Colonic self-expandable metal stent placement is widely used for palliation of obstructive colorectal cancer. The European recommendations for stent placement as a bridge to elective surgery in obstructive colorectal cancer were recently reviewed. The aim of this study was to evaluate the efficacy and safety of stent placement in obstructive colorectal cancer and to discuss these recent guidelines.
Materials and methodsDemographic characteristics, procedure indications, complications and final outcome in patients with obstructive colorectal cancer who underwent endoscopic stent placement between January 2012 and June 2015 were retrospectively analyzed. Statistical analysis was performed with SPSS V22.
ResultsThirty-six patients were included, 20 (56%) women, mean age 70.6±10.9 years. Stent placement as a bridge to elective surgery was performed in 75% (n=27) of patients and with palliation intent in 25% (n=9). In 94% (n=34) of procedures, technical and clinical success was achieved. A total of eleven (11%) complications were observed: 2 migrations and 9 perforations. No procedure related death was recorded. When stents were placed as a bridge to surgery, average time between endoscopic procedure and surgery was 11.7±9.4 days (excluding three patients who underwent neoadjuvant chemotherapy). Six perforations were recorded in this group: one overt and five silent (three during surgery and two after histopathological examination of the resected specimen). Twenty-one patients underwent adjuvant chemotherapy. During the follow-up period of 14.7±10.9 months recurrence was observed in five patients. None of the recurrence occurred in the group of patients with perforation.
ConclusionsIn this study, stent placement was an effective procedure in obstructive colorectal cancer. It was mainly used as a bridge to elective surgery. However, a significant rate of silent perforation was observed, which may compromise the oncological outcome of these potentially curable patients. Prospective real life studies are warranted for a better definition of actual recommendations.
A colocação de próteses metálicas autoexpansíveis é um procedimento endoscópico amplamente realizado como tratamento paliativo do cancro colo-rectal. As recomendações europeias para a colocação de prótese como ponte para a cirurgia na obstrução por cancro colo-rectal foram revistas recentemente. O objetivo deste estudo foi avaliar a eficácia e segurança da colocação de próteses na obstrução maligna por cancro colo-rectal e discutir as últimas recomendações publicadas.
Materiais e métodosAnálise retrospectiva das características demográficas, indicações, complicações e resultados da colocação de próteses metálicas autoexpansíveis em doentes com cancro colo-rectal obstrutivo entre janeiro de 2012 e junho de 2015. A análise estatística foi realizada com SPSS V22.
ResultadosForam incluídos 36 doentes, 20 (56%) do sexo feminino, com idade média de 70.6±10.9 anos. As próteses foram colocadas como ponte para cirurgia em 75% (n=27) dos casos e com intuito paliativo em 25% (n=9). Em 94% (n=34) dos procedimentos obteve-se sucesso técnico e clínico. No total registaram-se 11 (31%) complicações: 2 migrações e 9 perfurações. Não se registou mortalidade associada ao procedimento. Nos casos como ponte para a cirurgia, o tempo médio entre o procedimento endoscópico e a cirurgia foi de 11.7±9.4 dias (excluídos três doentes submetidos a quimioterapia neoadjuvante). Observaram-se seis perfurações neste grupo de doentes: uma perfuração clínica e cinco silenciosas (três intra-operatoriamente e duas após avaliação anatomopatológica da peça operatória). Vinte e um doentes foram submetidos a quimioterapia adjuvante. Após um tempo médio de seguimento de 14.7±10.9 meses, registaram-se cinco casos de recorrência. Nenhum dos casos de recorrência ocorreu no grupo de doentes com perfuração.
ConclusõesNesta amostra, a colocação de prótese revelou-se um procedimento endoscópico eficaz. Na maioria dos doentes foi utilizada como ponte para a cirurgia. No entanto, verificou-se uma taxa significativa de perfuração silenciosa que poderá comprometer o resultado oncológico de doentes tratados com intuito curativo. Estudos prospetivos da prática real podem ser úteis para uma melhor definição das recomendações atuais.
