Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) is a debilitating condition that affects 0.2–0.4% of the population. Health focussed anxiety is common across medical conditions, and may be relevant in CFS/ME. This study sought to identify the prevalence and impact of health anxiety (HA) in CFS/ME and evaluate the effectiveness of Cognitive Behavioural Therapy for HA in CFS/ME. Cross-sectional questionnaire methods and case-series design were used to achieve study aims. Analysis indicated that 41.9% of the CFS/ME clinic sample experienced threshold levels of health anxiety, which was associated with elevated symptom severity across several dimensions. Stepwise multiple regression indicated physical functioning and depression accounted for 23.8% of variance in fatigue; depression, fatigue and HA, accounted for 32.9% of variance in physical functioning. Large effect sizes and clinically significant changes were generated in the treatment study. HA is common in CFS/ME and likely to exacerbate fatigue and physical functioning. This study identifies HA as an important target for treatment, trial findings should be further replicated on a larger scale.
El Síndrome de Fatiga Crónica/Encefalomielitis Miálgica (SFC/EM) es una enfermedad que afecta al 0,2-0.4% de la población. La ansiedad por la salud (AS) es común en condiciones médicas y puede ser relevante en el SFC/EM. El objetivo de este estudio fue identificar la prevalencia e impacto de la AS en el SFC/EM y evaluar la efectividad de la terapia cognitivo conductual para tratar la AS en el SFC/EM. Se utilizaron cuestionarios en base a un diseño transversal y de estudio de casos. El 41.9% de la muestra clínica de SFC/EM experimentó niveles umbrales de HA, lo que se asoció a una mayor gravedad de algunos síntomas. Modelos de regresión lineal múltiple indicaron que el funcionamiento físico y la depresión representaron el 23.8% de la varianza en la fatiga; la depresión, la fatiga y la AS representaron el 32.9% de la varianza en el funcionamiento físico. Se identificaron grandes tamaños de efecto y cambios clínicamente significativos por el tratamiento. Lla AS es común en el SFC/EM y podría empeorar la fatiga y el funcionamiento físico. Este estudio identifica la HA como factor importante para el tratamiento del SFC/ME. Los resultados deberían replicarse a mayor escala.
Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) is a debilitating condition characterised by excessive fatigue, malaise, muscle pain and unrefreshing sleep, with prevalence rates in the region of 0.2-0.4% of the population (National Institute for Health and Care Excellence NICE, 2007). It is accepted that the pathogenesis is likely to be a complex interaction which may include genetic predisposition, somatic triggers and biopsychosocial factors. However, the aetiology of CFS/ME remains poorly understood. CFS/ME can lead to significant disability with subsequent economic costs (Rimbaut, Van Gutte, Van Brabander, & Vanden Bossche, 2016).
Despite a number of large CFS/ME treatment trials, a recent meta-analysis (Castell, Kazantzis, & Moss-Morris, 2011) suggests current treatment options such as Cognitive Behavioural Therapy (CBT) (National Institute for Health and Care Excellence NICE, 2007) result in only moderate improvements (g = 0.33), contrasting with larger effect sizes for CBT in the treatment of other conditions such as anxiety (Olatunji, Cisler, & Deacon, 2010). It has been suggested that both the heterogeneity of the condition and moderate treatment response may be attributable to phenotypes within the condition which are not being adequately accounted for (Collin, Heron, Nikolaus, Knoop, & Crawley, 2018). Treatment success inevitably depends on accurate identification of the presenting clinical problem, which is particularly challenging in conditions where co-morbidity is high; a recent systematic review and meta-analysis reported around half of the CFS/ME population report anxiety and/or depression (Caswell & Daniels, 2018) which supports existing findings. These rates are comparable with other long-term health conditions such as Diabetes and Chronic Obstructive Pulmonary Disorder (Campbell-Sills et al., 2013).
The overlapping characteristics of depression and CFS/ME continue to be discussed and supported (Janssens, Zijlema, Joustra, & Rosmalen, 2015) yet anxiety in CFS/ME has been largely neglected. Health anxiety has gained significant interest in medical settings due to high prevalence rates of up to 24.7% (Tyrer et al., 2011), compared to 0.26-8.5% in the primary care population (Creed & Barsky, 2004). A recent study reported incidence of health anxiety in CFS/ME at 42% (Daniels, Brigden, & Kacorova, 2017), however this study was limited in sample size and scope of investigation. There is a growing body of evidence supporting the use of the CBT health anxiety model (CBT-HA) in medical populations, showing effective outcomes across samples (Tyrer et al., 2017). CBT-HA is based on the notion that ambiguous health-related stimuli are subject to interpretation which hence informs behavioural responses such as hypervigilance to physiological sensations, avoidance, bodily monitoring and reassurance seeking in order to prevent realisation of health concerns. However, these strategies often increase distress. The behavioural strategies may feel useful, necessary and justified in a condition such as CFS/ME where diagnosis is often protracted and delegitimising. The utility of the CBT-HA model in CFS/ME has recently been tested in a single case study, reporting successful outcomes at 12 months (Daniels & Loades, 2017). With current recommended interventions for CFS/ME offering modest outcomes and emerging reports of elevated rates of health anxiety, CBT-HA may offer an alternative treatment to those presenting with co-morbidity in CFS/ME.
