used to estimate their association with the outcome (occurrence of at least one AE). Variables with statistical significance below 0.25 in the bivariate analysis were included in the multivariate analysis, which was done using a significance level of 0.05.
For the description of variables with missing data, sample size was adjusted accordingly. In the logistic regression model, only registries with complete data were included.
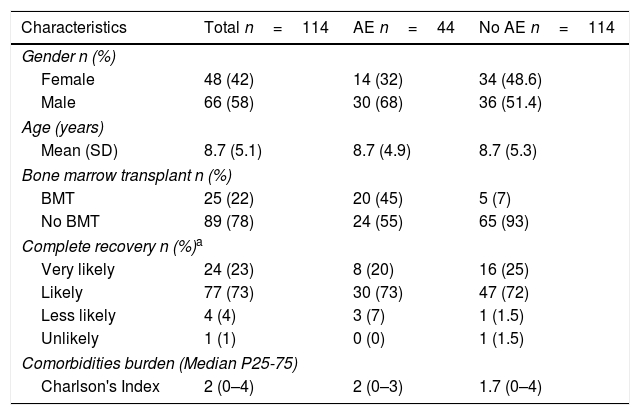
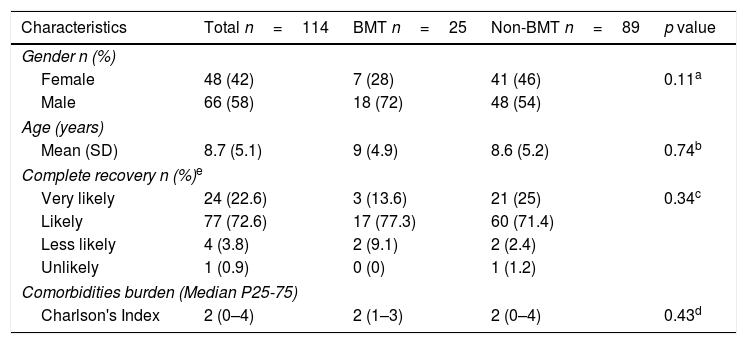
Results114 patients were included in the study, most of them were male, average age was 8.7 years and 25 were BMT patients. 44 of them had at least one AE during the study period. Among these, 20 were BMT patients and for most of them, complete recovery was considered as “likely”. Median for Charlson index was two for all patients and for those with at least one AE, it was slightly lower for those without AE (Table 1). Table 2 shows the characteristics of BMT and non-BMT patients. Registries with missing data were less than 10%.
Patient Characteristics.
| Characteristics | Total n=114 | AE n=44 | No AE n=114 |
|---|---|---|---|
| Gender n (%) | |||
| Female | 48 (42) | 14 (32) | 34 (48.6) |
| Male | 66 (58) | 30 (68) | 36 (51.4) |
| Age (years) | |||
| Mean (SD) | 8.7 (5.1) | 8.7 (4.9) | 8.7 (5.3) |
| Bone marrow transplant n (%) | |||
| BMT | 25 (22) | 20 (45) | 5 (7) |
| No BMT | 89 (78) | 24 (55) | 65 (93) |
| Complete recovery n (%)a | |||
| Very likely | 24 (23) | 8 (20) | 16 (25) |
| Likely | 77 (73) | 30 (73) | 47 (72) |
| Less likely | 4 (4) | 3 (7) | 1 (1.5) |
| Unlikely | 1 (1) | 0 (0) | 1 (1.5) |
| Comorbidities burden (Median P25-75) | |||
| Charlson's Index | 2 (0–4) | 2 (0–3) | 1.7 (0–4) |
Characteristics of BMT and non-BMT patients.
| Characteristics | Total n=114 | BMT n=25 | Non-BMT n=89 | p value |
|---|---|---|---|---|
| Gender n (%) | ||||
| Female | 48 (42) | 7 (28) | 41 (46) | 0.11a |
| Male | 66 (58) | 18 (72) | 48 (54) | |
| Age (years) | ||||
| Mean (SD) | 8.7 (5.1) | 9 (4.9) | 8.6 (5.2) | 0.74b |
| Complete recovery n (%)e | ||||
| Very likely | 24 (22.6) | 3 (13.6) | 21 (25) | 0.34c |
| Likely | 77 (72.6) | 17 (77.3) | 60 (71.4) | |
| Less likely | 4 (3.8) | 2 (9.1) | 2 (2.4) | |
| Unlikely | 1 (0.9) | 0 (0) | 1 (1.2) | |
| Comorbidities burden (Median P25-75) | ||||
| Charlson's Index | 2 (0–4) | 2 (1–3) | 2 (0–4) | 0.43d |
One hundred and forty-eight adverse events were documented in 44 patients during the study period, 81.7% presented more than one adverse event, with a maximum of 9 AE occurring in two patients. Overall cumulative incidence of patients with at least one AE was 38.6% (44/114) (95%CI 29.7–47.5). This estimate was higher in BMT patients, where it reached 80% (20/25) (95%CI 64.3–95.7) while in non-BMT patients it was 27% (24/89) (95%CI 17.7–36.2), (p<0.001).
