En Perú se realiza la presentación de trastuzumab en presentación endovenosa (TZM-IV) y trastuzumab en presentación subcutánea (TZM-SC). Pero no hay datos de costos comparativos por vía de administración. El objetivo de nuestro estudio fue conocer los costos de las pacientes con cáncer de mama, comparando las vías de administración en un centro oncológico regional del Perú.
Material y métodosEn 2020 a los pacientes que se pautó tratamiento con trastuzumab (TZM) de manera prospectiva se registraron los datos clínicos, demográficos y de transporte, y se obtuvieron los costos médicos de los expedientes de historia médica y farmacia. Con estos datos se realizó la simulación a 100 pacientes que recibieron 18 ciclos del fármaco.
ResultadosEl principal contribuyente al costo de la diferencia fue el costo del medicamento en sí, siendo S/. 4.711,11 (1.323,35 USD) y S/. 4.680,30 (1.314,69 USD) para TZM-IV y TZM-SC, respectivamente. Los costos de administración para tratar a 100 pacientes con ciclos completos de TZM-IV y TZM-SC fueron S/. 334.488,53 (93.957,45 USD) y S/. 207.455,33 (58.873,97 USD), respectivamente. Los costos indirectos indican que los pacientes perdieron en total, S/. 1.123,28 (315,53 USD) y S /. 1.148,60 (322,64 USD) en TZM-IV y TZM-SC por paciente, respectivamente.
ConclusionesSe recomienda el uso de TZM-SC, en el escenario de un menor costo del medicamento y menor duración del tiempo de administración. Sobre todo, en un país con bajo financiamiento, que solo permite subsidiar los costos directos del tratamiento del cáncer.
In Peru, the presentation of TZM-IV and TZM-SC is carried out. But there is no comparative cost data by route of administration. The objective of our study was to know the costs of patients with breast cancer, comparing the routes of administration in a regional cancer center in Peru.
Material and methodsIn 2020, patients who were prescribed TZM treatment were prospectively recorded clinical, demographic and transport data, and medical costs were obtained from medical history and pharmacy records. With these data, the simulation was performed in 100 patients who received 18 cycles of the drug.
ResultsThe main contributor to the cost of the difference was the cost of the drug itself, being S/. 4,711.11 (1,323.35 USD) and S/. 4,680.30 (1,314.69 USD) for TZM-IV and TZM-SC, respectively. The administration costs to treat 100 patients with complete cycles of TZM-IV and TZM-SC were S/. 334,488.53 (93,957.45 USD) and S/.207,455.33 (58,873.97 USD), respectively. Indirect costs indicate that patients lost in total, S/. 1,123.28 (315.53 USD) and S/. 1,148.60 (322.64 USD) in TZM-IV and TZMSC per patient, respectively.
ConclusionsThe use of TZM-SC is recommended, in the scenario of a lower cost of the drug and a shorter duration of administration time. Especially in a country with low funding, which only allows subsidizing the direct costs of cancer treatment.
A nivel mundial, el cáncer de mama (BC) es una de las neoplasias malignas más prevalentes entre las mujeres. Cada año se diagnostican más de un millón de nuevos casos de cáncer de mama, que afectan a 43,3 / 100.000 mujeres y provocan más de 500.000 muertes con una tasa de mortalidad de 12,9 / 100.000 mujeres1. En el Perú se registra 6.985 casos nuevos anuales con una tasa estandarizada de 40,0 por 100.000 habitantes, siendo considerado un problema de salud pública nacional y mundial1,2. El fenotipo con sobre expresión del receptor ligado a la tirosina quinasa, denominado receptor de factor 2 de crecimiento epidérmico humano (HER2), representan el 20 al 25% de los cánceres de mama3. Dicho fenotipo son más agresivos y menos sensibles a la quimioterapia estándar que lo tumores HER2 negativos. Por tanto, dirigirse al HER2 mediante un anticuerpo monoclonal, como el trastuzumab (TZM), tiene un efecto más significativo que la terapia hormonal sobre la supervivencia libre de enfermedad (SLE) supervivencia global (SG)4.
Los pacientes con cáncer de mama precoz deben ser tratados con TZM durante un año o hasta recaída de la enfermedad, lo que ocurra primero, no se recomienda prolongar el tratamiento en cáncer de mama precoz más de un año, mientras que los pacientes con cáncer de mama metastásico deben ser tratados con TZM hasta la progresión de la enfermedad.
El costo del tratamiento con TZM, es una limitante, sin embargo, este se debe comparar con los beneficios, en la disminución de costos futuros en el tratamiento en enfermedad progresiva, como en la mejora de sobrevida y calidad de vida. En este contexto el desarrollo de una evaluación económica de uso de las vías de administración del TMZ en el Perú, brindaría información importante de costo-efectividad y permitiría evaluar las estrategias más costo-efectivas de implementación de este tratamiento.
La eficacia de TZM subcutáneo (TZM-SC) frente a intravenoso (TZM-IV) como terapia neoadyuvante para el cáncer de mama HER2 positivo, presenta perfiles similares en múltiples ensayos, y actualmente está en proceso el ensayo fase III de HannaH5–7. También se dispone de algunos datos de eficacia similar, en ensayos de fase III y IV8–10, incluidos dos ensayos abiertos de seguridad de fase III SafeHER11 y MetaPHER12.