This study seeks to replicate and advance previous findings to examine the presence, impact and treatment of health anxiety in CFS/ME.
STUDY 1Aims were to replicate earlier findings and seek supportive evidence for the treatment of co-morbid health anxiety in CFS/ME. More specifically, to (a) identify the prevalence of anxiety, depression and health anxiety in CFS/ME and (b) assess the relative impact of health anxiety on the primary outcome measures in treatment for CFS/ME, fatigue and physical functioning.
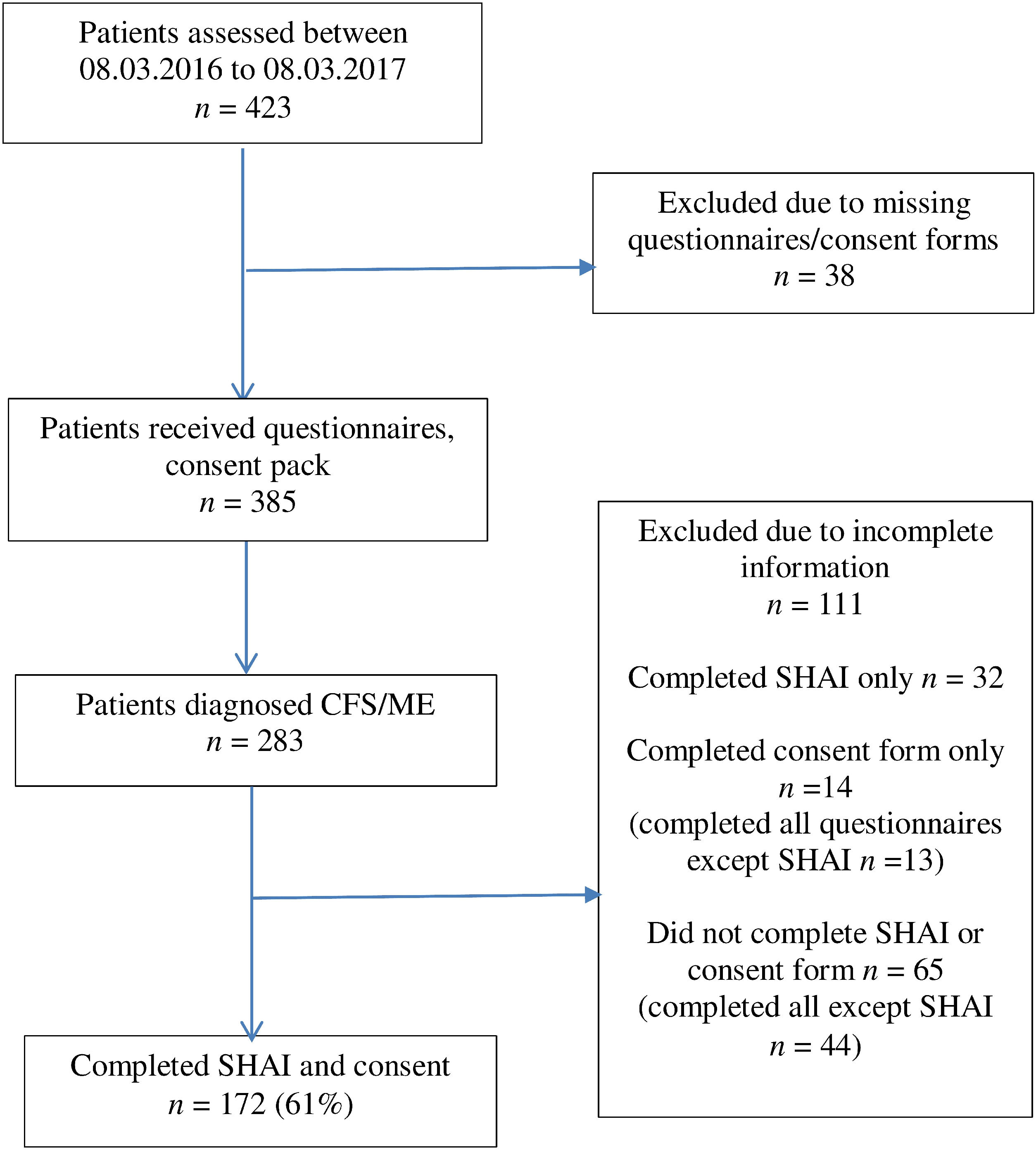
MethodParticipantsAll patients referred within the sampling period were invited to complete questionnaires prior to initial assessment. This formed the battery of measures for the study. Inclusion criteria were: (a) aged 18 or over (b) definite diagnosis of CFS/ME (c) literate in the English language. During the 12 month sampling period, 423 patients were referred to the service. Of those, 283 participants were eligible for participation due to receiving a definite primary diagnosis of CFS/ME (Fig. 1, CONSORT diagram), falling within the age range and indicating sufficient understanding of the English language. Sixty-one percent (N = 172) both consented and completed the SHAI; the remaining declined without reason. Standard imputation methods were required for missing data in eight cases.
