Vedolizumab (VDZ), a human monoclonal antibody that binds specifically to α4β7-integrin, and is approved for the treatment of Crohn's disease (CD) and ulcerative colitis (UC), has demonstrated its efficacy in controlled clinical trials.
ObjectiveTo describe a population treated with VDZ and to evaluate its long-term efficacy and safety in clinical practice.
MethodsAn observational and multicentre study was carried out on patients with inflammatory bowel disease treated with VDZ for at least one year. An evaluation was performed on the activity indices, faecal calprotectin and C-reactive protein levels, hospital admissions, surgeries, and adverse events.
ResultsA total of 73 patients were analysed (43 UC and 30 CD). More than one anti-TNF and more than one immunosuppressive was previously used by 74 and 23%, respectively, of UC patients, and 90 and 37%, respectively of CD patients. VDZ was stopped in 17 (23%) patients, 10 UC and 7 CD, due to a lack or loss of response before the first year, or due to adverse events. An intensification of the dose was required in 26 (63%) UC, and 16 (53%) CD patients. At 6 months, 70 and 42% of UC patients, and 80 and 43% of CD patients achieved a clinical response and remission, respectively. At one year, 58 and 35% of UC patients and 47 and 43% of CD patients, maintained the clinical response and remission, respectively. The C-reactive protein decreased significantly in both CD and UC patients. However, the decrease in faecal calprotectin was only achieved during follow-up in UC, but not in CD patients. Eight patients with CD that had been treated previously with ustekinumab avoided surgery at one year. A colectomy was performed on 8 (18.6%) UC patients, and 4 (13.3%) CD patients needed surgery. Six patients (8%) (5 UC and 1 CD) had adverse events. The concomitant use of corticosteroids or immunomodulators did not increase the efficacy. Those with a higher number of previous anti-TNF treatments showed less remissions in UC and responses in CD.
ConclusionsAfter one year of VDZ, a clinical response and remission was induced in a considerable percentage of patients refractory to different biological or immunosuppressive therapies. VDZ can be considered as an alternative in those intolerant to immunosuppressives, with few adverse events.
Vedolizumab (VDZ), un anticuerpo monoclonal humanizado que se une específicamente a -α4β7-integrina, aprobado para el tratamiento de la enfermedad de Crohn (EC) y la colitis ulcerosa (CU), ha demostrado su eficacia en ensayos clínicos controlados.
ObjetivoDescribir una población tratada con VDZ y evaluar su efectividad y seguridad a largo plazo en práctica clínica.
MétodosEstudio observacional y multicéntrico en pacientes con enfermedad inflamatoria intestinal tratados con VDZ durante al menos un año. Se evaluaron los índices de actividad, niveles de calprotectina fecal y proteína C reactiva, hospitalizaciones, cirugías y eventos adversos.
ResultadosSe analizaron un total de 73 pacientes (43 CU y 30 EC). El 74 y 23% de CU y el 90 y 37% de EC habían llevado previamente más de un anti-TNF y más de un inmunosupresor respectivamente. VDZ se suspendió en 17 pacientes (23%), 10 CU y 7 EC, debido a la falta o pérdida de respuesta antes del primer año o a eventos adversos. Veintisiete (63%) CU y 16 (53%) pacientes con EC requirieron intensificación de la dosis. A los 6 meses, el 70 y 42% de CU y el 80 y 43% de EC lograron respuesta clínica y remisión respectivamente. Al año, el 58 y 35% de CU y el 47 y 43% de EC mantuvieron la respuesta clínica y la remisión, respectivamente. La proteína C reactiva disminuyó significativamente tanto en la EC como en la CU. Sin embargo, la disminución de la calprotectina fecal se logró durante el seguimiento solo en CU pero no en EC. Ocho pacientes con EC que habían sido tratados previamente con ustekinumab evitaron la cirugía al año. En 8 CU (18,6%) se realizó colectomía y 4 EC (13,3%) necesitaron cirugía. Seis pacientes (8%) (5 UC y una enfermedad de Crohn) tuvieron eventos adversos. El uso concomitante de corticoides o inmunomoduladores no aumentó la efectividad. A mayor número de anti-TNF previos, menos remisión en la CU y respuesta en la EC.
ConclusionesTras un año de VDZ se induce respuesta y remisión clínica en una no desdeñable proporción de pacientes refractarios a diferentes biológicos o inmunosupresores. VDZ puede considerarse una alternativa en intolerantes a inmunosupresores con pocos eventos adversos.