Rate of AE occurrenceOverall occurrence of AEs was 2.5 cases per 100 person-days (95%CI 2.15–2.98), a similar rate was found in non-BMT patients (2.5 cases per 100-persons-day (95%CI 1.98–3.1)) and it was slightly higher in BMT patients (2.8 cases per 100 persons-day (95%CI 2.2–3.6)). Cumulative length of stay (LOS) for BMT patients was 2446 days, while in non-BMT patients it was 3158 days. Rate ratio was 1128 (95%CI 0.80–1.58, p=0.46).
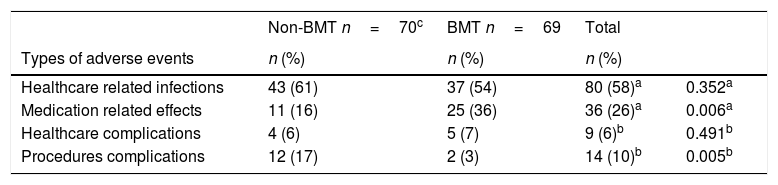
Characteristics of the AETable 3 summarizes type of AE. Healthcare related infection was the most frequent AE (80/139, 9 missing data) and this was similar for both groups. Among them, device associated bacteremia was the most frequent one (26/80) (data not shown).
Types of adverse events in BMT and non-BMT patients.
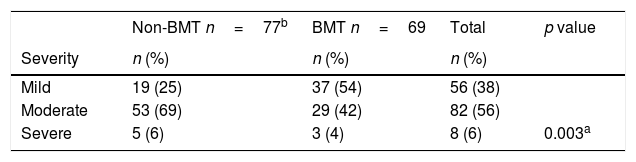
Table 4 describes the severity of the AE. Most of AE were classified as moderate overall (82/146, 2 missing data) and in non-BMT patients (53/77), specifically in BMT patients the majority of AE were categorized as mild (37/69).
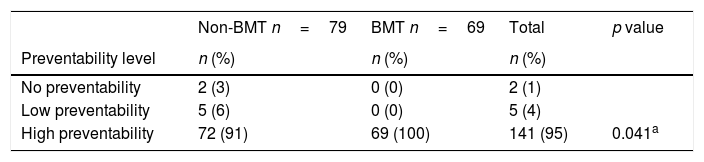
Regarding preventability, most AE were classified in the category of “high preventability” in both BMT and non-BMT patients (141/148), being all the AE in this category for BMT patients (Table 5).
Preventability of adverse events.
| Non-BMT n=79 | BMT n=69 | Total | p value | |
|---|---|---|---|---|
| Preventability level | n (%) | n (%) | n (%) | |
| No preventability | 2 (3) | 0 (0) | 2 (1) | |
| Low preventability | 5 (6) | 0 (0) | 5 (4) | |
| High preventability | 72 (91) | 69 (100) | 141 (95) | 0.041a |
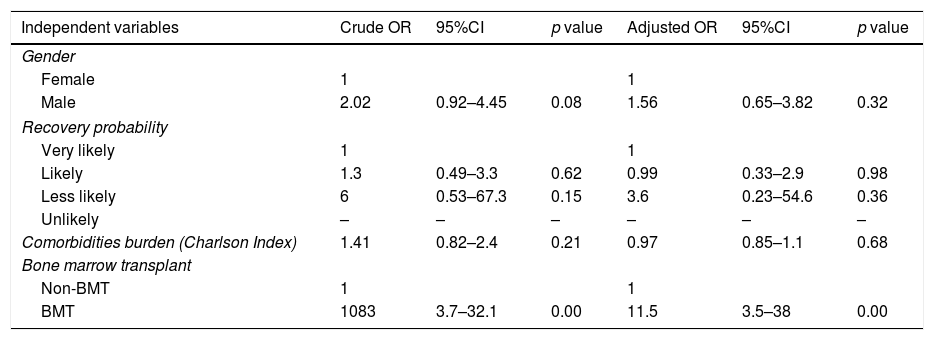
Bivariate logistic analysis showed that the main factor for having at least one AE was being a BMT patient (OR=10.83, p<0.001). Male gender had twice the odds of having at least one AE without reaching statistical significance (OR=2.02, p=0.08).
In the multivariate analysis, being a BMT patient was the only risk factor that remained statistically significant for having at least one AE regardless of age, sex, recovery probability and comorbidities measured by the Charlson index (OR=11.5, p<0.001). The results are showed in Table 6.