Colorectal cancer (CRC) is the third most common cancer in males and the second in females worldwide.1 Malignant large bowel obstruction is reported in up to 20% of colonic cancer patients.2 The management of this severe clinical condition remains controversial.3
Malignant colonic obstruction may be managed by emergent surgery with resection and/or diversion procedures or by endoscopy with self-expanding metal stents (SEMS) placement.4
In the latest European Society of Gastrointestinal Endoscopy (ESGE) guidelines published in 2014, SEMS placement is recommended as the preferred treatment for palliation of malignant and metastatic colonic obstruction3,5,6 but the role of SEMS as a bridge to elective surgery in obstructive CRC was largely modified.3 Preoperative SEMS placement can prevent high-risk emergent surgery allowing patient stabilization and staging workup before surgical intervention.7,8 This approach showed more favorable short-term outcomes in terms of permanent stoma formation, primary anastomosis and overall morbidity and similar postoperative mortality when compared to emergent surgery.9–11 However, a Dutch multicentric randomized trial showed an increased morbidity and mortality in the group of patients with SEMS as bridge to surgery when compared to the surgical approach and due to these interim results the study was interrupted.12 This study was criticized by several authors who questioned study design and mainly the experience of some of the centers in the use of metal stents due to the low clinical and endoscopic success rate reported.
Even if short-term benefits of stent placement are unquestionable, oncological outcomes are yet undetermined since SEMS insertion may promote tumor progression and metastasis.3,13,14 A meta-analysis recently published by Erichsen et al.15 reported a 5-year recurrence risk of 39% after SEMS placement compared with 30% after urgent resection. Although not completely proven, some studies have shown an association between recurrence and stent related perforation.14 Even silent perforations detected only on the surgical specimen may have oncological impact, potentially resulting in tumor cell seeding and dissemination.9 Sabbagh et al.16 reported higher rates of tumor ulceration, perineural and lymph node invasion after stent placement, suggesting that these microscopic alterations may also account for a worse oncological outcome in these groups of patients.
Therefore recent ESGE guidelines published in October 2014 no longer recommend colonic stenting as a bridge to surgery as standard treatment. It may be considered as an acceptable option in patients with an increased risk of postoperative mortality (over the age of 70 years and/or American Society of Anesthesiologists (ASA) score of ≥3) since the potentially impaired oncological outcome associated to stent-related perforation does not seem to outweigh the risk of postoperative death.3,11,14 These recommendations were felt to be very conservative by most gastroenterologists.
The aim of the present study was to critically evaluate the efficacy and safety of SEMS placement in a retrospective series of patients with obstructive colorectal cancer and to discuss current recommendations of this procedure.
2Materials and methodsWe conducted a retrospective study of all patients with obstructive CRC who underwent endoscopic stent placement between January 2012 and June 2015 in a secondary hospital, Hospital Beatriz Ângelo, in Lisbon area. ESGE guidelines were published in October 2014 and we modified our therapeutic strategy accordingly, in June 2015.
Acute colorectal obstruction was diagnosed by the absence of any flatus or bowel movements in the preceding 24h, abdominal distension and/or the presence of dilated colonic loops on abdominal radiograph. All patients underwent a contrast-enhanced computed tomography (CT) before endoscopic procedure in order to confirm the presence of obstruction, define the level of the stenosis, exclude perforation as well as local and distant staging.
Patient selection criteria for stenting were obstructive CRC located at or distal to the splenic flexure and absence of clinical or imagiologic evidence of perforation. Age, general physical condition or disease stage were not considered exclusion criteria for stenting as most cases occurred before the publication of ESGE guidelines. Patients with other causes of colonic obstruction were excluded from the study.
Stents were inserted as a bridge to elective surgery or with palliation intent. In our study, bridge to surgery was defined as elective surgery after optimization of physiological parameters and recovery of normal bowel function, regardless of the length of time between SEMS insertion and surgery.