Until the introduction of the new biological agents, vedolizumab (VDZ) and ustekinumab, there was no alternative treatment for patients with inflammatory bowel disease (IBD) who were intolerant or did not respond to conventional therapy with anti-tumour necrosis factor alpha (anti-TNF) biological drugs and immunosuppressants, and surgery was unavoidable in the majority of cases. These drugs provide the answer to the need for new therapeutic targets, as approximately one third of patients with IBD who receive anti-TNF agents do not respond at all (primary failure) and a significant proportion (more than a third) experience a loss of response (secondary failure) or intolerance to treatment.1 The lack of response to anti-TNF agents can be related to the complex pathophysiology of IBD, but also to the particular pharmacokinetics and pharmacodynamics of these treatments. In cases of lack or loss of response with normal levels, TNF-α will not be the main cytokine involved in altering immune response. There will be other pathways and different proinflammatory molecules at play which perpetuate the continuation of disease activity.2
VDZ is a humanised monoclonal antibody that binds specifically to α4β7-integrin expressed in helper T lymphocytes which migrate to the gastrointestinal tract and induce the inflammation in ulcerative colitis (UC) and Crohn's disease (CD).3,4 By binding to the lymphocyte α4β7, VDZ inhibits the migration and adhesion of these cells to mucosal addressin cell adhesion molecule 1 (MAdCAM-1) expressed mainly in the endothelial cells of the intestine.5 With all these factors, VDZ is a highly gastrointestinal-selective drug with fewer adverse events (AE) and less immunogenicity than the immunosuppressants (thiopurines) and anti-TNF agents (mainly infliximab and adalimumab) previously available for the treatment of IBD.3,4
VDZ has been approved since May 2014 for the treatment of UC and CD after its efficacy and safety were demonstrated in the pivotal clinical trials GEMINI I6 and II,7 respectively. The GEMINI I results showed VDZ to be more effective than placebo as induction and maintenance therapy in active UC, with remission rates at six weeks of 16.9% in patients treated with VDZ vs 5.4% in the placebo group and, at 52 weeks, of 41.8% in patients treated with VDZ every eight weeks vs 15.9% in the placebo group.6 Efficacy was greater in patients not previously treated with anti-TNF.8
The GEMINI II study assessed the efficacy of the drug in patients with CD. It also showed superiority of VDZ versus placebo, both for induction, with remission rates of 14.5% in treated patients vs 6.8% with placebo (week 6), and long-term maintenance (week 52), with remission rates of 39% in treated patients vs 21.6% in the placebo group.7 Efficacy was also greater in anti-TNF-naive patients.9
Since the pivotal trials there have been very few post-marketing studies in clinical practice. Data from observational studies have shown that VDZ is effective in inducing steroid-free clinical remission as early as week 14 in both CD (18–31%) and UC (19–36%).10–13 The Swedish SWIBREG study14 and the French group GETAID11 series have shown clinical remission rates of more than 60% at one year. Moreover, VDZ achieves mucosal healing in a high percentage of patients,15 has a good safety profile16–18 and can improve the quality of life of patients with IBD in the short and long term.12,15
In view of the above, the primary objective of this study is to describe the clinical and safety outcomes in a population of patients treated with VDZ in the medium and long term (6 and 12 months) in real clinical practice in our area. The secondary objectives are to assess hospitalisation and surgery rates, determine the impact of VDZ on C-reactive protein (CRP) and faecal calprotectin (FC) values and identify factors predictive of effectiveness.
Material and methodsMulticentre, observational study involving three hospitals in Valencia with IBD Reference Units. Patients diagnosed with either CD or UC who agreed to participate and gave consent for their medical records to be reviewed were included according to the usual criteria.19 The study was approved by the ethics committee for the hospitals. The patients included had to have been on treatment with VDZ for at least a year and, if they had stopped the treatment early, they had to have been followed up for at least a year. The efficacy data were obtained using the entire initial cohort, including the patients considered “failures” when the drug was withdrawn within the first year.
VDZ was administered intravenously at doses of 300mg in weeks 0, 2 and 6 and then every 8 weeks in all patients, in accordance with the clinical practice protocol. In the case of lack or loss of response, we determined the management and timing of drug dose intensification where necessary.
We collected demographic data (gender, age, smoking history), type of IBD (CD or UC) and Montreal classification, data on the disease, such as time since onset, and previous biological and immunosuppressant therapies.
We analysed endoscopic activity at the start of treatment (baseline) and clinical activity, FC levels and CRP at baseline, 6 months and 12 months, when available. For CD, endoscopic and/or radiological severity was defined as mild, moderate or severe at the discretion of the clinician. We assessed clinical activity using the Harvey-Bradshaw Index (HBI) for CD and the partial Mayo Score (pMS) for UC. Clinical remission in CD was considered as HBI ≤4 and in UC, a pMS ≤2, with all the scores a maximum of one and a rectal bleeding subscore of 0. Clinical response was defined as a reduction in the pMS ≥3 points and a decrease ≥30% compared to baseline, with a decrease of ≥1 point in the rectal bleeding subscore (absolute score 0–1). For CD, response was defined as a decrease in the HBI ≥3 points without achieving remission.
The definition of treatment failure included the following: (1) clinical recurrence, considered as the presence of clinical worsening, quantified by HBI in CD and pMS in UC, in conjunction with an increase in inflammatory parameters (CRP and FC) over the initial values; and (2) the need to introduce steroids, the impossibility of withdrawing them or hospital admission or surgery because of disease activity.
The addition of oral or topical salicylates or the addition of topical corticosteroids was not considered as failure. The need for an extra dose in week 10 or shortening of the interval was not considered as failure, provided there was a subsequent response.
In the event of loss or lack of response, changes in treatment were made at the discretion of the doctor responsible for patient follow-up.
We also analysed the number of hospitalisations, surgical interventions and AE per year, in addition to possible predictors of treatment response.
Statistical analysisThe statistical analysis of data was performed using Microsoft Excel 2016. We carried out a descriptive analysis of the variables. Continuous variables are expressed as mean and standard deviation or median and first and third quartiles. The categorical variables are presented as frequencies and percentages. A multivariate study (Cox regression) was performed to determine the factors predictive of response. A p value <0.05 was considered significant for all tests.