En el contexto peruano, se cuenta en el mercado, la presentación de TZM-IV de 440 mg/420 mg y TZM-SC de 600 mg. Sin embargo, a nivel de los centros oncológicos del Ministerio de Salud (MINSA), solo está indicado en pacientes con cáncer de mama metastásico o cáncer de mama precoz, cuyos tumores sobre expresen HER2, hasta un año de tratamiento13. En la Resolución Ministerial N° 545-2019/MINSA, menciona que se encuentra el TZM para el uso exclusivo para el tratamiento de pacientes con cáncer de mama HER2 positivo en adyuvancia13, pero no es referida la vía de administración el cual es motivo de análisis.
El instituto, actualmente utiliza la presentación de TZM-IV, pero se proyecta a implementar el uso de presentación TZM-SC, debido a que no se cuenta con dichos datos nacionales, se tiene la necesidad de conocer la repercusión. El objetivo de nuestro estudio fue conocer los costos de los pacientes con cáncer de mama, con el uso de TZM-IV vs. TZM-SC en un centro oncológico regional en Perú. Evaluando los costos médicos directos e indirectos, y los tiempos involucrados del paciente.
Material y métodosEn el año 2020, a los pacientes con cáncer de mama que se pautó tratamiento con TZM, cada sesión de administración de medicamento se consideró como un registro, obteniéndose los datos de manera prospectiva, obteniéndose las características clínicas con las demográficas, mediante la historia clínica, mientras que los gastos de transporte, se recolectó mediante un cuestionario al paciente de su gasto diario por acudir al instituto. Se obtuvieron los costos médicos a partir de los archivos de historia clínica y farmacia. Con este proceso se identificó los medicamentos y consumibles utilizados en la preparación y administración. Así mismo se entrevistó a los médicos, farmacéuticos y personal de enfermería, para conocer los tiempos durante la preparación y administración del medicamento. Previamente el estudio fue aprobado por el comité de ética institucional y solo se analizó los datos completos de los pacientes que aceptaron previamente el consentimiento informado.
Horizonte de tiempoEste estudio se basó en un solo año de terapia, lo que significa un total de 18 ciclos de dosis de TZM. Los costos se expresaron en nuevos soles, con equivalente de 3,56 para dólares americanos (USD). La evaluación económica de este estudio cubre un horizonte temporal de 1 año, proyectado en la atención de 100 pacientes; se escogió esta cantidad, porque se acerca al número aproximado de pacientes con HER2, diagnosticados de la macrorregión centro al año.
Costo estimadoSe creó un modelo de hoja de cálculo para proyectar los costos de TZM-IV vs. TZM-SC, a partir de los promedios de los pacientes que recibieron TZM-IV, adaptando los procesos a las recomendaciones internacionales de preparación y administración del TZM-SC. Se incluyó los costos médicos directos e indirectos, asociado con el tiempo de trabajo de los profesionales de salud, medicamentos y consumibles utilizados para preparar y administrar TZM-IV vs. TZM-SC, y los costos no médicos asociados con el tiempo involucrado de los pacientes en el establecimiento, como el tiempo involucrado en el transporte desde su hogar.
Por lo tanto, el modelo asumió que todos los pacientes se sometieron a los mismos protocolos de preparación y administración. Siendo calculados por paciente nuevo y continuador como único y también para 100 pacientes.
Costo de preparaciónLos costos de preparación incluyeron los costos de medicamentos, equipos y consumibles, y el tiempo del farmacéutico. Debido a que las dosis de carga (8 mg/kg) y mantenimiento (6 mg/kg) de TZM-IV dependen del peso corporal, utilizamos datos anónimos de peso corporal recopilados para los pacientes en nuestro centro oncológico para calcular la cantidad de viales de TZM utilizados para cada paciente en cada ciclo de tratamiento de TZM-IV. El contenido restante de los viales de TZM-IV después de un único ciclo de tratamiento no se puede utilizar para ciclos de tratamiento posteriores ni para otros pacientes. No se hizo ningún ajuste en el análisis por el posible desperdicio de materiales asociado con el descarte de porciones remanentes de medicamento de viales parcialmente usados. Se estimaron los costos de todos los materiales consumibles utilizados para la reconstitución de TZM-IV y otras actividades de preparación y almacenamiento de medicamentos. El costo del tiempo del farmacéutico se calculó multiplicando el tiempo dedicado a la reconstitución, preparación y almacenamiento de medicamentos por el salario del farmacéutico. Para calcular el costo del farmacéutico, se multiplicó el número de minutos de trabajo por el salario del establecimiento.
Costo de administraciónLos costos de administración incluyeron los consumibles utilizados para la infusión o inyección del medicamento, el tiempo de la silla de infusión, el tiempo de la enfermera requerido para la infusión o inyección, monitorización y culminación. Los consumibles incluían traje de protección del personal, solución salina, tubos intravenosos, agujas de puerto, jeringas, guantes estériles, gasas, hisopos para limpiar la piel y equipos de infusión. Ningún paciente tenía dispositivos de acceso venoso central para infusión de quimioterapia, y ninguno desarrollo efectos adversos. El tiempo de la silla de infusión fue el tiempo desde que los pacientes se sentaron por primera vez en la silla de infusión hasta que se levantaron de la silla al final de cada ciclo de tratamiento. El costo de tiempo de las enfermeras asociado a la administración del tratamiento se estimó multiplicando los salarios del establecimiento.