As described in Table 1, the final sample were mostly female, white British with ages ranging from 17-70 years. Months since onset of fatigue was available for 135 participants (78%) and ranged from 5-312 months, with a mean duration of 60 months. Age and months since onset were correlated using Spearman’s Rho (due to lack of normality in the data and multimodal distribution) however no significant association was found (rs=.14, p= .93) and therefore not further controlled for. Of those who responded to the demographic assessment questions on co-morbidity (average of 64% across questions), 30% indicated comorbid migraine, 41.5% irritable bowel syndrome, 16.5% fibromyalgia; <1% chronic regional pain disorder, 44.2% anxiety, depression 48% and ‘other’ co-morbidities uncategorised 28.4% (n = 29). The latter included pain related disorders (n=12), neurological problems (n=4), mental health diagnosis (n=4), and vitamin deficiencies (n=3).
Demographic and clinical variables for Study 1 and Study 2.
| Study 1N < 172Mean (SD) | Study 2N = 10Median (IQR) | |
|---|---|---|
| Age (years) | 38.6 (12.7) | 32 (12.41) |
| Female, n (%) | 147 (86%) | 10 (100%) |
| Median duration of illness, months (IQR) | 59.55 (14, 72) | 36 (12–96) |
| British ethnicity | 167 (97%) | 10 (100%) |
| SHAI | 16.65 (6.45) | 26.3 |
| Number of Pts reaching case level SHAI (%) | 41.9 | 100 |
| HADS-A | 9.73 (4.45) | 12 |
| Number of Pts with HADS-A (%) score >11 | 40.7 | 80 |
| HADS-D | 9.36 (4.06) | 8.8 |
| Number of Pts with HADS-D (%) score >11 | 38.6 | 40 |
| Chalder fatigue questionnaire | 27.11 (4.71) | 10.5 |
| SF-36 | 45.18 (25.91) | 39.3 |
Note. SD: Standard Deviation; IQR: Interquartile Range; SHAI: Short Health Anxiety Inventory; HADS-A: Hospital Anxiety and Depression Scale-Anxiety; HADS-D: Hospital Anxiety and Depression Scale-Depression; SF-36: Short-Form-36.
Information regarding demographic and existing medical information was collected using the routine clinic assessment form. In addition to this, the questionnaire battery included the following standardised instruments.
The 11-item Chalder Fatigue Questionnaire (Chalder et al., 1993) measures physical and mental fatigue. Reliability and validity of this measure is supported in CFS/ME (Deale, Chalder, Marks, & Wessely, 1997). The 0-3 scoring method was used as it is considered more sensitive to change.
The 14-item Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith, 1983) measures anxiety and depression in two sub-scales. Reliability has been demonstrated CFS/ME samples (α=.80, .84 respectively) (Daniels et al., 2017).
The Short Form-36 (SF-36) physical functioning scale measures functioning as a result of physical/emotional difficulty (Jenkinson, Coulter, & Wright, 1993). The SF-36 has good test–retest reliability score (r=.75) (Brazier et al., 1992). Higher scores indicate better functioning.
The Epworth Sleepiness Scale (Johns, 1991) measures likelihood of sleepiness in specific situations, rating 0-3. Test–retest reliability (r = .82), and internal reliability (α = .88) were good (Johns, 1992).
In addition to the standard battery of questionnaires, the 14-item Short Health Anxiety Inventory (SHAI; Salkovskis, Rimes, Warwick, & Clark, 2002) was included for the purposes of this study. This measure was deemed the most suitable measure to assess health anxiety due to the robust theoretical basis of the measure: items are directly derived from the evidence-based empirically-grounded clinical model of health anxiety (Salkovskis, Warwick, & Deale, 2003) aligning with the now commonly accepted anxiety foundation of health anxiety (rather than somatization or more generic underpinnings seen in other similar measures; see Salkovskis et al., 2002). According to the original paper, the conceptual construct of health anxiety is based on the principle that distress arises due to an enduring predisposition to misinterpret ambiguous normal bodily variations or physiological stimuli as indicators of physical illness (which may also extend to medical information). This results in the employment of behaviours which serve to maintain rather than reduce distress. The items relate to the specific dimensions of the cognitive model as set out in Salkovskis et al. (2003), including preoccupation with health concerns, vigilance to bodily sensations, interpretation of ambiguous physical sensations and disbelief in medical reassurance. The original paper utilised standard deviations of normative data to produce clinical cut-offs to indicate case (>18) and non-case levels of health anxiety and differentiate generic anxiety. Subsequent studies and reviews have established test-retest reliability, concurrent, convergent and discriminant validity of the SHAI (Daniels et al., 2017; Hedman et al., 2015). Overall internal consistency of the measure is very good (α=.89) (Rabiei, Kalantari, Asgari, & Bahrami, 2013; Salkovskis et al., 2002) and the measure has also been demonstrated as reliable in CFS/ME (α =.89) (Daniels et al., 2017) and other medical conditions (Tyrer et al., 2011).