All colonic SEMS were inserted endoscopically under fluoroscopic guidance with sedation performed mainly by anesthesiologist. Biopsies of the tumor were obtained in case of previously unknown diagnosis. SEMS placement was performed using through-the-scope/over the guidewire technique. After reaching the stenosis, a standard biliary cannula or stone extraction balloon with a 0.035in. Hydra Jagwire™ Guidewire (Boston Scientific) was advanced. Soon after it overcame the stenosis full strength contrast was injected under fluoroscopic guidance. The extent of the stenosis was measured with the stone extraction balloon or estimate through fluoroscopy. Clips were used as fluoroscopic markers to identify stenosis distal ends. Under endoscopic and fluoroscopic guidance, the stents were positioned above the stricture and proximally deployed. Stent length ranged from 6cm to 10cm and was inserted to include at least 2cm on each side of the lesion. After deployment its correct position was confirmed with fluoroscopic images. Uncovered colonic stents with proximal release system (Boston Scientific – WallFlex® or Cook Medical – Evolution®) were used in all patients. Balloon dilatation was not performed in any patients either before or after SEMS insertion. Abdominal radiographs were taken afterwards to check full deployment of the stent and colonic decompression.
After resolution of obstruction, pre-operative staging was completed and patients with potentially curable disease were offered elective resection. Neoadjuvant and/or adjuvant chemotherapy were performed whenever indicated. Those with incurable disease were referred for consideration of palliative chemotherapy according to international guidelines.4
Data collected included patient demographics, endoscopic findings (tumor anatomical site and length), local and distant staging, technical and clinical success, stent-related complications and mortality as well as details of further interventions performed after stenting (such as stent reinsertion, surgery or chemotherapy). For those patients with endoscopic stent placement as a bridge to surgery we also recorded time to surgery, perforations observed during surgery and on the surgical specimen as well as recurrence rate.
Technical success was defined as successful stent deployment across the obstructing tumor with radiographic confirmation of flaring of the stent both proximally and distally. Clinical success was defined as colonic decompression with visible flatus or stool passage within 48h after the procedure.
Stent complications were defined as those leading to new symptoms, characterized by perforation, re-obstruction and stent migration.