Bivariate and multivariate logistic model.
| Independent variables | Crude OR | 95%CI | p value | Adjusted OR | 95%CI | p value |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 1 | 1 | ||||
| Male | 2.02 | 0.92–4.45 | 0.08 | 1.56 | 0.65–3.82 | 0.32 |
| Recovery probability | ||||||
| Very likely | 1 | 1 | ||||
| Likely | 1.3 | 0.49–3.3 | 0.62 | 0.99 | 0.33–2.9 | 0.98 |
| Less likely | 6 | 0.53–67.3 | 0.15 | 3.6 | 0.23–54.6 | 0.36 |
| Unlikely | – | – | – | – | – | – |
| Comorbidities burden (Charlson Index) | 1.41 | 0.82–2.4 | 0.21 | 0.97 | 0.85–1.1 | 0.68 |
| Bone marrow transplant | ||||||
| Non-BMT | 1 | 1 | ||||
| BMT | 1083 | 3.7–32.1 | 0.00 | 11.5 | 3.5–38 | 0.00 |
Adverse events frequency is highly variable and depends on several factors: the clinical area where the study has been conducted14 the type of institution15 and the method of measurement.16
In this study we found higher AE cumulative incidence (38.6%) when compared to similar studies, such as the one conducted in Argentina (11% CI 10.2–12.6)6 and the Canadian study,4 where an overall incidence of 9.2% (CI 5.1–13.3) was observed. The incidence rate in our study (2.5 per 100 person-days) was also higher than that reported by Fajreldines et al. (1.5 per 100 patient-days). These studies were done including all type of pediatric patients, while this study was done only in HO patients, whose disease processes and clinical care are typically more complex. The study by Matlow et al. describes children with complex clinical conditions as a group with increased vulnerability to AE. Other studies have reported healthcare complexity association with adverse events risk.17
The most frequent adverse events in this study were infections (58%), these findings are different from those of Fajreldines et al., where the most common AE were associated with medication use (48.6%) followed by infection (42.8%). This may be explained by the fact that HO patients are frequently immunosuppressed by both their diseases and the treatments, making them more susceptible to infections.
Regarding severity, most AEs were classified as moderate (56%), followed by mild and severe, while in the article by Fajreldines et al., mild AEs were the most frequent (54.2%). Even though this could be explained by the high complexity of patients included in our study, the fact that among BMT patients the majority of AE were classified as mild is not explained by this theory. One of the reasons could be related to the definition of AE. According to the Common Terminology Criteria for Adverse Events and the adapted version of the National Coordinating Council for Medication error Reporting and Prevention (NCC MERP),10 mild event is defined as that event that causes any lesion or complication without increasing LOS. In BMT patients AE resolved within the time required for leaving the BMT ward and their LOS was not increased due to AE.
In relation to preventability, we found that nearly all AEs were classified as preventable, this is different from the findings of Woods et al. who analyzed pediatric hospitalizations in the Colorado and Utah Medical Practice Study and reported only 59% AEs as preventable.18 Even though preventability is measured using a validated scale, its measurement may be somewhat subjective and this could explain the difference in the results.
Being a BMT patient was found to be strongly associated in the multivariate analysis to the occurrence of at least one AE, regardless of age, sex, likelihood of recovery of the underlying disease and comorbidities burden. The study of Eshetie et al. about adverse drug events done in a pediatric service including wards and ICUs, found that the occurrence of ADEs increased with age, LOS and use of CNS, endocrine and antihistamine medicines.5 In our study, LOS did not meet statistical significance to be included in the multivariate analysis, furthermore the occurrence rate of AE was similar in both groups, and cumulative LOS was greater in non-BMT patients, suggesting that LOS does not explain this finding. We hypothesized that even though the time of exposition may increase the probability of having an AE, specifically the complexity of the care provided to the BMT patients plays a bigger role.
Among the limitations of our study are its retrospective nature, and that it was based on medical records, which could impact the quality of the data. Nevertheless, the retrospective method of data collection by review of medical records has been identified as effective as the prospective method for estimating adverse events rates.16 Our team made a thorough process reviewing weekly the recorded data, and the inconsistencies were reviewed by an experienced pediatric hematologist oncologist. Additionally, the preventability of the AE can be subjective and difficult to measure, which can lead to bias. We tried to minimize this risk by using a validated scale that was applied by a pediatric hemato-oncologist with expertise in Epidemiology and Patient Safety.
Furthermore, we also analyzed the characteristics of children with HO diseases having an AE, and did not find many studies evaluating this variable in this type of patients. We consider this is precisely one of the strengths of the study, and our findings suggest that BMT patients are at greater risk of having an AE, and probably strategies focused on patient safety need to account for the specific characteristics of this population.
Accurate reporting of AE is necessary to design strategies and programs aimed to increase patient safety in children. In many countries, initiatives such as the Pediatric Trigger tool have been very useful in detecting adverse events in pediatric patients.19 Implementation of a computerized handoff tool linked to the electronic medical records and family participation in hospital incident reports and systematic surveillance, are also options to consider in order to improve reporting.20–22 Additionally, although general pediatric triggers can identify ADEs in HO patients,23 the complexity of their treatments and interventions may deserve a separate trigger tool for medication surveillance.24
ConclusionOur findings suggest that AE tend to be moderate and preventable in HO pediatric patients. BMT patients seem to be at greater risk of having an AE and strategies focused on patient safety need to account for the specific characteristics of this population. As far as we know, there are not many studies about the occurrence of AE in this type of patients. Further research needs to be done regarding the best method to detect and report AEs, in order to develop strategies to improve the safety of this vulnerable population.
Conflict of interestNone declared.
Acknowledgment to Comité e Instituto de Investigaciones de Clínica Imbanaco.