Costo indirectoPara los pacientes, incluyeron los costos relacionados con el tiempo presente desde que ingresa y hasta que se retira del establecimiento. Se adicionó el gasto promedio del transporte desde su vivienda hasta el establecimiento. Nuestro análisis no incluyó los costos asociados con un compañero acompañante, porque las terapias que recibieron los participantes del estudio no suelen afectar la autonomía del paciente.
Análisis estadísticoTodos los datos y el análisis estadístico se realizaron con SPSS versión 21. Siendo la estadística descriptiva, se presenta como media o proporciones, y para evaluar la mejor opción en escenarios con variable independiente alterados como el tiempo de administración y costo de medicamento, se utilizó el análisis de sensibilidad por costos, mediante el programa Microsoft Excel 2020.
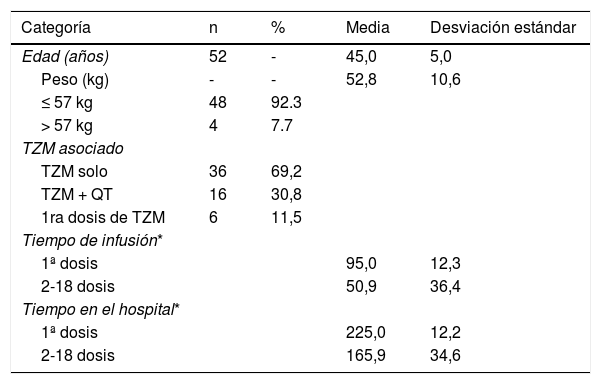
ResultadosSe obtuvieron 52 dosis de TZM, que contenían información completa para el estudio. Siendo sus características presentadas en la tabla 1. Asimismo en la tabla 2, se presenta el costo unitario de los recursos empleados para las proyecciones comparativas.
Características de las sesiones administradas de TZM
| Categoría | n | % | Media | Desviación estándar |
|---|---|---|---|---|
| Edad (años) | 52 | - | 45,0 | 5,0 |
| Peso (kg) | - | - | 52,8 | 10,6 |
| ≤ 57 kg | 48 | 92.3 | ||
| > 57 kg | 4 | 7.7 | ||
| TZM asociado | ||||
| TZM solo | 36 | 69,2 | ||
| TZM + QT | 16 | 30,8 | ||
| 1ra dosis de TZM | 6 | 11,5 | ||
| Tiempo de infusión* | ||||
| 1ª dosis | 95,0 | 12,3 | ||
| 2-18 dosis | 50,9 | 36,4 | ||
| Tiempo en el hospital* | ||||
| 1ª dosis | 225,0 | 12,2 | ||
| 2-18 dosis | 165,9 | 34,6 | ||
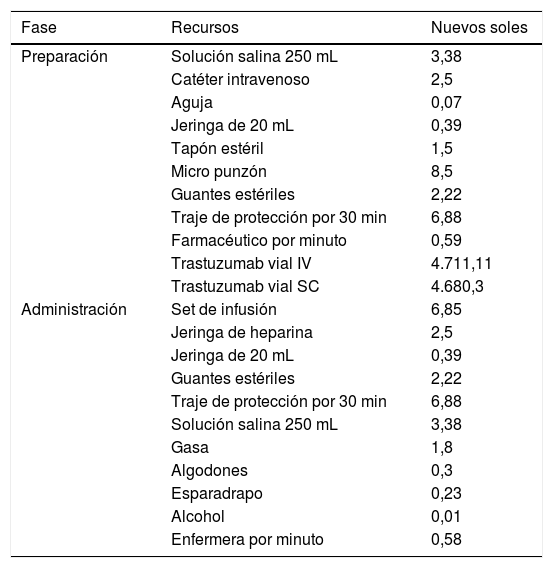
Costos unitarios asociados a la preparación de TZM
| Fase | Recursos | Nuevos soles |
|---|---|---|
| Preparación | Solución salina 250 mL | 3,38 |
| Catéter intravenoso | 2,5 | |
| Aguja | 0,07 | |
| Jeringa de 20 mL | 0,39 | |
| Tapón estéril | 1,5 | |
| Micro punzón | 8,5 | |
| Guantes estériles | 2,22 | |
| Traje de protección por 30 min | 6,88 | |
| Farmacéutico por minuto | 0,59 | |
| Trastuzumab vial IV | 4.711,11 | |
| Trastuzumab vial SC | 4.680,3 | |
| Administración | Set de infusión | 6,85 |
| Jeringa de heparina | 2,5 | |
| Jeringa de 20 mL | 0,39 | |
| Guantes estériles | 2,22 | |
| Traje de protección por 30 min | 6,88 | |
| Solución salina 250 mL | 3,38 | |
| Gasa | 1,8 | |
| Algodones | 0,3 | |
| Esparadrapo | 0,23 | |
| Alcohol | 0,01 | |
| Enfermera por minuto | 0,58 |
La preparación de una dosis única de TZM-SC cuesta S/. 66,54 (18.69 USD) menos por paciente que la preparación de una dosis única de TZM-IV (tabla 3). El principal contribuyente al costo de la diferencia fue el propio costo del medicamento, siendo S/. 4.711,11 (1.323,35 USD) y S/. 4.680,30 (1.314,69 USD) para TZM-IV y TZM-SC, respectivamente. Sin embargo, si el paciente superaba los 57 kg, en la primera dosis de TZM-IV incrementaría el costo a S/. 9.490,59 (2.665,90 USD) ya que necesitarían dos viales de TZM, mientras que la primera dosis de TZM-SC se mantendría el costo de preparación igual. Los datos de peso mostraron que el 92,3% tenían peso menor a 57 kg. En base a esto, los costos proyectados de preparación para 18 dosis de TZM-IV para 100 pacientes excedieron en S/. 246.812,20 (69.329,27 USD).