Statistical analysis was performed using SPSS V22.0. All continuous variables were described as median and range, while categorical variables were expressed as frequency and percentage. To explore univariate associations in the distribution of categorical data, the Chi-squared test or Fisher's exact test was used as appropriate. Differences in mean continuous variables between groups of patients were analyzed by the t-test. A p value<0.05 was considered statistically significant.
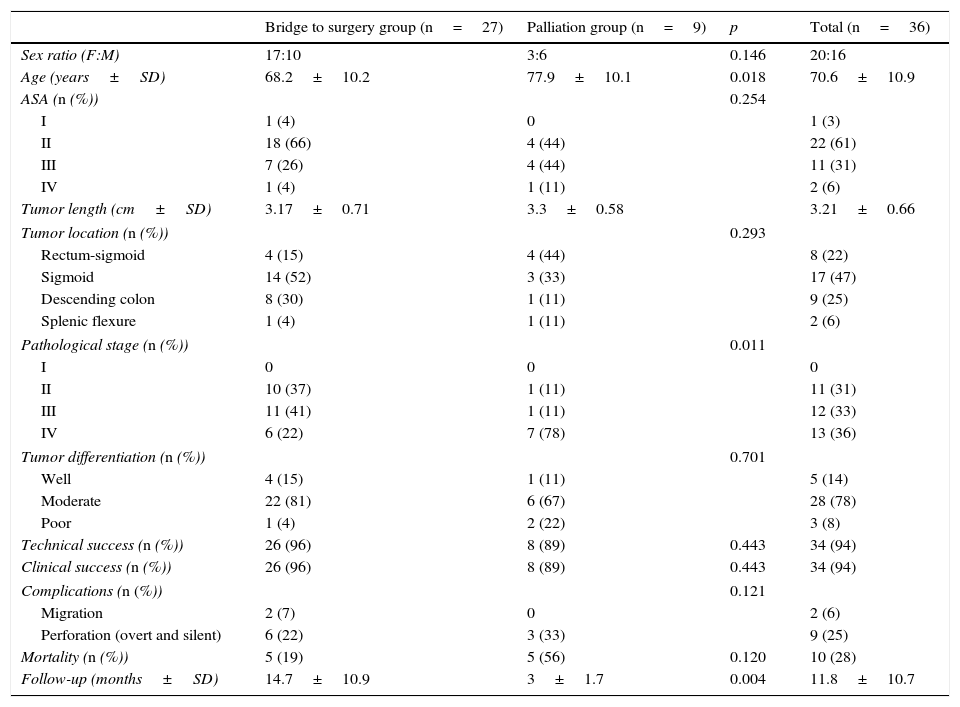
3ResultsStent placement was attempted in 36 patients, 20 (56%) women, mean age 70.6±10.9 years. Twenty-seven patients (75%) were stented as a bridge to surgery and the remaining nine patients (25%) underwent SEMS insertion with palliative intent. Patient demographics, clinical and endoscopic data of all patients are summarized in Table 1.
Demographics and clinicopathological parameters of all patients who underwent SEMS insertion.
| Bridge to surgery group (n=27) | Palliation group (n=9) | p | Total (n=36) | |
|---|---|---|---|---|
| Sex ratio (F:M) | 17:10 | 3:6 | 0.146 | 20:16 |
| Age (years±SD) | 68.2±10.2 | 77.9±10.1 | 0.018 | 70.6±10.9 |
| ASA (n (%)) | 0.254 | |||
| I | 1 (4) | 0 | 1 (3) | |
| II | 18 (66) | 4 (44) | 22 (61) | |
| III | 7 (26) | 4 (44) | 11 (31) | |
| IV | 1 (4) | 1 (11) | 2 (6) | |
| Tumor length (cm±SD) | 3.17±0.71 | 3.3±0.58 | 3.21±0.66 | |
| Tumor location (n (%)) | 0.293 | |||
| Rectum-sigmoid | 4 (15) | 4 (44) | 8 (22) | |
| Sigmoid | 14 (52) | 3 (33) | 17 (47) | |
| Descending colon | 8 (30) | 1 (11) | 9 (25) | |
| Splenic flexure | 1 (4) | 1 (11) | 2 (6) | |
| Pathological stage (n (%)) | 0.011 | |||
| I | 0 | 0 | 0 | |
| II | 10 (37) | 1 (11) | 11 (31) | |
| III | 11 (41) | 1 (11) | 12 (33) | |
| IV | 6 (22) | 7 (78) | 13 (36) | |
| Tumor differentiation (n (%)) | 0.701 | |||
| Well | 4 (15) | 1 (11) | 5 (14) | |
| Moderate | 22 (81) | 6 (67) | 28 (78) | |
| Poor | 1 (4) | 2 (22) | 3 (8) | |
| Technical success (n (%)) | 26 (96) | 8 (89) | 0.443 | 34 (94) |
| Clinical success (n (%)) | 26 (96) | 8 (89) | 0.443 | 34 (94) |
| Complications (n (%)) | 0.121 | |||
| Migration | 2 (7) | 0 | 2 (6) | |
| Perforation (overt and silent) | 6 (22) | 3 (33) | 9 (25) | |
| Mortality (n (%)) | 5 (19) | 5 (56) | 0.120 | 10 (28) |
| Follow-up (months±SD) | 14.7±10.9 | 3±1.7 | 0.004 | 11.8±10.7 |
Results for both groups are shown and discussed separately.
3.1Palliation groupNine patients (25%) underwent SEMS insertion with palliative intent.
Technical and clinical success was achieved in 8 out of 9 (89%) procedures. In the remaining case, the stent could not overcome the stenosis, probably related to stricture angulation, and the patient underwent urgent surgery.
