Acute-on-chronic liver failure (ACLF) is a recently defined syndrome characterised by acute decompensation of chronic liver disease, associated with organ failures and high mortality. ACLF is a common condition and may affect up to 30% of patients admitted to hospital for cirrhosis complications. Bacterial infections, alcoholism and reactivation of viral hepatitis are the most common precipitating factors in ACLF, although in up to 40% of patients no precipitating factor can be identified. Although the pathophysiology of ACLF is not completely understood, the presence of an excessive inflammatory response appears to play a key role. There is no specific treatment for patients with ACLF and management is based on organ support and liver transplantation. New treatment strategies based on liver support systems and immunomodulatory treatments are being evaluated but existing data are still limited.
La insuficiencia hepática aguda sobre crónica (ACLF, acute-on-chronic liver failure) es un síndrome definido recientemente y caracterizado por una descompensación aguda de una hepatopatía crónica, asociada al fallo de diferentes órganos y a una elevada mortalidad. La ACLF es frecuente, y afecta al 30% de los pacientes ingresados por complicaciones de la cirrosis. Las infecciones bacterianas, el alcoholismo y la reactivación de hepatitis virales representan los factores precipitantes más frecuentes, aunque hasta en un 40% de los pacientes no se identifica ningún factor precipitante. La fisiopatología no es completamente conocida, pero se considera que la existencia de una respuesta inflamatoria excesiva juega un papel clave en su desarrollo. No existe ningún tratamiento específico para la ACLF y su manejo se basa en el tratamiento de soporte y el trasplante hepático. Actualmente se están evaluando nuevas estrategias de tratamiento, como mecanismos de soporte hepático y tratamientos inmunomoduladores, pero los datos son todavía limitados.
Liver cirrhosis evolves from compensated cirrhosis until the onset of decompensated cirrhosis, characterised by the development of the typical complications of the disease (ascites, hepatic encephalopathy, bacterial infections and gastrointestinal bleeding) and is associated with a poorer prognosis.1,2 Patients with acute decompensated cirrhosis with no other associated factors are identified in daily clinical practice, while other patients present with acute decompensation associated with the rapid onset of multiple organ failure and a poor short-term prognosis. Traditionally, this concept has been called acute-on-chronic liver failure (ACLF). To summarise, and based on clinical experience, ACLF has been defined as acute decompensation in a patient with chronic liver disease, associated with the failure of organs other than the liver and a high mortality rate. However, this has been and continues to be a heterogeneous concept since, until recently, there was no established definition and the existing definitions were based on consensus rather than data from prospective studies.
In 2009, the Asian Pacific Association for the Study of the Liver (APASL) established the first agreed-upon definition for ACLF: “acute liver damage manifested as jaundice (bilirubin ≥5mg/dL) and clotting (INR ≥1.5), complicated in the space of 4 weeks with ascites or encephalopathy”.3 More recently, two prospective studies aimed at establishing a definition for ACLF were published. The first study was conducted by the North American Consortium for the Study of End-Stage Liver Disease (NASCELD) in the United States and Canada, and included only patients with cirrhosis and bacterial infections; thus, it did not consider the remaining patients.4 The second study was the CANONIC study conducted by the EASL-Chronic Liver Failure (EASL-CLIF) Consortium, which included 1343 consecutive patients with liver cirrhosis admitted to 21 European hospitals for acute decompensation of the disease.5 Therefore, the CANONIC study is currently the prospective study conducted with the largest number of patients, which includes all patients admitted for cirrhosis-related complications, of any aetiology, and is aimed at establishing a definition for ACLF.