ProcedureCross-sectional questionnaire data were taken from a specialist CFS/ME service over a 12 month period from March 2016 to March 2017. The service offers assessment and treatment in accordance with National Institute for Health and Care Excellence NICE (2007) guidance, patients are diagnosed using the Fukuda criteria checklist (Fukuda et al., 1994). Ethical approval was granted for the prevalence study and case series from the University of Bath and Cornwall and Plymouth Research Ethics Committee (REC no: 13/SW/006) and East Essex Research Ethics Committee (13/EE/0301) respectively.
Data analysis was completed using Statistical Package for Social Sciences (SPSS) v23. Internal consistency of the SHAI was tested using Cronbach’s alpha and item-total correlations to support the utility and reliability of the SHAI in the CFS/ME population.
To assess prevalence of health anxiety, a score of >18 was used as a cut off for definite cases of health anxiety, replicating previous work (Daniels et al., 2017) and research in similar fields (Carrigan, Dysch, & Salkovskis, 2018). To assess prevalence of anxiety and depression using the HADS, the predefined cut-off of >11 was used to indicate case level distress (Brennan, Worrall-Davies, McMillan, Gilbody, & House, 2010). A chi-squared test compared the prevalence rates of health anxiety in CFS/ME in comparison to other medical settings.
Independent samples t-tests examined differences between those with high health anxiety (HiHA) versus those with low health anxiety (LoHA) across all variables. An upper cut-off of definite case ≥18 on the SHAI was used, with a ≤14 lower cut-off allowing a comparison of distinct groups at either end of the distribution. Scores of 15-17 on the SHAI represent sub-clinical borderline health anxiety and were thus excluded from the sub-group analysis.
Planned a priori Pearson’s tests of association were performed to examine the relationship between health anxiety, fatigue and physical functioning, plus age and duration to assess whether the latter should be controlled for in analysis.
Stepwise multiple regressions was performed to establish the r2 variance of each of the independent predictor variables on the pre-specified dependent criterion variables. The predictor variables were HADS-A, HADS-D and of focal interest, health anxiety as measured by the SHAI. The criterion variables were the Chalder fatigue questionnaire and SF-36 for physical functioning, respectively. Given the theoretical and evidenced link between fatigue and physical functioning, fatigue was also included as a predictor variable for physical functioning, and vice versa.
A significance value of p < .05 was planned for all analyses except tests of association where multiple comparisons indicated a significance threshold of p < .0125.
ResultsData from the SHAI were normally distributed. The SHAI was found to have high internal-consistency in this population (14 items; α = .86); tests of convergent validity between the HADS-A and SHAI confirmed moderate convergence (r = .48, n=169, p<.001), similar to previous tests of convergence in CFS/ME (Daniels et al., 2017). Score on the SHAI ranged from 4-38 (M = 16.65, SD = 6.45), with 41.9% of the sample reaching SHAI clinical cut-off for definite case health anxiety (≥18). This is significantly higher (p<.05) than prevalence in a Neurology outpatient sample (N = 3,205, 24.7%) (Tyrer et al., 2011), the most elevated across outpatient medical clinics.
Analysis of HADS data indicated 40.7% (n = 70) and 38.6% (n = 66) reached ‘definite’ caseness in terms of anxiety and depression respectively (≥11), (excluding missing data cases), consistent with rates of self-reported diagnosis of anxiety (33.1%) and depression (35.5%) on the clinic assessment form.
Independent t-test comparisons between HiHA and LoHA groups (n = 69, n = 72 respectively) were performed with equal variances noted on all variables except age and SHAI where variance adjustments were made. Significant differences were found between groups in the expected directions on all measures of interest: Chalder fatigue questionnaire (t(135) = -3.24, p = .002), SF-36 (t(138)=4.83, p < .001). Analysis indicated that those with case level health anxiety demonstrated significantly lower physical functioning and mood and higher levels of fatigue, sleepiness and anxiety than those without case level health anxiety. Age between groups was not significant (t(136.12)=1.59, p =.115). HiHA group mean scores for the HADS-A and HADS-D fell within the ‘severe’ range (≥11) whereas LoHA group means did not.
Pearson’s test of association indicated significant relationships between the SHAI and the SF-36 (r = -.34, n = 170, p < .001) and Chalder fatigue questionnaire (r = .20, n = 167, p = .01), HADS-A (r = .47, n = 169, p < .001) and HADS-D (r = .38, n = 171, p < .001) in expected directions. The association between the SHAI and Epworth Sleepiness Scale was non-significant at predetermined levels (r = .18, n = 167, p = .018).
Stepwise regression analysis indicated physical functioning and depression accounted for 23.8% of the variance of fatigue (R2 = .23, F(2, 160)=24.61, p < .001). Physical functioning accounted for 20.7% of the variance (β=.-45, p < .001), depression accounted for an additional R2 change of 3% (β=.19, p =.014) (see Table 2). All other variables entered were excluded from the model.
Summary of stepwise multiple regression analysis for variables predicting fatigue.