Three overt perforations were observed 22.7±6 days after stent placement, requiring urgent surgery. No stent-related death was recorded.
Five patients underwent palliative chemotherapy. In the remaining four cases, patients performance status was 4 and best supportive care was decided. All patients with perforations were receiving chemotherapy – two with 5-fluouracil and iritnotecan (and cetuximab) and one with capecitabine. None of the patients was treated with bevacizumab.
With a mean time of follow-up of 3±1.7 months, 5 out of 9 patients (56%) had died.
3.2Bridge to surgery groupTwenty-seven patients (75%) had potentially curable disease on staging and were stented as a bridge to surgery. Fourteen patients were ≤70 years and/or ASA<III (all treated before June 2015) and 13 were >70 years and/or ASA≥III.
In 96% (26/27) of cases, technical and clinical success was achieved. In one patient the stent did not fully expand after being correctly placed and the patient was operated.
Eight stent-related complications were recorded: two migrations and six perforations. One stent migration occurred during the endoscopic procedure and a second stent was inserted. The other stent migration occurred two days after colonoscopy requiring surgery. One out of six perforations was overt and occurred six days after stent placement requiring urgent surgery. The other five perforations were silent: three observed during open surgery and two were recognized on the surgical specimen only. No procedure-related death was recorded.
Mean time between endoscopic procedure and surgery was 11.7±9.4 days, excluding three patients who underwent neoadjuvant chemotherapy – one patient to downstage his rectal tumor and two patients who had resectable synchronous liver metastases. One patient refused surgery and was lost in follow-up.
Twenty-one out of 26 (81%) patients underwent adjuvant chemotherapy. Of the remaining five patients, one died 11 days after surgery, and four did not meet clinical conditions to undergo chemotherapy.
With a mean follow-up time of 14.7±10.9 months, 15% (4/26) of patients had recurrence. One patient, who had not received adjuvant chemotherapy due to his poor clinical condition, had local recurrence with distant metastasis. One other patient had isolated local recurrence and in the other two cases distant metastasis were observed without local recurrence. All these three patients had received adjuvant chemotherapy.
During the mean time of follow-up five deaths were observed: two from medical causes (pneumonia and chemotherapy related complications), one secondary to septic shock after anastomosis leakage, one from disease progression and in the other no direct cause was determined.
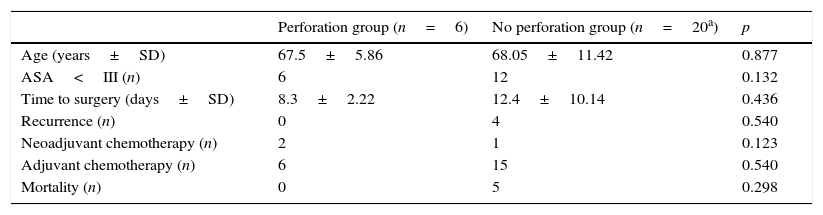
3.2.1Perforation versus no perforationIn the bridge to surgery group six perforations were recorded. There was no statistically significant difference either in age (67.5±5.86 years vs 68.05±11.42 years, p=0.877) or in ASA classification<III (6/6 vs 12/20, p=0.132) between the group of patients with stent-related perforation when compared with those without perforation.
There was also no difference between the two groups in terms of mean time between endoscopic procedure and surgery (12.4±10.1 days vs 8.3±2.2 days, p=0.436).
All cases of recurrence and death occurred in the group of patients without perforation. There was no statistical difference between the two groups (p=0.540 and p=0.298, respectively). These results are summarized in Table 2.