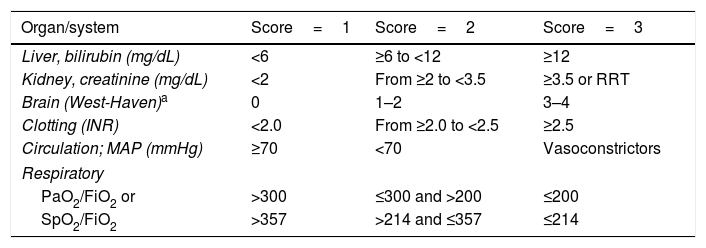
Definition and diagnosisAccording to the results of the CANONIC study, ACLF is defined as a syndrome characterised by acute decompensated cirrhosis, associated with the failure of various organs and a high short-term mortality rate (mortality at 28 days ≥15%). In that study, the existence of organ failure was evaluated using a modified version of the Sequential Organ Failure Assessment (SOFA) score, an index that is widely used to evaluate organ failure in critical patients. In that case, the SOFA index was adapted to the characteristics of patients with cirrhosis and was called CLIF-SOFA, or its simplified version, CLIF-C Organ Failure score (CLIF-C OF) (Table 1).5,6 The presence of ACLF was identified according to the number and type of organ failure, while its severity was classed into 3 stages (Table 2).6
CLIF-C OF index for diagnosing ACLF.
| Organ/system | Score=1 | Score=2 | Score=3 |
|---|---|---|---|
| Liver, bilirubin (mg/dL) | <6 | ≥6 to <12 | ≥12 |
| Kidney, creatinine (mg/dL) | <2 | From ≥2 to <3.5 | ≥3.5 or RRT |
| Brain (West-Haven)a | 0 | 1–2 | 3–4 |
| Clotting (INR) | <2.0 | From ≥2.0 to <2.5 | ≥2.5 |
| Circulation; MAP (mmHg) | ≥70 | <70 | Vasoconstrictors |
| Respiratory | |||
| PaO2/FiO2 or | >300 | ≤300 and >200 | ≤200 |
| SpO2/FiO2 | >357 | >214 and ≤357 | ≤214 |
The grey area outlines the diagnostic criteria for the failure of each organ.
FiO2: fraction of inspired oxygen; INR: international normalised ratio; MAP: mean arterial pressure; PaO2: partial pressure of oxygen in arterial blood; SpO2: oxygen saturation; RRT: renal replacement therapy.
Diagnostic criteria for ACLF.
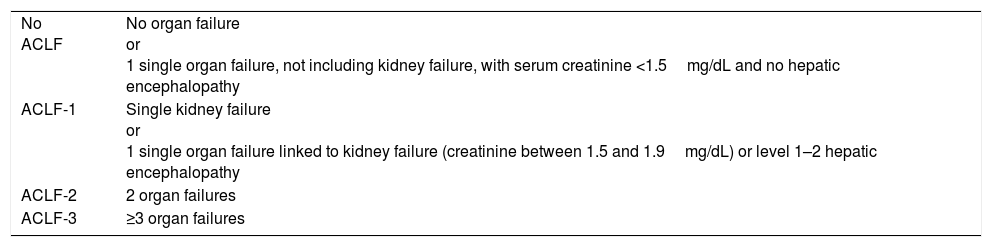
| No ACLF | No organ failure or 1 single organ failure, not including kidney failure, with serum creatinine <1.5mg/dL and no hepatic encephalopathy |
| ACLF-1 | Single kidney failure or 1 single organ failure linked to kidney failure (creatinine between 1.5 and 1.9mg/dL) or level 1–2 hepatic encephalopathy |
| ACLF-2 | 2 organ failures |
| ACLF-3 | ≥3 organ failures |
Although the prospective studies only included patients with liver cirrhosis, it should be noted that in reality ACLF can appear in patients with both compensated and decompensated cirrhosis, as well as in patients with chronic liver disease without cirrhosis. In this regard, and in an attempt to clarify the concept, in a recent consensus meeting aimed at unifying the diagnostic criteria for ACLF, it was suggested that ACLF should be defined as “a syndrome that occurs in patients with chronic liver disease, with or without previously diagnosed cirrhosis, which is characterised by acute liver decompensation that leads to liver failure (jaundice and clotting) and is associated with the failure of one or more extrahepatic organs”.7 It was suggested that ACLF be classified into 3 types according to the stage of the underlying chronic liver disease: Type A ACLF (patients with chronic liver disease without cirrhosis); Type B ACLF (patients with compensated cirrhosis); and Type C ACLF (patients with decompensated cirrhosis). The CANONIC study included patients with type B and type C ACLF.5,7 Patients with type A ACLF are patients with underlying chronic liver disease, without cirrhosis, and which typically occurs as acute hepatitis associated with chronic liver disease or as the reactivation of viral hepatitis. As described in the previous section, bearing in mind that viral hepatitis is the most common precipitating factor for ACLF in Asia, type A ACLF would be the most common in this region. However, this classification and the concept of type A ACLF should be validated in future prospective studies to confirm whether type A ACLF truly has characteristics similar to those of patients with type B ACLF and type C ACLF.