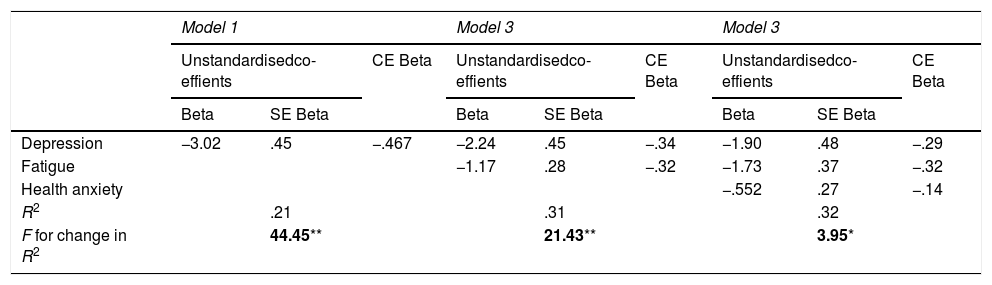
Three significant predictors accounted for 32.9% of the variance of physical functioning (R2 = .32, F(3,160)=25.62, p < .001). Depression explained 21.8% (β = -.46, p < .001) followed by fatigue (β = -.32, p < .001) and health anxiety (β = -.14, p =.044) (Table 3). Health anxiety contributed R2 change of an additional 3.2% after accounting for depression and fatigue, indicating health anxiety was a significant independent predictor of physical functioning. All other variables entered were excluded from the model.
Summary of stepwise multiple regression analysis for variables predicting physical functioning.
| Model 1 | Model 3 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unstandardisedco-effients | CE Beta | Unstandardisedco-effients | CE Beta | Unstandardisedco-effients | CE Beta | ||||
| Beta | SE Beta | Beta | SE Beta | Beta | SE Beta | ||||
| Depression | −3.02 | .45 | −.467 | −2.24 | .45 | −.34 | −1.90 | .48 | −.29 |
| Fatigue | −1.17 | .28 | −.32 | −1.73 | .37 | −.32 | |||
| Health anxiety | −.552 | .27 | −.14 | ||||||
| R2 | .21 | .31 | .32 | ||||||
| F for change in R2 | 44.45** | 21.43** | 3.95* | ||||||
Aims were to assess the relative effectiveness of a CBT-HA intervention for CFS/ME and co-morbid health anxiety, replicating earlier findings on a larger scale.
MethodParticipantsPatients were eligible for referral to the treatment trial if they met criteria for study one, plus a score of ≥18 on the SHAI at assessment. Those with co-morbidities were not excluded if their primary concern was CFS/ME. An idiosyncratic CBT-HA treatment derived formulation collaboratively developed with each individual provided the basis for the treatment intervention, integrating CFS-associated symptoms and health anxiety-related symptoms. The CBT-HA treatment comprised twelve individual face-to-face 60minute sessions, replicating earlier work described by Daniels and Loades (2017). Treatment was delivered by the first author, an experienced clinical psychologist and accredited cognitive behaviour psychotherapist, with in-vivo supervision and protocol fidelity monitoring from health anxiety expert Professor Paul Salkovskis. Both participants and clinician audio recorded all treatment sessions.
InstrumentsParticipants completed all study one battery of questionnaires, with the addition of the three level EQ-5D health related quality of life questionnaire (EuroQol Group, 1990). Measures were repeated weekly during the baseline period to establish stability of symptoms, and at each treatment session.
ProcedureA consecutive case-series with phased AB design was used, with phase ‘A’ representing a six week baseline phase requiring completion of weekly measures only, and phase ‘B’ representing the intervention phase. The n of 1 design is a suitable approach to pilot and test feasibility of complex interventions as fore-runners to large multi-centre randomised controlled trials (Medical Research Council, 2007).
For analysis, reliable and clinically significant change on each measure was calculated using the Jacobson and Truax (1991) method. Change is calculated based on pre-treatment measures (session 1) and final treatment measures (session 12). Cohen’s d was calculated per measure for the combined group to give an indication of overall treatment effect, dividing the mean change in individual scores by the pooled standard deviation of scores at time points (pre-treatment, end of treatment). Further statistical analyses were not planned as this was not considered to offer further meaningful interpretation of the data and was considered scientifically unjustified given sample size and appropriateness of aforementioned planned analysis.
Description of treatment: CBT-HACBT-HA is a formulation-driven cognitive behavioural intervention for health anxiety as described in Salkovskis et al. (2003). Following assessment of relevant factors such as onset, duration and relevant background, the initial sessions were used to formulate an idiosyncratic CBT-HA model that integrated individual patients’ reported CFS/ME-associated symptoms (e.g., fatigue and pain in ‘physical’ domains) and health anxiety-related symptoms (e.g., palpitations). Details of a recent episode of CFS/ME symptoms, cognitions, associated behavioural and emotional responses (e.g., anxiety) were elicited to populate an individualised formulation. In CBT-HA, cognitions related to health concerns (e.g., I will collapse) trigger behavioural responses designed to prevent the feared outcome. However, these (safety seeking) behaviours (SSB) serve to prevent disconfirmation of the feared outcome, thus reinforcing the behaviour through operant mechanisms. The CBT-HA formulation draws out the unintended consequences of the SSB (e.g., anxiety/frustration, intensified pain/deconditioning) and how these further reinforce fear of ‘collapse’, for example. This creates a vicious self-reinforcing cycle of distress which is maintained by SSB originally employed to reduce the likelihood of physical collapse or other feared outcome.