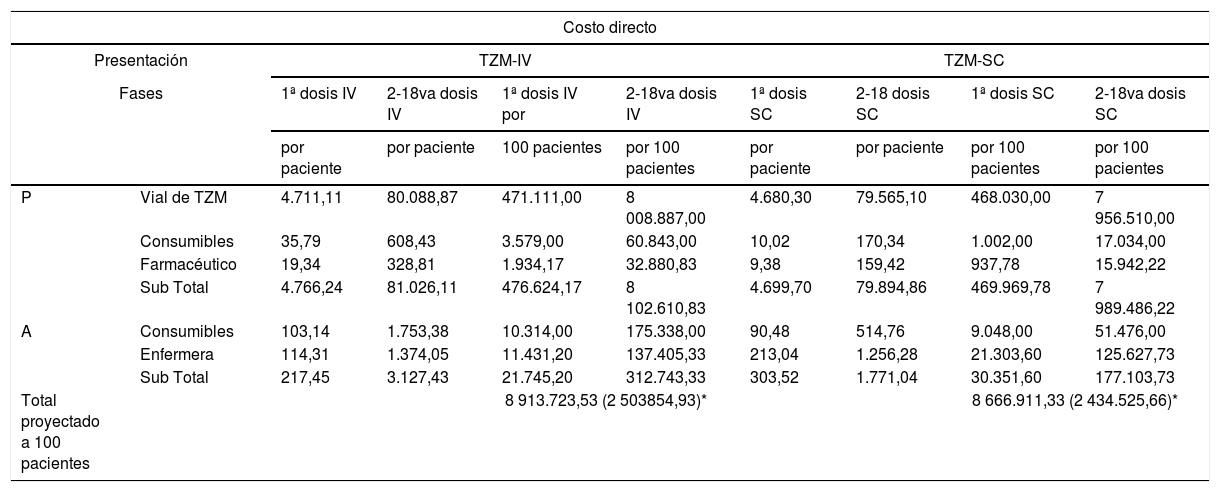
Comparación de costos directos e indirectos simulados por dosis de TZM-IV vs. TZM-SC
| Costo directo | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Presentación | TZM-IV | TZM-SC | |||||||
| Fases | 1ª dosis IV | 2-18va dosis IV | 1ª dosis IV por | 2-18va dosis IV | 1ª dosis SC | 2-18 dosis SC | 1ª dosis SC | 2-18va dosis SC | |
| por paciente | por paciente | 100 pacientes | por 100 pacientes | por paciente | por paciente | por 100 pacientes | por 100 pacientes | ||
| P | Vial de TZM | 4.711,11 | 80.088,87 | 471.111,00 | 8 008.887,00 | 4.680,30 | 79.565,10 | 468.030,00 | 7 956.510,00 |
| Consumibles | 35,79 | 608,43 | 3.579,00 | 60.843,00 | 10,02 | 170,34 | 1.002,00 | 17.034,00 | |
| Farmacéutico | 19,34 | 328,81 | 1.934,17 | 32.880,83 | 9,38 | 159,42 | 937,78 | 15.942,22 | |
| Sub Total | 4.766,24 | 81.026,11 | 476.624,17 | 8 102.610,83 | 4.699,70 | 79.894,86 | 469.969,78 | 7 989.486,22 | |
| A | Consumibles | 103,14 | 1.753,38 | 10.314,00 | 175.338,00 | 90,48 | 514,76 | 9.048,00 | 51.476,00 |
| Enfermera | 114,31 | 1.374,05 | 11.431,20 | 137.405,33 | 213,04 | 1.256,28 | 21.303,60 | 125.627,73 | |
| Sub Total | 217,45 | 3.127,43 | 21.745,20 | 312.743,33 | 303,52 | 1.771,04 | 30.351,60 | 177.103,73 | |
| Total proyectado a 100 pacientes | 8 913.723,53 (2 503854,93)* | 8 666.911,33 (2 434.525,66)* | |||||||
| Costo indirecto | ||||
|---|---|---|---|---|
| Presentación | TZM-IV | TZM-SC | ||
| Costo | 1ª dosis IV | 2-18va dosis IV | 1ª dosis SC | 2-18 dosis SC |
| por paciente | por paciente | por paciente | por paciente | |
| Costo por tiempo de tratamiento | 16,50 | 206,78 | 30,43 | 218,17 |
| Traslado | 50 | 850 | 50 | 850 |
| Total por paciente | 1.123,28 (315,52)* | 1.148,60 (322,64)* | ||
P: fase de preparación, A: fase de administración, TZM-IV: trastuzumab en presentación endovenosa, TZM-SC: trastuzumab en presentación subcutánea, *: equivalente en dólares americanos (3.56).