EpidemiologyACLF is a common complication in patients with liver cirrhosis, which is a common reason for hospital admission and one of the most common causes of death in these patients. Overall, the prevalence of ACLF is approximately 30%. Studies conducted in different populations show relatively similar prevalence levels. The results of the CANONIC study, conducted on a European population, revealed a prevalence rate of 30%, with 20% of the patients presenting with ACLF on admission to hospital and 10% developing it during their stay. ACLF-1 and ACLF-2 were the most common (16% and 11%, respectively), whereas ACLF-3 represented just 4%.5 A study conducted in North America using the NASCELD diagnostic criteria and, therefore, including only patients with bacterial infections, reported a prevalence of 24%.4 Finally, a study conducted in Asia, which included patients with liver cirrhosis caused by hepatitis B virus (HBV) and used the CANONIC diagnostic criteria, reported a prevalence of 34%.8
Precipitating factorsIn many cases, a precipitating factor linked to the development of ACLF can be identified. The precipitating factors can be classified as “intrahepatic”, such as alcohol consumption, reactivation of HBV or acute hepatitis due to HAV or HEV, or “extrahepatic”, which are mainly bacterial infections or gastrointestinal bleeding, among others.9 Despite the fact that the precipitating factors may have a key role in the development of ACLF, existing data show that the presence and type of precipitating factor are not linked to prognosis, which indicates that prognosis depends on factors other than the precipitating factors, such as clinical progress and the number of organ failures.5,10–13
In general, the most common precipitating factors are bacterial infections, followed by active alcoholism and the reactivation of HBV.10–13 However, the prevalence of the precipitating factors varies according to geographic location. In Europe and the United States, bacterial infections and alcoholism are the most common identifiable precipitating factors, which represent around 30% and 20% of the cases of ACLF, respectively.5 In Asia, reactivation of HBV followed by bacterial infections are the most common precipitating factors, with 36% and 30%, respectively.9 However, it is important to highlight that for a relatively significant number of patients (up to around 20–40%) a precipitating factor was not able to be identified.5
PathophysiologyAlthough the mechanisms that characterise the pathophysiology that leads to the development of ACLF are still unknown, we do know that ACLF occurs in the context of an intense systemic inflammatory response.11–15 In the CANONIC study it was observed that patients with ACLF presented with a significant increase in C-reactive protein and the number of leukocytes, which are proinflammatory markers that are also correlated with the prognosis of the disease.5 These findings were what led to the hypothesis that the excessive systemic inflammatory response is a basis for explaining the pathogenesis of ACLF. This hypothesis is widely accepted nowadays and, although detailed data on its characteristics are still limited, it is the area that has advanced the most in the study of ACLF.