This formulation formed the basis for treatment. Standard CBT techniques such as use of ‘hypothesis A vs. hypothesis B’, verbal reattribution, cognitive restructuring and so forth, were used to appraise negative self-referential beliefs, test SSB and develop optimal adaptive self-management strategies. The CBT-HA approach was not adapted for the CFS/ME population; physical symptoms, beliefs and behaviours associated with CFS/ME were included within the standard dimensions of the model, with the same techniques used for those with and without medical problems, as seen in Tyrer et al. (2017). This does not infer HA adequately accounts for or explains CFS/ME; the model is regarded as transdiagnostic when applied in medical settings.
ResultsSample characteristicsThirty consecutive referrals were made to the treatment trial by clinicians within the service. Of these, seven did not meet inclusion criteria at screening, a further four reported logistical reasons for declining therapy (e.g., competing work demands) and one preferred a standard CFS/ME intervention. This left eighteen meeting eligibility criteria who consented to entry into the trial. Thirteen progressed to assessment after five were lost to follow up. Prior to treatment commencement, two further participants were withdrawn due to no longer meeting the eligibility criteria (n = 2) and one withdrew for personal/social reasons (n = 1). The final sample consisted of the pre-specified target of n = 10 participants, 63% of the eligible sample. Recruitment ceased once n = 10 had been reached.
Data from the clinical assessment form indicated all participants were female, ages ranging from 19-56 years, with duration of onset of CFS/ME 6-96 months (Table 1). Participants reported pain (n = 3), anxiety (n = 6) and depression (n = 2) in addition to CFS/ME. None had previous experience of therapy. Four participants were in full time (>30hours per week) or graded return to full time employment (40%), four in part-time employment (40%), one was a student (10%) and one was unemployed (10%).
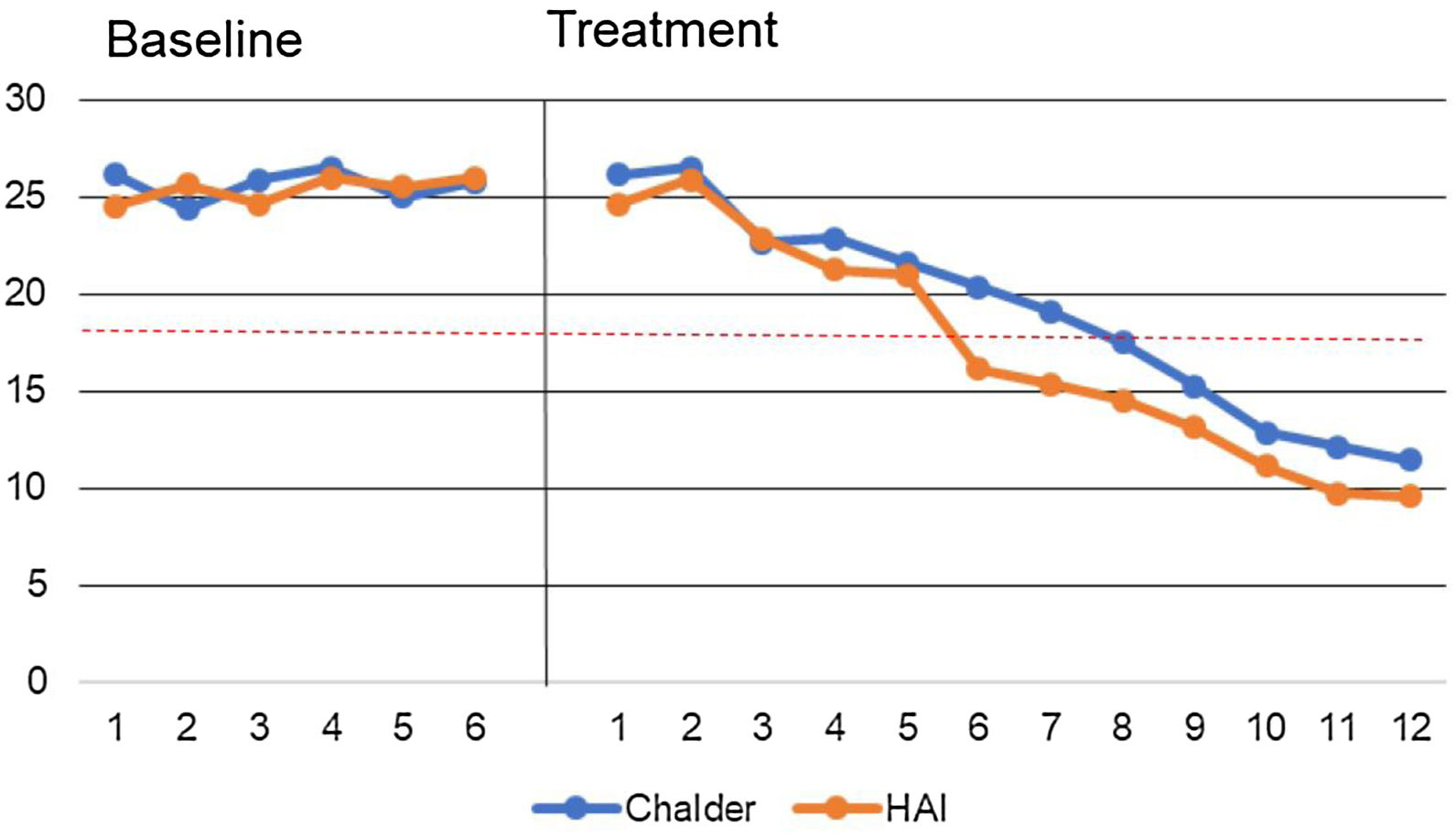
Eight participants completed the full course of therapy (80%, n = 8). All those who completed treatment demonstrated reliable change (RC) and clinically significant change (CSC) on at least one primary outcome: six of eight achieved RC and seven of eight CSC on the Chalder fatigue questionnaire; all treatment completers (n = 8) achieved both RC and CSC on the SHAI and moved to non-case status. Relevant data required to calculate the RC on the SF-36 or EQ-5D was not available, however treatment completers improved by 20-50 points, with six of eight achieving CSC (see Table 4). For those who did not complete treatment (n=2; withdrawal at session 5 and 6) scores remained stable: neither participant demonstrated point change on the Chalder fatigue questionnaire; no follow-up data was returned on the SF-36 or EQ-5D. The SHAI either remained the same or reduced by 2 points. CSC and RC were not calculated for these participants as it was evident that a partial intervention had elicited no change.