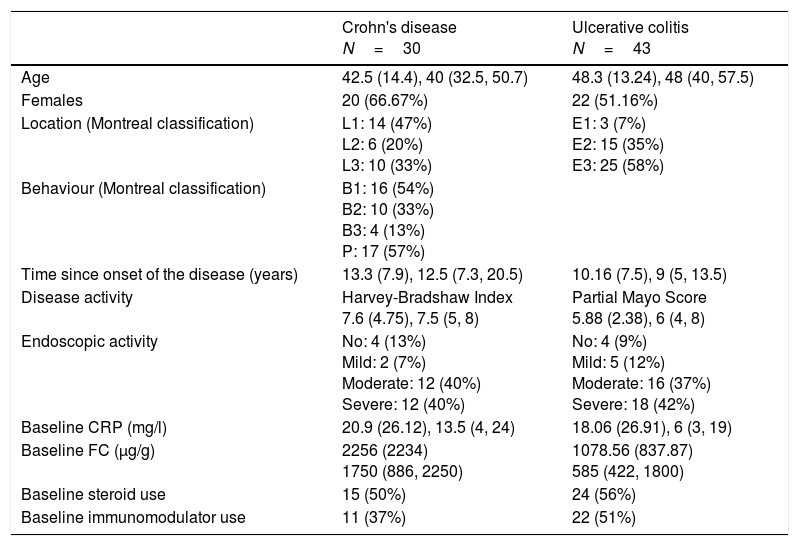
ResultsDemographic data, phenotype and history of previous treatmentsWe analysed a total of 73 patients (43 UC and 30 CD). Table 1 shows the characteristics of the patients when starting treatment. The majority of patients with CD had ileal location (47%) and inflammatory behaviour (54%) and 57% had a history of perianal involvement. In UC, the majority were pancolitis (58%).
Patient demographic and clinical characteristics.
| Crohn's disease N=30 | Ulcerative colitis N=43 | |
|---|---|---|
| Age | 42.5 (14.4), 40 (32.5, 50.7) | 48.3 (13.24), 48 (40, 57.5) |
| Females | 20 (66.67%) | 22 (51.16%) |
| Location (Montreal classification) | L1: 14 (47%) L2: 6 (20%) L3: 10 (33%) | E1: 3 (7%) E2: 15 (35%) E3: 25 (58%) |
| Behaviour (Montreal classification) | B1: 16 (54%) B2: 10 (33%) B3: 4 (13%) P: 17 (57%) | |
| Time since onset of the disease (years) | 13.3 (7.9), 12.5 (7.3, 20.5) | 10.16 (7.5), 9 (5, 13.5) |
| Disease activity | Harvey-Bradshaw Index 7.6 (4.75), 7.5 (5, 8) | Partial Mayo Score 5.88 (2.38), 6 (4, 8) |
| Endoscopic activity | No: 4 (13%) Mild: 2 (7%) Moderate: 12 (40%) Severe: 12 (40%) | No: 4 (9%) Mild: 5 (12%) Moderate: 16 (37%) Severe: 18 (42%) |
| Baseline CRP (mg/l) | 20.9 (26.12), 13.5 (4, 24) | 18.06 (26.91), 6 (3, 19) |
| Baseline FC (μg/g) | 2256 (2234) 1750 (886, 2250) | 1078.56 (837.87) 585 (422, 1800) |
| Baseline steroid use | 15 (50%) | 24 (56%) |
| Baseline immunomodulator use | 11 (37%) | 22 (51%) |
The data are expressed as absolute number (%), mean (standard deviation) and median (1st, 3rd quartiles).
Baseline: at start of treatment; CRP: C-reactive protein; FC: faecal calprotectin.
Clinical and endoscopic disease activity was generally moderate to severe (80% in CD and 79% in UC). Both CRP and FC were elevated and were higher at the start of treatment in CD (CRP 20.9 and 18.06mg/l and FC 2256 and 1078μg/g in CD and UC, respectively) (Table 1).
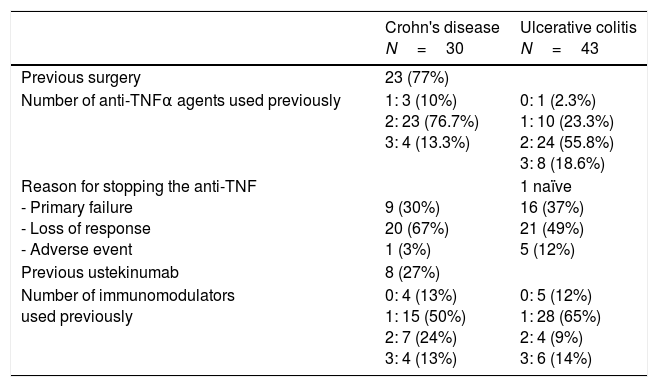
With regard to time since disease onset when administering VDZ, in both cases the mean was over 10 years. In CD, 23 patients (77%) had previously had surgery; 15 patients one operation, five patients two operations and three patients three or more. The patients had also previously been on multiple different treatments: 74% of UC and 90% of CD had previously taken more than one anti-TNF agent; the majority were following on from loss of response to anti-TNF (67% CD and 49% UC) (Table 2); eight patients with CD (27%) had been treated with ustekinumab, administered subcutaneously and at the recommended doses, before it was approved for CD; 23% of UC and 37% of CD had previously been prescribed more than one immunosuppressant (Table 2); and 47% of the patients had had to stop taking azathioprine as a result of an AE (9 patients for pancreatitis, 9 for gastrointestinal intolerance, 6 for hepatotoxicity, 4 for leukopenia and 6 for other less common events).