Two recent studies evaluated the behaviour of a large number of cytokines in patients with ACLF, compared to patients with decompensated cirrhosis and healthy patients.16,17 The results of these studies showed that in patients with ACLF there is a significant increase in various proinflammatory cytokines and chemokines (IL-6, IL-8, TNF-α, MCP-1, etc.), compared to patients with decompensated liver cirrhosis without ACLF.16,17 Furthermore, it was observed that the levels of some of these cytokines were correlated with the progression of the disease and its prognosis. In this respect, low levels of IL-6 and IL-8, for example, were associated with an improvement in ACLF, whereas high levels were associated with worsening of the disease and a high short-term mortality rate.16
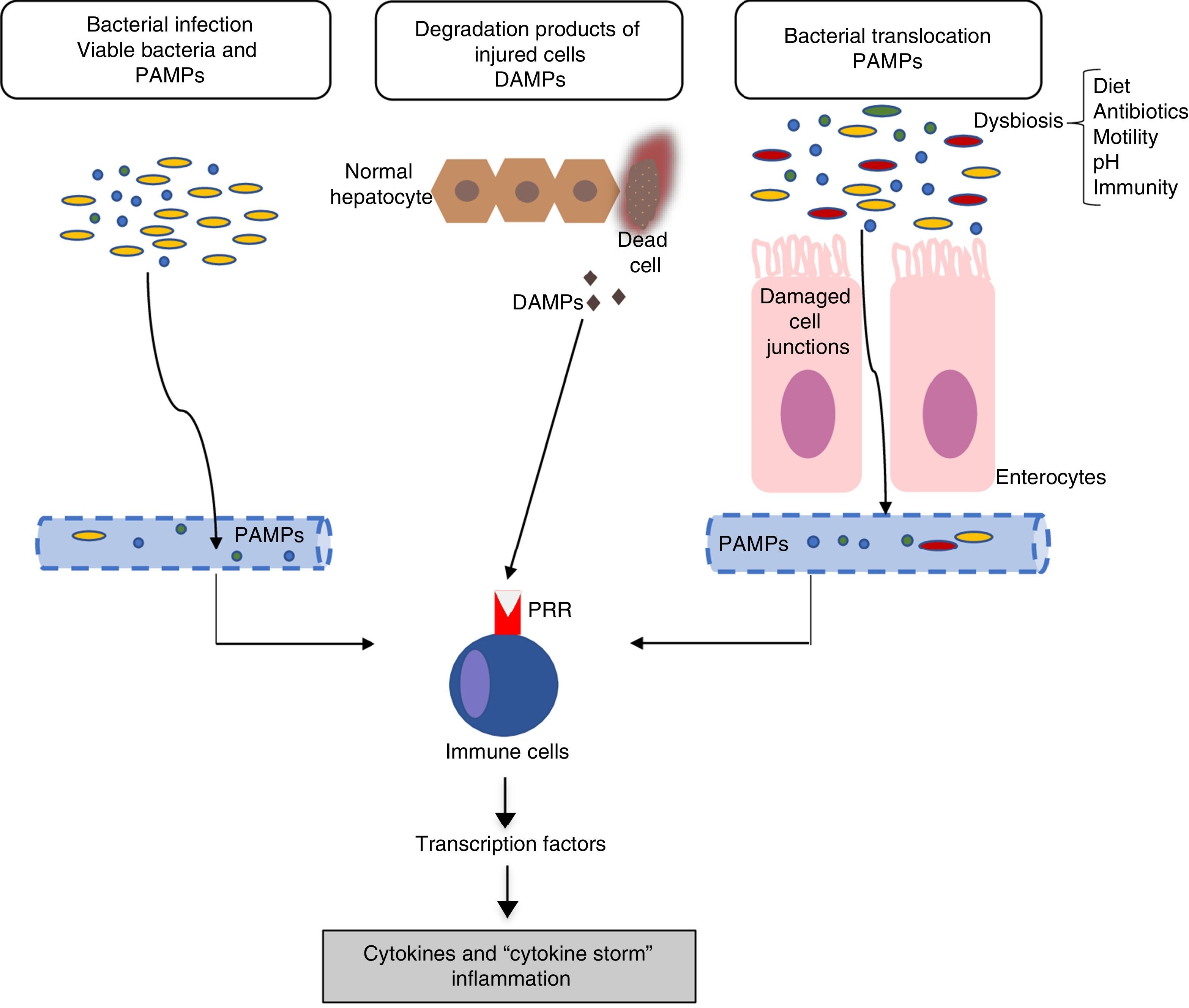
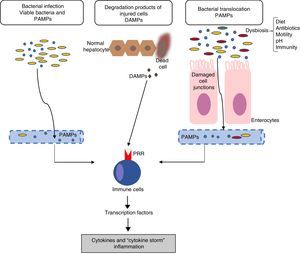
Inflammation mechanisms in acute-on-chronic liver failureThe mechanisms responsible for inflammatory response are not completely clear. In general, the inflammatory inducers can be classified as: (a) exogenous inducers (especially, bacterial infections) and (b) endogenous inducers (molecules from necrotic cells).18 The current hypothesis indicates that the intense systemic inflammatory reaction that occurs in patients with ACLF can be induced by exogenous factors, such as pathogen-associated molecular patterns (PAMPs) originating from bacterial products existing in the systemic circulation, or endogenous factors, such as damage-associated molecular patterns (DAMPs) originating from cells from the damaged liver (Fig. 1).