Calculation of effect size, reliable and clinically significant change of CBT-HA for CFS/ME.
| Measure | Baseline 1M (SD) | Last baseline/pre-treatmentM (SD) | End of therapyM (SD) | ChangePre-postM (SD) | ReliableChangen | ClinicallySignificantChangen | Effect size pre-post d |
|---|---|---|---|---|---|---|---|
| Chalder Fatigue Score | 26.70 (4.52) | 26.70 (4.79) | 14.90 (10.26) | 11.80 (9.45) | 6 | 7 | 1.47 |
| SHAI | 25.70 (5.31) | 26.40 (6.98) | 14.30 (11.38) | 12.10 (7.87) | 8 | 8 | 1.28 |
| HADS-A | 13.40 (3.27) | 13.10 (3.35) | 7.6 (6.54) | 5.5 (4.50) | 5 | 7 | 1.06 |
| HADS-D | 11.20 (3.99) | 10.20 (3.99) | 6.6 (5.54) | 3.60 (3.78) | 3 | 6 | 0.75 |
| EQ-5Da | – | 8.88 (1.36) | 7.38 (1.85) | 1.5 (0.92) | – | – | 0.93 |
| SF-36a | – | 46.88 (25.35) | 74.38 (22.43) | 27.50 (13.63) | – | 6 | 1.15 |
Calculations of Cohen’s d included all participants, including those who withdrew from treatment. Analysis of data indicated large effect sizes of > 0.8 on each measure with the exception of HADS-D which fell marginally shy of the cut-off (0.75). Cohen’s d for the effect of treatment on the EQ-5D and SF-36 were based on the data available for the n = 8 treatment completers as these were pre/post measures.
Fig. 2 reports the data path of the median values of key measures (SHAI, Chalder fatigue questionnaire) during phase A (baseline) and phase B (treatment) for those who completed treatment (dotted line denotes clinical cut off of the SHAI). Physical functioning is not represented due to scaling.
DiscussionThe overarching objectives of this study were to replicate and extend previous findings examining the prevalence, relative impact and treatment of CFS/ME with co-morbid health anxiety using larger-scale more robust studies to inform future treatment development and advance research in the field.
Findings from study one indicate that health anxiety in CFS/ME is common and significantly associated with symptom severity. Participants with high health anxiety were more fatigued, anxious and depressed, demonstrating lower levels of physical functioning than those without; health anxiety was confirmed as a significant predictor in a three factor model of physical functioning, but not at all in fatigue. Overall, findings from study one support, replicate and extend previous work (Daniels et al., 2017) suggesting health anxiety in CFS/ME is highly prevalent, significantly more so than in other medical settings (Tyrer et al., 2011) but similar to those found in the chronic pain population (Rode, Salkovskis, Dowd, & Hanna, 2006). The commonality between the chronic pain and CFS/ME population may be attributed to the absence of a unifying theory to underpin and explain physical symptoms that are heterogeneous in nature; the chronicity associated with ‘chronic’ pain and ‘chronic’ fatigue syndrome/ME may present fertile breeding ground for health focussed anxiety where other relevant factors co-exist, particularly considering the stigmatisation and deligitamization reported in CFS/ME (Dickson, Knussen, & Flowers, 2007).