Los costos de administración del medicamento, para tratar a 100 pacientes con ciclos completos de TZM-IV y TZM-SC fueron S/. 334.488,53 (93.957,45 USD) y S/. 207.455,33 (58.273,97 USD), respectivamente. La diferencia principal fue por los costos derivados del tiempo del personal de enfermería, seguido por los consumibles (tabla 3).
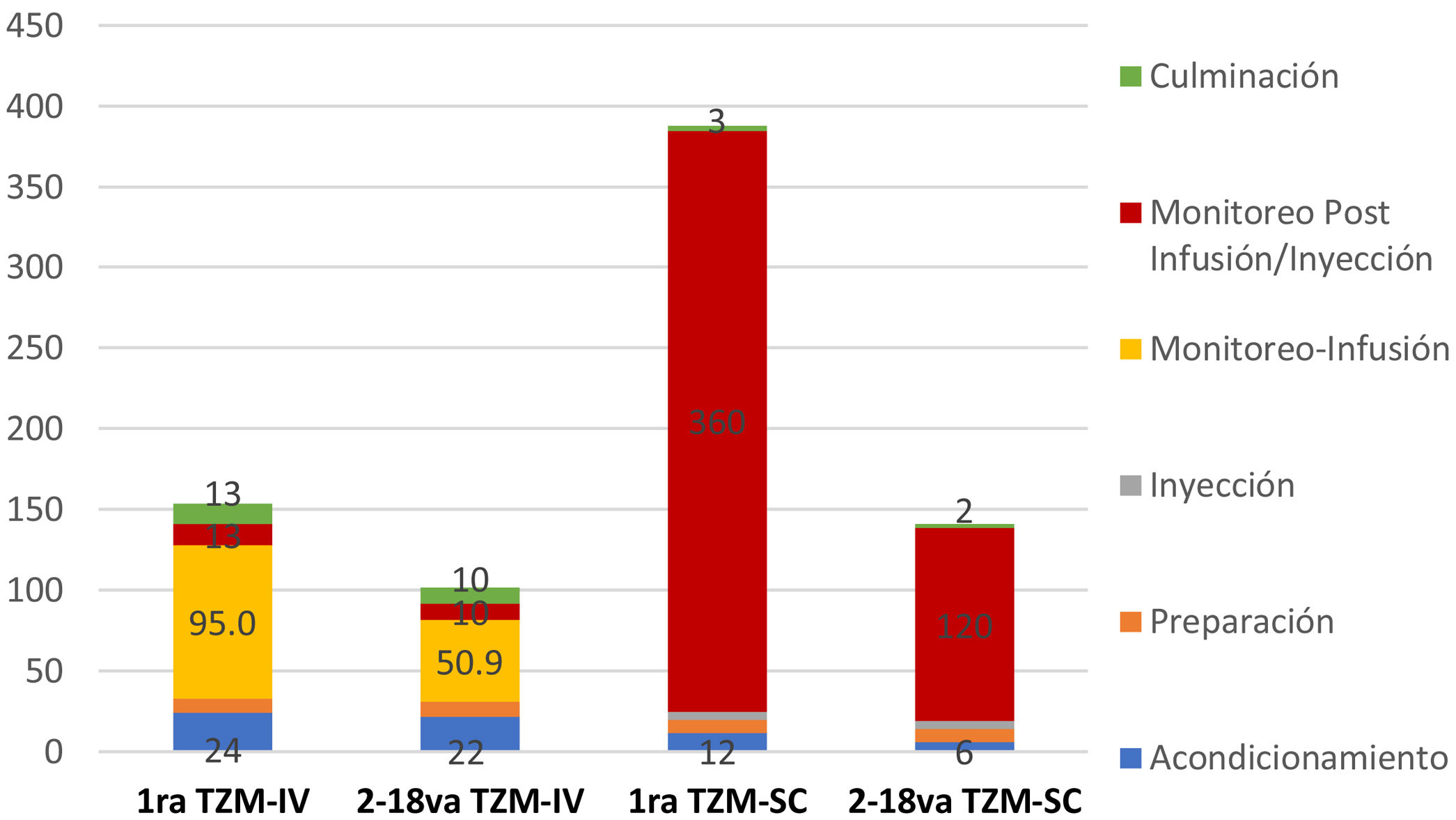
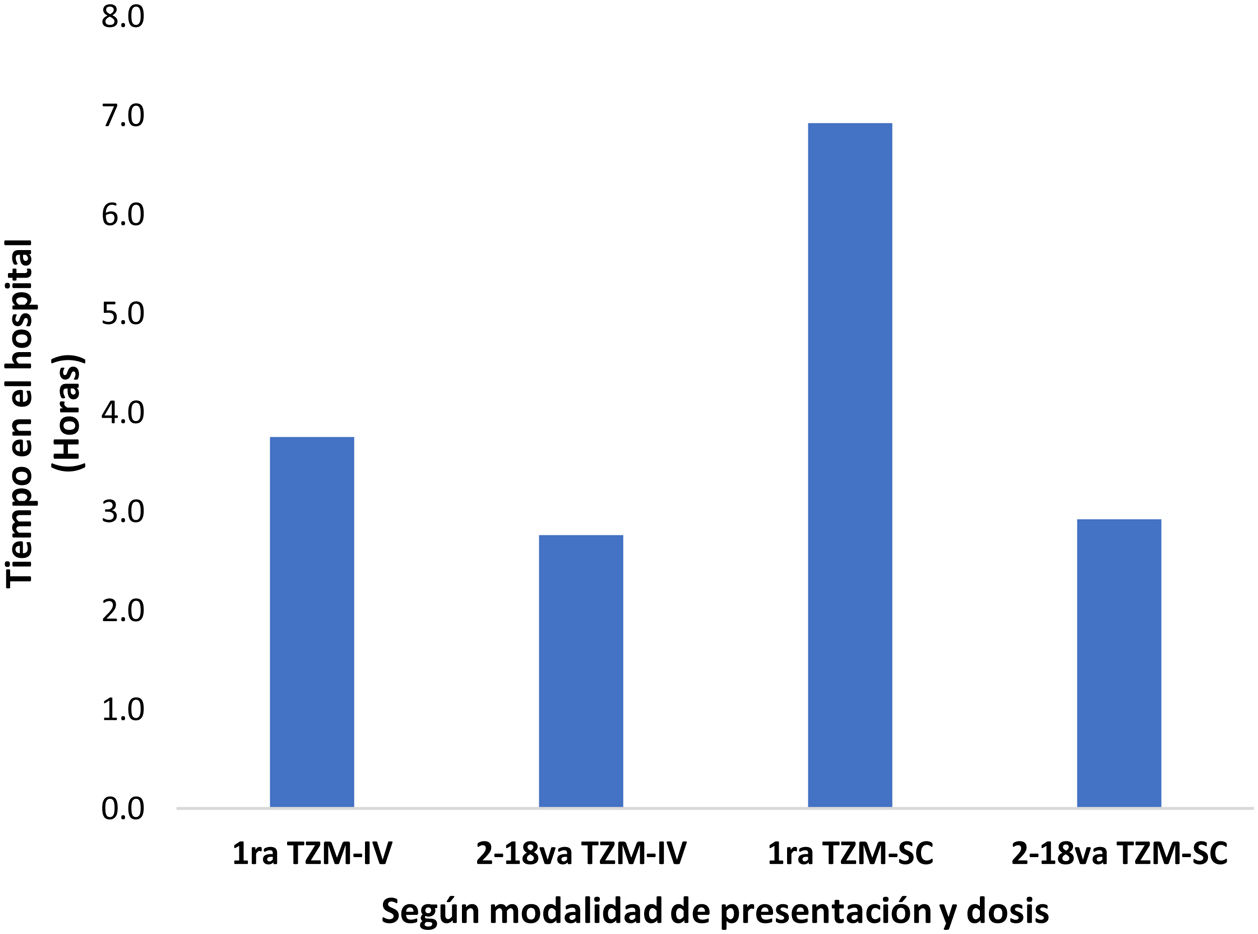
Respecto a la distribución de los tiempos de los profesionales en el tratamiento por dosis de TZM, se observa que tanto la primera dosis de TZM-IV y TZM-SC, es mucho mayor que en las dosis subsecuentes. El tiempo promedio de monitorización post infusión o inyección, es 75, 60, 360 y 120 minutos para la primera dosis de TZM-IV, 2 da a 18va dosis de TZM-IV, primera dosis de TZM-SC, 2 da a 18va dosis de TZM-SC, respectivamente (fig. 1). Asimismo, este tiempo, influye sobre el tiempo del paciente en las instalaciones del establecimiento de salud, siendo estos 225, 166, 415 y 175 minutos para la primera dosis de TZM-SC, 2 da a 18va dosis de TZM-SC, respectivamente (fig. 2).