History of previous treatments.
| Crohn's disease N=30 | Ulcerative colitis N=43 | |
|---|---|---|
| Previous surgery | 23 (77%) | |
| Number of anti-TNFα agents used previously | 1: 3 (10%) 2: 23 (76.7%) 3: 4 (13.3%) | 0: 1 (2.3%) 1: 10 (23.3%) 2: 24 (55.8%) 3: 8 (18.6%) |
| Reason for stopping the anti-TNF - Primary failure - Loss of response - Adverse event | 9 (30%) 20 (67%) 1 (3%) | 1 naïve 16 (37%) 21 (49%) 5 (12%) |
| Previous ustekinumab | 8 (27%) | |
| Number of immunomodulators used previously | 0: 4 (13%) 1: 15 (50%) 2: 7 (24%) 3: 4 (13%) | 0: 5 (12%) 1: 28 (65%) 2: 4 (9%) 3: 6 (14%) |
The data are expressed as absolute number (%).
Approximately half of the patients were taking steroids and immunomodulators during induction (Table 1).
Response and clinical remissionVDZ was withdrawn in 17 patients (23%) within the first year, 10 UC and 7 CD (mean treatment time 22±14 and 26±12 weeks respectively) due to lack or loss of response (13 patients) or because of AE (4 patients).
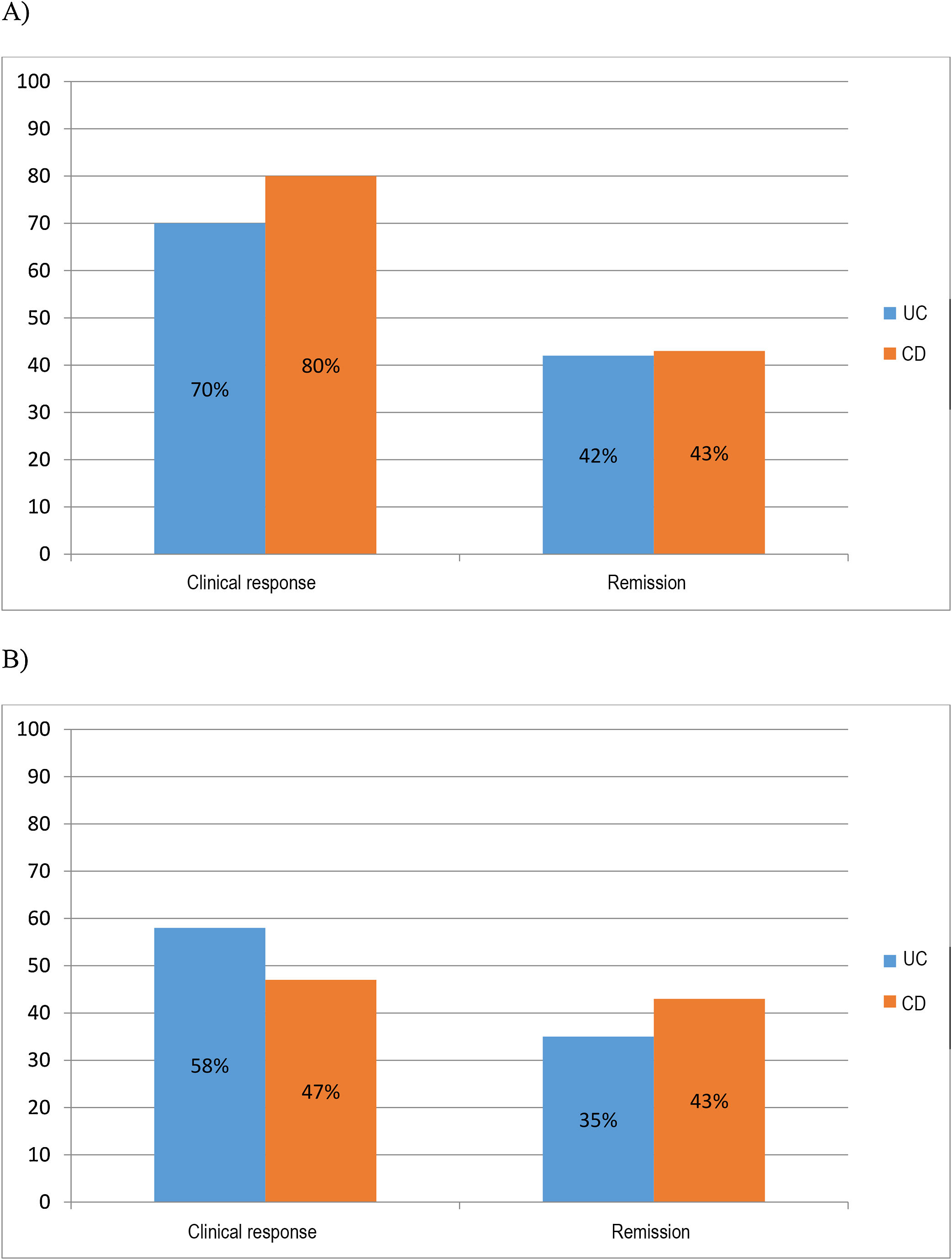
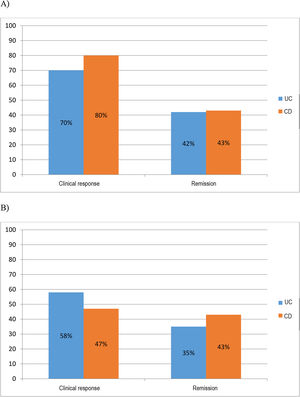
At six months 70% (30/43) of UC and 80% (24/30) of CD patients had obtained response and 42% (18/43) of UC and 43% (13/30) of CD patients had achieved clinical remission. At one year 58% (25/43) of UC and 47% (14/30) of CD patients had sustained clinical response and 35% (15/43) of UC and 43% (13/30) of CD patients continued to be in remission (Fig. 1A and B).