Physical functioning accounted for 20.7% of the variance of fatigue, supporting current theories of a relationship between these factors and established clinical notions, suggesting that reduced physical activity may perpetuate and exacerbate fatigue on exertion, as seen in other conditions such as arthritis (Hegarty, Conner, Stebbings, & Treharne, 2015). Reciprocally, the presence of fatigue is likely to both reduce motivation and compromise capacity for physical functioning, presenting as a vicious cycle which may form a key barrier to clinical intervention. This assumption mirrors the basis of behavioural and physical activity based interventions for CFS/ME. Depression was a secondary significant predictor of fatigue at a lower proportion of variance (an additional 3%), and was the most significant predictor of physical functioning. This is unsurprising given the common association between low mood and reduction in physical activity, the subsequent muscle deconditioning that follows from low levels of physical activity, and then increased fatigue at lower levels of exertion (Browne & Chalder, 2006). Chronic health problems such as CFS/ME are likely to impair mood and physical functioning, which perpetuate both physical symptoms such as fatigue and psychological distress. These findings coupled with rates of co-morbidity suggest that screening for depression in CFS/ME may be warranted.
General anxiety was not found to be a significant predictor of fatigue or physical functioning, where health anxiety has been found to be an independent significant predictor of the latter, albeit accounting for only a marginal proportion of the variance. Taken with significant findings relating to higher physical and psychological distress in the high health anxiety group, it is evident this area warrants further exploration. Previous research suggests that anxiety and health anxiety are distinct yet related constructs (Daniels et al., 2017), with the present findings offering replication and new advancement in the precision of our current understanding of the potential impact and co-morbid of anxiety and health anxiety in CFS/ME. More specifically: we suggest that due to largely unexplained physical sensations, lack of effective reassurance from medical practitioners (due to the condition being poorly understood) and the complex anxiety provoking and problematic nature of managing fatigue, some patients with CFS/ME may be prone to employing strategies to prevent worsening of symptoms such as restricting and/or avoidance of physical activity, paying attention to ‘warning signs’ or excessively resting (Daniels & Loades, 2017; De Gucht, Garcia, den Engelsman, & Maes, 2017) as part of a health anxiety cycle, subsequently resulting in lower levels of physical functioning and fatigue on exertion. This is consistent with models of health anxiety (Salkovskis et al., 2003), fear-avoidance models (Vlaeyen, Crombez, & Linton, 2016) and is seen elsewhere in other medical conditions such as Multiple Sclerosis (Hayter, Salkovskis, Silber, & Morris, 2016) where there is a clearly understood pathogenesis; indeed we suggest that CFS/ME presents similarly. Findings support these clinical hypotheses, however further work is needed to elucidate the mechanisms and direction of effects when health anxiety co-occurs with CFS/ME.
Study two replicates and further tests the utility of CBT-HA in a larger CFS/ME sample. Outcomes are consistent with recent findings indicating that CBT-HA is an appropriate and effective treatment for health anxiety comorbid with medical conditions (Cooper, Gregory, Walker, Lambe, & Salkovskis, 2017), despite the potential complex interaction between CFS/ME and health anxiety. Treatment outcomes demonstrate high levels of both reliable and clinically significant change in the target measures, with large effect sizes across the majority of measures indicating effectiveness of the intervention. Notwithstanding the complex symptomatic presentation of CFS/ME and health anxiety co-occurring, the intervention was unproblematic and protocol driven. This offers preliminary evidence that a protocolised intervention for co-morbidity in CFS/ME demonstrates utility and a credible basis for psychological intervention. It is imperative that those receiving the intervention understand that the implicit assumptions of the model does not discriminate between conditions; health anxiety is prevalent and responsive to effective treatment across medical conditions. The focus is distress, and does not infer causality.
Limitations and future researchA proportion of clinicians and patients within the prevalence study expressed scepticism relating to the SHAI. This may have subjected recruitment to selective bias from clinicians with positive/neutral views of the SHAI, leaving opportunity for inadvertent sampling bias of psychologically receptive participants only. Data was collected prior to diagnosis (a potentially anxious time), however the SHAI requires data based on the ‘past week’ and has good test-retest reliability in medical settings. Health anxiety was not confirmed using formal diagnostic procedures: a larger, longitudinal treatment study using diagnostic interviews such as the structured clinical interview for the diagnostic statistical manual (SCID) or similar would advance a more complex and robust understanding of the impact and potential mediating role of health anxiety in treatment. These findings serve as a firm theoretical basis for development in this area.
The case-series n of 10 represents an adequately sized sample proportionate to the methodology, with significant outcomes across several dimensions. However, a RCT using formal diagnostic procedures and control comparisons would generate further evidence to support the utility of CBT-HA in CFS/ME and would both satisfy current study limitations and offer a logical next step in the field.
ConclusionsThis is the first study to robustly examine prevalence rates of health anxiety in CFS/ME, acting as a larger scale replica of previous studies by the same group. Outcomes from both the prevalence study and treatment trial make a novel contribution to the current understanding and treatment of health anxiety and co-morbidity in CFS/ME. This study provides a clear rationale and platform for further research to replicate and enhance treatment options to this clinical population. Screening for comorbidity is strongly recommended.
FundingThis work was funded by North Bristol NHS Trust, Bristol UK (grant numbers 3045 and 3178). Financial support was received to facilitate the conduct and dissemination of both studies described in this paper.
Thanks are extended to participants and clinicians who participated in the study, the steering committee, Laura Medina Perucha for translation work and Matthew Harland, Stephanie Ennis and Tzeamara Goddard for assisting on the projects.