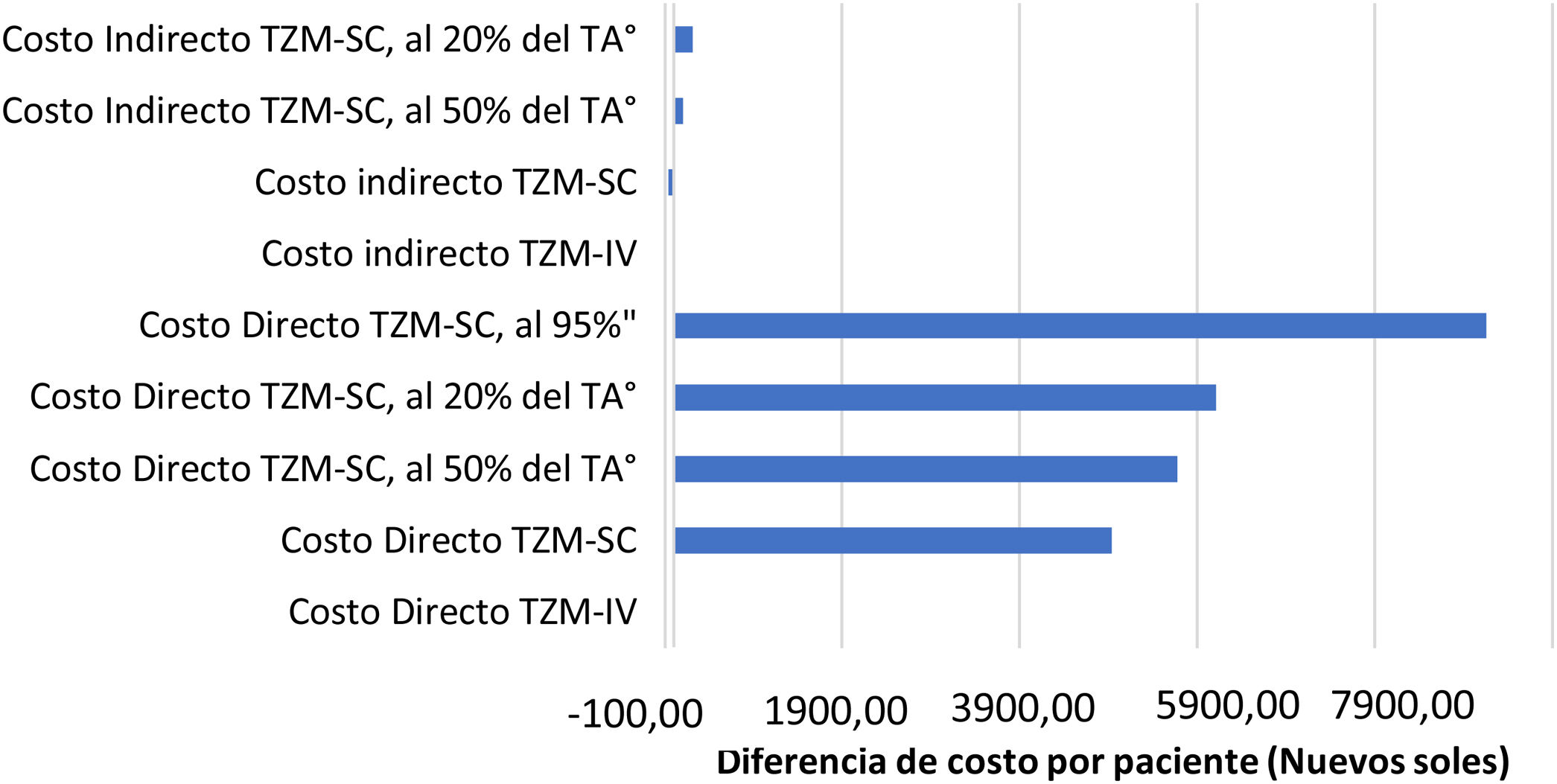
Los costos indirectos están representados en la tabla 3, indicando que los pacientes perdieron en total, S/. 1.123,28 (315,53 USD) y S/. 1.148,60 (322,64 USD) en TZM-IV y TZM-SC por paciente, respectivamente. Por tiempo no laborado, proyectado a partir de los 4,4 soles por hora que corresponde por sexo y ubicación geográfica, resulta S/. 223,28 (62,72 USD) y S/. 248,60 (69,83 USD), en TZM-IV y TZM-SC por paciente, respectivamente. Mientras que el traslado fue igual en ambas presentaciones. En el análisis de sensibilidad de costo, sin modificar ninguna variable, el ahorro es cero. Sin embargo, al modificar el factor de tiempo de administración disminuyéndolo al 50% del TZM-SC, se obtiene un ahorro de S/. 98,98 por paciente con ciclo completo. Y al disminuir el costo absoluto de la presentación de TZM-SC solo en un 5%, se obtiene un ahorro de S/. 9.148,49 por paciente con ciclo completo. Ambos resultados comparados a su costo directo respectivo (fig. 3).
Análisis de sensibilidad de costos TZM-IV vs. TZM-SC.
La diferencia del costo entre la modalidad TZM-IV menos la TZM-SC, modificando el factor de tiempo de administración y si hubiera una disminución del costo de la presentación del TZM-SC.
TÅ: Tiempo de administración, 95%”: Costo de medicamento al 95%.
El instituto, es un establecimiento público, ubicado a 284 km de la capital del país, asume una cobertura de la mayor parte de la zona central de los andes peruanos. Las pacientes con cáncer de mama tributarios a TZM y que gozan del seguro integral de salud (SIS), se encuentra subvencionado los costos directos de la atención médica. En el caso de la terapia con anticuerpo monoclonal, el estado peruano, asume el costo del medicamento, los consumibles asociados y la actividad de los profesionales de salud. Sin embargo, su financiamiento, es limitado por lo que se debe tomar decisiones gerenciales, con el soporte de la eficacia y además de los costos asociados, ya que permitirá el aumento de la oferta de tratamientos oncológicos14.
Comparamos el costo estimado de 18 ciclos de TZM-IV vs. TZM-SC, para pacientes con cáncer de mama HER2 positivo, en adyuvancia. Encontramos que, para el tratamiento de 100 pacientes, el uso TZM-SC se obtuvo una reducción en S/. 246.812,20 (equivalente a 69.329,27 USD) en comparación con el uso de TZM-IV.