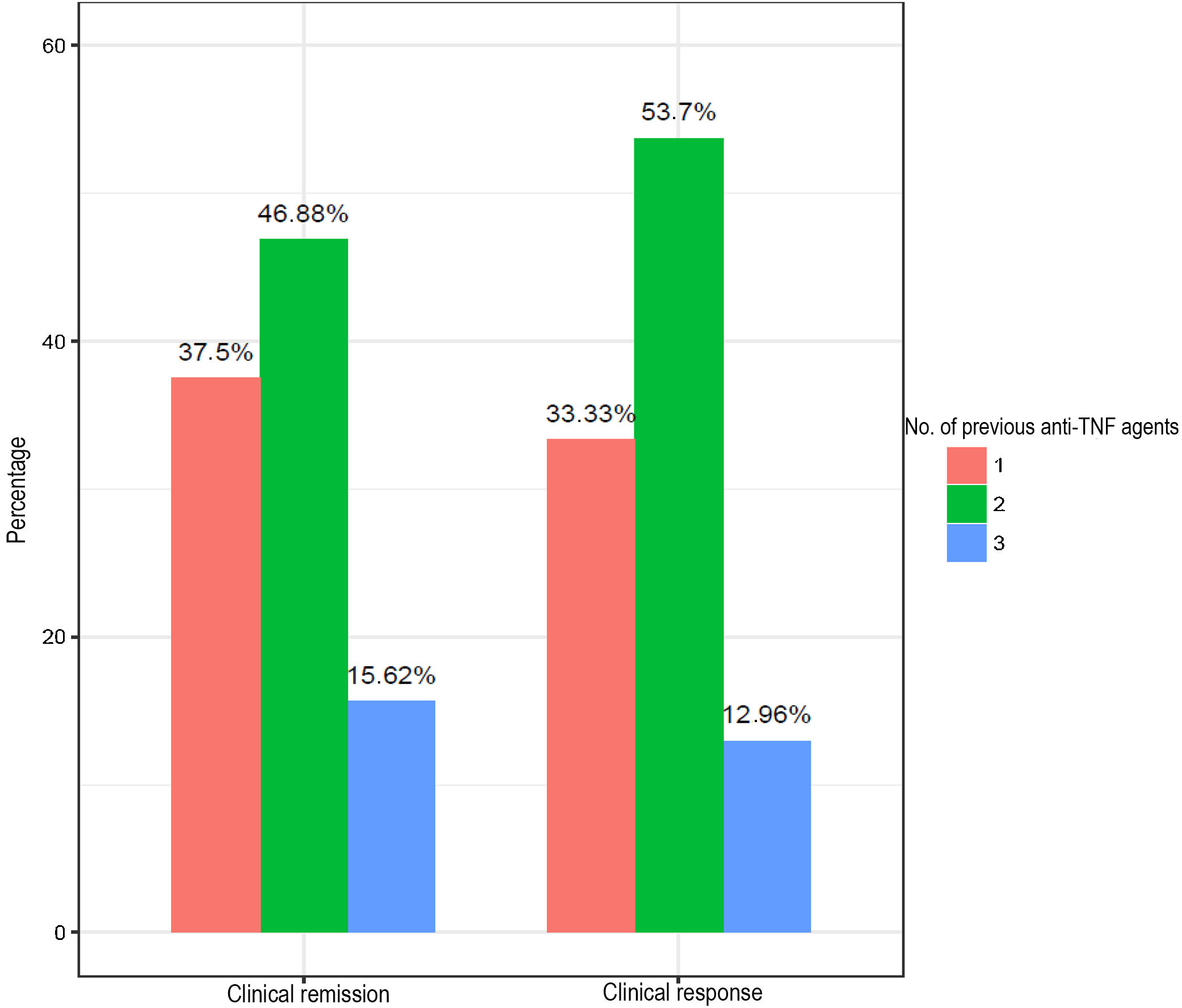
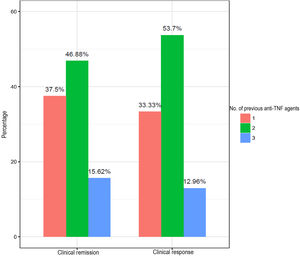
Fig. 2 shows the percentages for response and clinical remission according to the number of anti-TNF agents used previously. Patients with the worst response and remission rates (15.62% and 12.96%) were those with failure to three anti-TNF agents.
Management of lack and loss of responseVDZ was withdrawn in 13 patients (17.8%) (7 UC and 6 CD) within the first year due to lack or loss of response. During the follow-up period, 27 (63%) patients with UC experienced loss of response and required dose intensification: 13 in week 10, three in week 14, six in week 18, four in week 24 and one in week 50 (mean 16±9.2 weeks). The clinician proceeded to administer the drug every six weeks in three patients and every four weeks in 24 patients.
In CD, 15 (50%) patients experienced loss of response and required an additional dose or shortening of the interval: seven in week 10, two in week 14, two in week 18, three in week 24 and one in week 50 (mean 17.2±10.6 weeks). The drug was administered every six weeks in four patients and every four weeks in 11 patients.