Comparison of patients with stent-related perforation and without perforation in bridge to surgery group.
| Perforation group (n=6) | No perforation group (n=20a) | p | |
|---|---|---|---|
| Age (years±SD) | 67.5±5.86 | 68.05±11.42 | 0.877 |
| ASA<III (n) | 6 | 12 | 0.132 |
| Time to surgery (days±SD) | 8.3±2.22 | 12.4±10.14 | 0.436 |
| Recurrence (n) | 0 | 4 | 0.540 |
| Neoadjuvant chemotherapy (n) | 2 | 1 | 0.123 |
| Adjuvant chemotherapy (n) | 6 | 15 | 0.540 |
| Mortality (n) | 0 | 5 | 0.298 |
The management of malignant large bowel obstruction is challenging and controversial.3 The European Society of Gastrointestinal Endoscopy (ESGE) recently reviewed the endoscopic approach of obstructive colorectal cancer in order to provide practical guidance regarding the use of SEMS in these patients.3 Accordingly, the use of surgery vs SEMS in patients with distal obstructive colorectal cancer was changed in our Hospital in June 2015. In this study, we critically reviewed our experience with stent placement for malignant acute large bowel obstruction before we adopted the latest ESGE guidelines.
Stent placement has been shown to be an effective procedure to manage obstructive CRC.8,17–19 A recent prospective multicenter study of 513 cases of obstructive CRC showed a technical and clinical success superior to 95%.19 In our study, technical and clinical success was achieved in 94% (34/36) of patients. Overall 9 (25%) perforations were recorded: 4 (11%) patients had overt perforation whereas in 5 (14%) other patients perforation was detected during surgery (n=3) and on the surgical specimen (n=2).
According to ESGE guidelines, in patients with metastatic obstructive CRC SEMS placement is the preferred treatment since stoma formation, early complications (<30 days), hospitalization and 30-day mortality rates are lower after stent placement compared with palliative surgery.5,6 In our study 25% patients underwent SEMS insertion with palliative intent with a technical success rate of 89%, similar to that reported in the literature.5,6 No stent-related migration was recorded. However, 3 (33%) perforations were registered, requiring urgent surgery. The rate of perforation in patients with incurable CRC was higher than described in the literature (8 a 10%)5,6 which might relate to several patient or tumor related variables although the small numbers do not allow any definitive conclusions.
Colonic stenting as a bridge to elective surgery in obstructive colorectal cancer has been widely used during the last decade because it can prevent high-risk emergent surgery providing time for patient stabilization, staging workup and appropriate bowel preparation before elective surgery.7,8 Several studies compared the efficacy and safety of SEMS insertion as a bridge to elective surgery vs emergency surgery for acute left-sided malignant colonic obstruction.9–11 Recent meta-analysis showed lower rates of permanent stoma formation, higher rate of successful primary anastomosis and more favorable overall morbidity, with no statistically significant difference in the postoperative mortality between the two groups.9–11 Mean success rate of colonic stent placement in these meta-analysis ranges from 53 to 97%.9–11,20 In our study 75% patients were stented as a bridge to surgery, the large majority of these occurring before the publication of current ESGE guidelines in 2014. Technical and clinical successes of 96% were comparable to those reported in the literature.
Although immediate advantages of SEMS placement are undeniable, its impact on the oncological outcome of patients with potentially curable disease remains the main drawback.3,13,14 The literature regarding this issue is still conflicting. The meta-analysis by Matsuda et al.21 which included 11 studies compared 432 patients with SEMS as a bridge to surgery to 704 patients who had emergent surgery. Both groups had comparable recurrence rates (31.1% vs 27.2%, NS), 5-year overall survival (57.2% vs 67.1%, NS), and 5-year disease-free survival (48.4% vs 59%, NS), suggesting that SEMS as bridge to surgery could be a promising alternative strategy for obstructive colorectal cancer. In a recent study published by Erichsen et al.,15 581 patients underwent SEMS placement and 3333 patients were operated. The authors reported a higher 5-year recurrence risk in the SEMS group (39% vs 30%; adjusted incidence rate ratio 1.12, 95% confidence interval [CI] 0.99–1.28), but 5-year overall survival rate was similar between the two groups (49% vs 40%; adjusted mortality rate ratio 0.98, 95% CI 0.90–1.07). Whether the increased recurrence rate in the study by Erichsen et al. may have been influenced by perforation is unclear, as these data were not available. Moreover, the prevalence of stent-related complications is likely to be underestimated, because subclinical perforations may be identified only after histological examination of the surgical specimen.