La mayor parte de los costos, han sido por el propio valor económico del TZM, al menos en nuestro escenario, el costo de TZM-SC es menor que el de TZM-IV, en S/. 30,81(8,65 USD). Debido a las características clínicas de las pacientes, más del 92% de ellas, no superaban los 57 kg, para que usen un segundo vial de TZM-IV. Esto nos favorece económicamente, ya que el cálculo de dosis de TZM-IV, se realiza por peso, ocasionando la duplicidad del vial y consumibles en la preparación. Esto en estudios de minimización de costos en poblaciones con mayores índices de peso, plantearon que la reducción del 15% corporal de peso durante el curso del tratamiento, el TZM-IV podría ser la modalidad de tratamiento más económica comparado al TZM-SC1.
Se han venido reportando, más que nada en Europa y poca información de Latinoamérica, sobre hallazgos similares, sobre el beneficio del TZM-SC, en reducir costos15. Se ha informado ahorros del costo directo en 1,156 USD por paciente16,17. Asimismo, De Cock et al. y Lieutnenant et al. determinó que TZM-SC se asoció con costos más bajos que TZM-IV, pero las estimaciones de costos en esos estudios solo incluyeron el tiempo que los pacientes pasaron en las sillas de tratamiento y el tiempo activo dedicado por el personal de salud para brindar el tratamiento18,19. Por otro lado, presentación de efectos adversos son similares en ambas presentaciones, es así que Rojas y col. evaluó los costos de TZM de preparación, administración, costos no médicos, con resultado de ahorro a favor del TZM-SC de 485.089,80, 148.010,00 y 5.148,10 USD, respectivamente. Sin embargo, en la categoría efectos adversos que requirieron hospitalización o interrupción transitoria de la terapia, la presentación de TZM-SC, presentó un gasto adicional de 14.112,60 USD. Mientras que nuestro estudio reflejo a favor del TZM-SC, una disminución en 693,30 USD por paciente en el costo directo, no siendo tan marcado como los otros estudios, por la poca diferencia de costos de la presentación de vial en el mercado peruano, y los tiempos prolongados de monitorización post inyección de 6 y 2 horas para la primera y 2 da a 18va dosis del TZM-SC, respectivamente, recomendado por el MINSA13. Sugiere nuestro análisis de sensibilidad de costos, que si disminuimos el tiempo de administración y al menos un 5% del costo del medicamento, tendríamos un ahorro significativo en los costos directos.
Respecto a los tiempos de los profesionales, a pesar de eliminarse el tiempo de infusión, usando la presentación de TZM-SC, suele ser mayor que la presentación de TZM-IV, por la monitorización post inyección. Resalta la reducción en el tiempo que la enfermera utiliza para la culminación, a favor del TZM-SC, por carecer de dispositivos de infusión. Siendo estos resultados parcialmente compatibles con otros estudios, con un tiempo promedio de enfermería más corto para TZM-SC en comparación con el tratamiento con TZM-IV1,20.
Respecto a los costos indirectos, el tiempo por tratamiento con TZM en el establecimiento de salud, a partir del ingreso promedio por hora según el sexo y ubicación geográfica en el Perú21, presenta un gasto adicional de S/. 25,32 por paciente con la presentación de TZM-SC, que podría aumentar el ausentismo laboral, siendo este resultado contradictorio a otros estudios20, esto se debe, por los tiempos asignados a su monitorización post inyección. Sin embargo, al evaluar los costos de transporte, no hubo diferencia, ya que tienen que acudir al establecimiento de salud con la misma frecuencia en ambas presentaciones, cada 3 semanas. Cabe resaltar que la distancia de desplazamiento de los pacientes, desde su vivienda hasta el establecimiento de salud, suele ser variable por la propia cobertura regional, desde los 20 hasta los 200 km de distancia, por lo que un posible esquema que aumentará el intervalo de tiempo entre dosis, si reduciría los gastos asociados al traslado.
Nuestro análisis tiene limitaciones. En primer lugar, se basa en una simulación, en la cual todos los pacientes se sometieron a los mismos procedimientos con los tiempos promedios, lo cual puede haber diferencias en la práctica real. Todos los datos utilizados, se obtuvieron de una sola institución y por lo tanto se debería extrapolar con precaución, a pesar de no haberse presentado ningún efecto adverso en la muestra, sería favorable evaluar dichas reacciones. Los costos del Instituto son representativos de la mayoría de establecimientos públicos en el Perú, por lo que podría ser extrapolado en otros centros oncológicos públicos de la nación.
Nuestro estudio es una de las pocas evaluaciones de los costos de TZM, que ha incorporado el peso de los pacientes, los costos directos, y agregando el tiempo y costo indirecto del paciente. Además, este es el primer informe que incluye datos clínicos y económicos de un hospital público peruano.
La presentación de TZM-SC va a ser eficiente por disminuir tanto los costos directos, y aumentando la capacidad de oferta con el mismo presupuesto destinado a salud. Sin embargo, regirnos a las recomendaciones nacionales sobre los tiempos de monitorización del TZM-SC, aumentaríamos los gastos indirectos. El uso de TZM-SC, en el escenario que tuviera un costo más reducido, es recomendable en un país con un financiamiento bajo para el sistema de salud estatal, que solo permite la subvención de los costos directos del tratamiento oncológico.
FinanciamientoNo se ha recibido financiamiento alguno.
Conflicto de interesesNo existen potenciales conflictos de intereses con esta investigación.
Los autores agradecen a la Dirección Regional de Salud de Junín, Instituto Regional de Enfermedades Neoplásicas del Centro.