SafetySix patients out of the total (8%) (5 UC and 1 CD) suffered AE during follow-up which, in four of them (3 UC and 1 CD), led to withdrawal within the first year. The main adverse effects found were rhinopharyngitis, arthralgia, non-opportunistic infections and an infusion-related reaction.
Surgery and hospital admissionsOf the eight patients with CD who had previously been treated with ustekinumab, none required surgery in the year of follow-up. Out of the whole cohort analysed, 12 patients required surgery within the year; eight UC (18.6%) had colectomies and four CD (13.3%) required abdominal surgery with resection (3 ileocolic resections and one subtotal colectomy with ileorectal anastomosis). In the postoperative period, two patients had minor complications: one patient with UC had paralytic ileus and another with CD developed an abdominal wall abscess. Ten patients (23%) with UC and eight (27%) with CD had to be admitted to hospital during the year of follow-up.
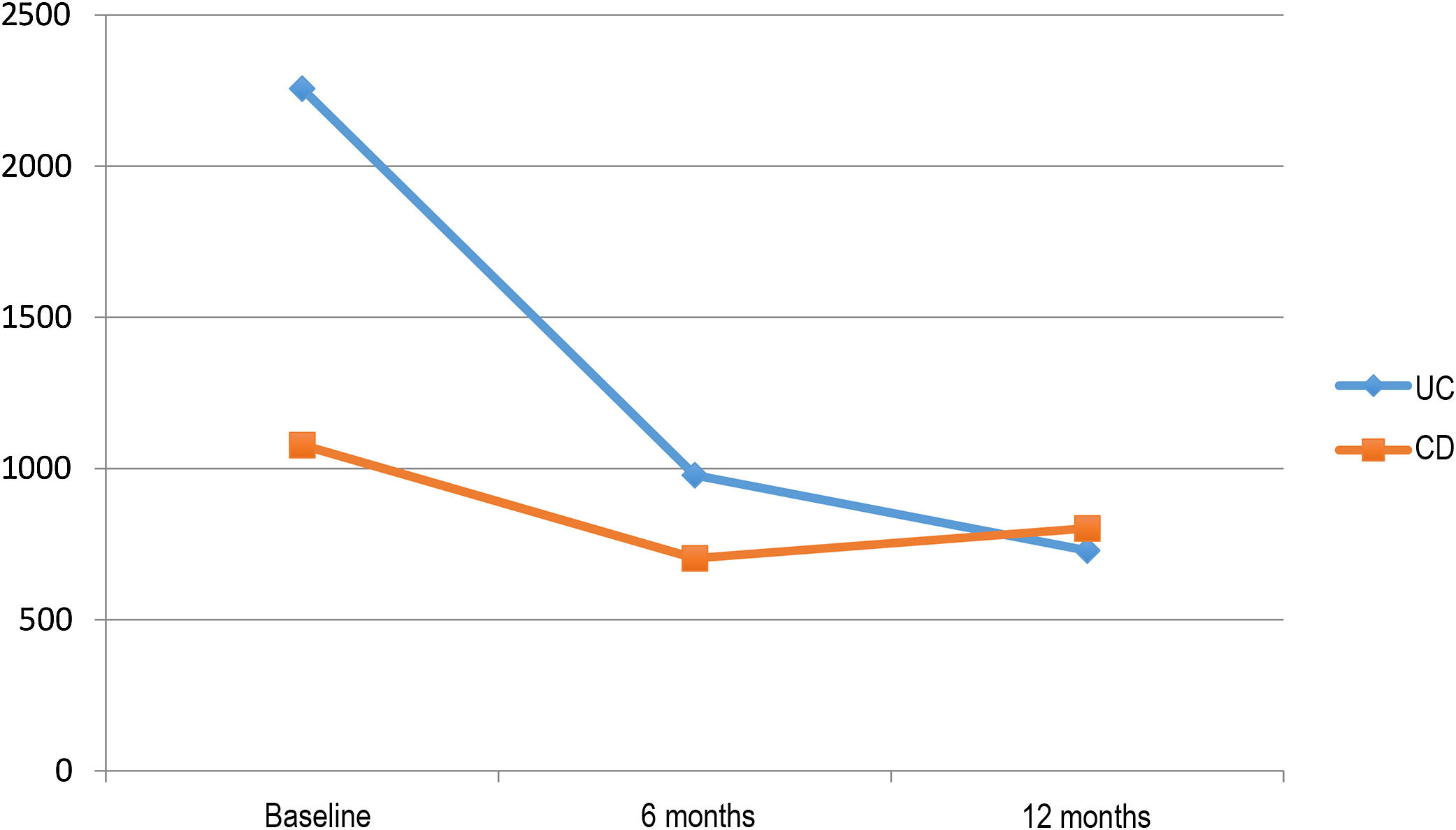
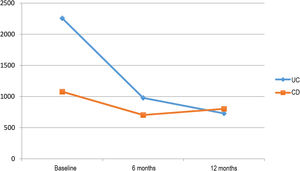
Impact on inflammatory biomarkersVDZ reduced CRP in both UC and CD over the one-year follow-up (20.9mg/l, 9.1mg/l and 7.9mg/l in UC vs 18mg/l, 11.23mg/l and 10.1mg/l in CD, at baseline, 6 months and 1 year, respectively). However, the reduction in FC over the follow-up period was greater in the patients with UC (2256, 978 and 729μg/g in UC vs 1078, 703 and 802μg/g in CD, at baseline, 6 months and 1 year, respectively) (Fig. 3).