The meta-analysis by Tan et al.9 reported a clinical perforation rate of 6.9% and silent perforation rate of 14% after stent placement as a bridge to elective surgery. In our study, stent-related perforation occurred in 6 (22.2%) patients in the bridge to surgery group. One perforation was overt (3.7%) and 5 perforations were silent (18.5%): 3 (11.1%) were diagnosed during surgery and 2 (7.4%) were seen only on histopathological examination of the resected specimen. Thus, in terms of complications our results are comparable to the ones published in the literature.
The Dutch Stent-In 2 trial14 suggested an association between stent perforation and recurrence. In this trial, a total of 6/26 (23%) perforations were recorded: 3 overt and 3 silent perforations. Recurrence rate was 83% (5/6) in the group of patients with stent-related perforation and 40% (8/20) in the group without perforation. The 4-year disease-free survival was worse in the former group (0% vs 45%, p=0.007). Although the numbers are strikingly different between the perforated and non-perforated groups, the authors were unable to draw conclusions due to the small sample size. Recently published ESGE guidelines were probably based on the fact that since there is no reduction in postoperative mortality after SEMS placement as a bridge to surgery11 and oncological outcome of potentially curable patients can be compromised with colonic stenting,14 emergent surgery should be considered as the preferred treatment strategy for patients without significantly increased operative risk (ASA<III and/or age≤70 years).3 On the other hand, in patients with an increased risk of postoperative mortality, stent placement may be the preferred treatment since the potentially impaired oncological outcome associated to stent-related perforation seems to be less important than the increased risk of postoperative death.3
These recommendations were perceived by most gastroenterologists and colorectal surgeons as being very conservative and unjustified. As some heterogeneity was observed in the Dutch trial,12 most of clinicians involved felt that if these studies had been performed in well trained and high volume centers this rate of perforation and late recurrence would not have occurred.
In our study, 13 of 27 patients who underwent stent placement as bridge to surgery had an increased risk of postoperative mortality (ASA≥III and/or age>70 years). Fourteen patients were ≤70 years and/or ASA<III and 4 out of 6 perforations were observed in this group. Fortunately, so far none of the patients with stent-related perforation had recurrence. Mean follow-up time was short (14.7±10.9 months) and it is worth noting that 14 patients still have a follow-up time lower than 12 months. Thus, considering previous studies namely the Dutch Stent-In 2 trial,14 these patients should be kept under close surveillance before any definitive conclusions are drawn.
Our study has some limitations, namely its retrospective nature, the small sample size and the short follow-up time which may limit our conclusions in terms of oncologic outcome. Nonetheless, it reflects real life practice and it was reassuring to observe that, although complications namely perforation rates are within those described in the literature, none of the recurrences observed so far were in patients in whom perforations occurred. Now that ESGE guidelines have changed and colonic stenting is no longer recommended as a bridge to surgery in all patients, it is important to call the attention of endoscopists that oncological outcome may be compromised by this practice. It is also worth noting that endoscopists may not be aware of silent perforations which are far more frequent than overt ones and which may very well increase rate of local or distant relapse. In fact, if not all patients are discussed in multidisciplinary meetings both pre and post-operatively, we believe that most gastroenterologists will not be aware of these silent perforations.
5ConclusionIn this study, SEMS placement was an effective procedure in obstructive CRC. Because ESGE guidelines changed within the last year, colonic stenting was mainly used as a bridge to elective surgery. However, a significant (22%) rate of macro and microscopic perforation was observed, which might increase recurrence rate and compromise oncological outcome of patients with potentially curable disease. Nevertheless, in our study all the recurrences occurred in patients in whom perforation was not observed. A prospective registry of all these complications should be mandatory in all endoscopy units.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.