Predictors of clinical effectivenessIn the multivariate analysis, the concomitant use of steroids or immunomodulators did not increase the effectiveness of the drug. Only the number of previous treatments with anti-TNF was related to the lack of remission in UC and the lack of response in CD.
DiscussionOur study evaluating the efficacy of VDZ after one year of follow-up has shown VDZ to be a safe drug, with few AE, capable of inducing clinical response and remission in patients with long-standing disease who have had surgical intervention and are refractory to other lines of treatment. It may also help to avoid surgery and the need for hospital admission. VDZ does not seem to require combined therapy to increase its efficacy and was found to more effective the lower the number of anti-TNF agents the patient had used previously.
The efficacy data obtained in our study are similar to those described in published real-life studies, although the assessment point was different. Unlike the pivotal studies which assess the efficacy at an early stage, most of the real-life studies assess the response at week 12 or 14.10–14 In the GEMINI 2 induction trial, the decrease in the Crohn's Disease Activity Index (CDAI) and CRP levels and the greater differences in efficacy compared to placebo appeared very late, only after a year of treatment,7 and in GEMINI 3, clinical remission was detectable in week 10 but not in week 6.20 Its particular pharmacokinetic properties mean that VDZ produces a slower response than anti-TNF. However, the data for VDZ as maintenance treatment show profound and sustained remission in patients who initially respond to the induction, with less loss of efficacy in the long term than with the anti-TNF agents.21 For that reason, we decided to assess the patients at six months (week 24) and one year (week 52).
Only two other studies have evaluated the efficacy of VDZ at six months and are therefore comparable with ours. The United States VICTORY Consortium study analysed the clinical response and steroid-free response and remission at six months in CD (32%, 26% and 18%, respectively), with their clinical response rates being somewhat lower than in our series.22 Their population had also been treated with various previous biological agents, anti-TNF and even ustekinumab. The second study was Finnish (FINVEDO) and assessed clinical remission at six months, obtaining the same results as us in UC (41.8% compared to our 42%). In CD, however, they had a much higher remission rate (73.3% vs 43% in our study).23 In that series, although larger than ours (108 CD and 139 UC), the characteristics of the patients with CD were similar in terms of age (42.5 vs 40.3), time since onset (13.3 vs 13.9 years) and disease behaviour (B1 54 vs 52.8%, B2 33 vs 41.7% and B3 13 vs 12%). The percentage of patients on concomitant treatment with immunosuppressants and steroids was also similar. They only differed in the location of the disease and the number of previous anti-TNF agents. In our population with CD the majority had ileal location (47%), while in the FINVEDO study only 12% were ileal, the majority having colon involvement (L2 26.9% and L3 59.3%).23 It has been found in vitro that the inhibition of α4β7 by VDZ does not produce the same response in CD and UC. While in UC there is a rapid increase in regulatory T lymphocytes, with a decrease in the effector T cell/regulatory T cell ratio, which potentially leads to the suppression of systemic inflammation, this does not occur in CD, thus supporting the idea that the effects of the inhibition of α4β7 in the two disorders are not identical.24 The pathophysiological basis of this effect seems to be that there are increased levels of α4β1 in the ileum and this impedes blockade of α4β7 by VDZ.25 In terms of previous treatments, 90% of patients with CD in our population had failed two or more anti-TNF agents and 25% had also been treated with ustekinumab. Our population, in principle, therefore had risk factors for poor response.26
As far as long-term efficacy is concerned, at 52 weeks, our results were similar to the pivotal clinical trials.6,7 This is surprising as, unlike clinical trials, in clinical practice, the drug dose can be intensified (in our cohort, more than half the patients required this, and at an early stage) and other treatments can be given concomitantly. In clinical practice, the Swedish SWIBREG study, which included 147 patients with CD and 92 with UC, found clinical response rates similar to our study in CD and UC at 52 weeks (59% vs 58% and 53% vs 47%, respectively). However, their clinical remission rates were much higher in both CD (64% vs 35%) and UC (60% vs 43%).14 In this case, the patients included in the SWIBREG study had a shorter time since disease onset than our patients (8.5 years in CD and 4 years in UC) and most had only failed one anti-TNF agent; these being factors that can make them more likely to respond to biological agents and which could therefore have contributed to their better outcomes.1,14,22,26 In our study, the number of previous anti-TNF agents was also correlated with the response to the drug. This is a factor which has already been investigated and is consistent in almost all published studies.26 Interestingly, the response to VDZ seemed to be better in those who had received two anti-TNF agents than in those who had received one or three, probably because the percentage of patients who had received two was higher in both disorders.
Our series included patients with CD who had been treated not only with anti-TNF but also with ustekinumab (IL12/23 inhibitor). However, the doses and route of administration were those recommended before ustekinumab was approved for marketing in CD and were therefore suboptimal as they were not adjusted to weight, meaning some of the potential efficacy of the drug may have been lost. VDZ was effective in these patients in terms of avoiding surgery after a year of treatment. To date, only the VICTORY study has included patients previously treated with another biological drug with a different mechanism of action from anti-TNF. However, it does not analyse the results in this subgroup.22 Although the inflammatory pathway most studied in IBD is reported to be TNF-α-dependent and blocking of that pathway is associated with improvement in inflammation, a large proportion of patients fail to respond or experience loss of response over time.27 The possible efficacy of a third mechanism of action after already attempting to block two different inflammatory pathways tells us that the pathophysiology of CD is very complex and that there are other mechanisms involved in perpetuating the chronic inflammatory state. Hence the value of using new drugs to try to block other inflammatory pathways.28
A large proportion of patients in our series had adverse events to azathioprine (47%) and concomitant use with steroids and immunomodulators was not associated with a better response to the drug. It is now known that combined treatment with anti-TNF plus an immunosuppressant is superior to monotherapy with anti-TNF in both CD29 and UC.30 This is thanks to the adjuvant effect exerted by the immunosuppressant, decreasing immunogenicity and the ability to develop antibodies against anti-TNF and, as a consequence, reducing loss of response to the drug. It has already been pointed out that VDZ could be a good alternative in patients with intolerance to thiopurines and refractory to medical treatment due to its low immunogenicity, its high degree of safety, and to the fact that concomitant use with immunosuppressants does not seem to affect the efficacy of the drug.31
VDZ was useful in preventing surgery and after one year only 12 patients (16%) required surgical intervention. Moreover, there were few postoperative complications; only affecting two patients (17%). There is, however, much debate surrounding this in the literature. There are reports from some series that patients with IBD who received VDZ within the 30 days prior to major abdominal surgery experienced more postoperative complications than patients who received anti-TNF or did not receive any biological therapy,32 and reports from others that patients did not have more complications with VDZ.33 More studies are therefore necessary which take into account all the clinical variables and the experience of the centres where the surgery is carried out.
At 8%, our rate of adverse events at one year was low, and similar to rates obtained in other real-life studies.11,13 The adverse events most commonly reported with VDZ are headache, paraesthesia, arthralgia, paradoxical skin reactions, fatigue, nasopharyngitis, upper respiratory tract infections, cough, gastrointestinal infection and abdominal pain.11,12,16 In general, both pivotal studies and clinical trials have shown the drug to have a good safety profile, with low incidence rates for serious infections and malignancies over a prolonged treatment period, and no cases of progressive multifocal leukoencephalopathy have been reported.16
In our cohort, CRP decreased in both CD and UC. However, despite much higher baseline levels, there was a greater decrease in FC in patients with UC. Nonetheless, these data should be interpreted with caution, as the number of patients included was not sufficient to perform a statistical analysis. There were also losses to follow-up, as a result of early withdrawal (before the year was completed) and the fact that these parameters were not documented at all assessment times (baseline and 6 months and 1 year of treatment). Other studies have measured the biological response through changes in CRP and FC during treatment with VDZ. The German study found that FC levels in UC decreased progressively at each of the times evaluated (week 0, 6 and 14). The decrease in CD, however, was slower and less significant.12 The Swedish study found that FC decreased significantly in CD and UC, but CRP only decreased in CD.14 The high CRP levels during induction,10,11 as well as the early decrease (in week 14) of CRP and FC34 have been correlated with the response to the drug. It would therefore seem that these biomarkers may be useful and so should be explored in more depth to determine how they might be affected by VDZ therapy.
The limitations of our study are mainly the retrospective nature of the data collection and the small number of patients included (only 30 CD). In addition, endoscopic remission was not assessed and, as it was a real-life study, the administration of concomitant treatments (oral and topical salicylates) was allowed and additional doses or intensifications have not been considered as failure. Also not considered, as would have been in a clinical trial, were washout periods for the previous biological drug. All these factors can influence the results in a positive sense. Despite those issues, we have described here for the first time the possible response to VDZ after failure to drugs with different mechanisms of action in patients with CD. Moreover, our cohort is highly representative of the current use of VDZ in our area. VDZ tends to be used in patients with long-standing disease, aggressive disease behaviour, previous history of surgical interventions and previous exposure to more than one anti-TNF agent and immunosuppressants; in other words, in a population with a low likelihood of responding to VDZ. In the future, we will probably use this drug at earlier stages and in certain patients with the need for a better safety profile. VDZ is likely to be a good alternative in patients with intolerance to thiopurines who are refractory to medical treatment, thanks to its low immunogenicity and its high degree of safety.
Conflicts of interest- •
Marisa Iborra collaborates with presentations or in an advisory capacity with Takeda and MSD.
- •
Belén Beltrán Niclos collaborates with presentations or in an advisory capacity with AbbVie, Ferrer, Otzuka, Pfizer and Takeda.
- •
Nuria Maroto Arce has given presentations and acts as a consultant for MSD, AbbVie, Takeda, Ferring, Tillotts Pharma.
- •
Isabel Ferrer-Bradley collaborates with presentations with AbbVie and Ferrer.
- •
Maia Bosca collaborates in an advisory capacity or with presentations with AbbVie, Ferring, Takeda, Kern Pharma and MSD.
- •
Miguel Mínguez collaborates in an advisory capacity or with presentations with Allergan, Takeda, AbbVie and Janssen.
- •
Joaquín Hinojosa del Val collaborates in an advisory capacity or with presentations with MSD, AbbVie, Ferring, Faes Farma, Shire Pharmaceuticals, Chiesi, Otsuka Pharmaceutical, Pfizer–Hospira, Kern Pharma, UCB Pharma, Vifor Pharma, Janssen, Takeda and Dr Falk Pharma.
- •
Pilar Nos Mateu collaborates with presentations or in an advisory capacity with AbbVie, Ferrer, MSD, Otsuka, Takeda, Kern, Biogen and Ferring.
Please cite this article as: Iborra M, Beltrán B, Maroto N, Navarro-Cortés P, Boscá-Watts M, Ferrer-Bradley I, et al. Vedolizumab, una opción en pacientes con enfermedad inflamatoria intestinal intolerantes a tiopurinas y refractarios a biológicos. Gastroenterol Hepatol. 2018;41:535–543.