Pathophysiology of acute-on-chronic liver failure (ACLF). The current hypothesis on the pathophysiology that promotes the development of ACLF is based on the existence of an excessive inflammatory response. The figure outlines the potential mechanisms involved in the induction of this systemic inflammatory response in patients with ACLF. DAMPs: damage-associated molecular patterns; PAMPs: pathogen-associated molecular patterns; PRRs: pathogen recognition receptors.
Bacterial pathogens can cause inflammation through two types of mechanisms: PAMPs or virulence factors. PAMPs are molecular signatures originating in bacteria that are recognised by pattern recognition receptors (PRRs) expressed in the immune or epithelial cells. PRRs include toll-like receptors (TLRs), among others.19 The binding of PAMPs with PRRs leads to a cascade of intracellular signals that activate the transcription factors (for example, NF-kβ), which, in turn, induce genes that code molecules involved in inflammation, such as proinflammatory cytokines. On the other hand, virulence factors are generally not recognised by a specific receptor, but are detected through recognition of the effects of a pathogen's activity (recognition of functional characteristics), for example, the activation of the NLRP3 inflammasome by bacterial exotoxins.18 Because bacterial infections are one of the most common causes of ACLF, in this case, PAMPs originating from these bacteria would be the cause of the inflammatory response. However, in patients with decompensated cirrhosis, the existence of PAMPs in the circulatory system is independent of the existence of bacterial infections. An increase in intestinal permeability and bacterial translocation in patients with advanced cirrhosis contributes to the presence of PAMPs in the circulation, which can cause an increase in systemic inflammation in the absence of an established bacterial infection.11,15
Endogenous inducersDAMPs are released by necrotic or damaged cells, or as a result of the rupture of the extracellular matrix, to alert the immune system of the existence of a tissue lesion. DAMPs are recognised by the host's receptors and this binding leads to the so-called sterile inflammation, i.e., inflammation in the absence of infection. For example, the high-mobility group-1 proteins are bonded to the receptors for the advanced glycosylation compounds and cause a systemic inflammatory response.20
Finally, systemic inflammation in response to DAMPs and PAMPs is also likely linked to genetic factors. Recently, two polymorphisms of a single nucleotide were described in genes that code for IL-1, which protect patients with decompensated cirrhosis from excessive systemic inflammation by reducing the probability of developing ACLF.21
Mechanisms of organ failure in acute-on-chronic liver failureExcessive systemic inflammation in ACLF is correlated with the number of organ failures.5 According to the current hypothesis, systemic inflammation could also be the cause of organ failure in these patients.
In summary, the main objective of inflammatory response in infections is to eliminate the infection, while in the context of sterile inflammation, the objective is to promote tissue repair. In both cases the inflammatory response can be excessive and may cause organ damage and even multiple organ failure. This is what happens, for example, in patients with sepsis, where systemic inflammation leads to organ failure as a direct result of inflammatory mediators on microvascular function. The negative impact of the host's immune response on tissue is known as immunopathology.22 For example, using an experimental approach, it was shown that liver failure in the context of sepsis and cirrhosis is the result of the death of hepatocytes caused by apoptosis induced by TNF-α or by necrosis induced by endothelin-1.23 In addition to immunopathology, tissue damage and organ failure could be related to the change in tissue homeostasis by bacteria, caused by the direct alteration of cell functions. Furthermore, organ failure has been linked to a failure in tissue tolerance mechanisms, which are mechanisms aimed at protecting against immunopathology and direct bacterial damage.11,22
In summary, cellular dysfunction, immunopathology and dysfunction of tissue tolerance mechanisms in the context of excessive inflammatory response could be mechanisms involved in organ failure in patients with ACLF.11,15
Immunosuppression in acute-on-chronic liver failureIn addition to an increase in proinflammatory cytokines, patients with ACLF also present with an increased production of certain anti-inflammatory cytokines, such as IL-10 and IL-1Ra.16,17 This could indicate the existence of a compensatory immune response that is not capable of mitigating the excessive inflammatory response. It has also been shown that the monocytes of patients with ACLF present with an ex vivo decreased production of inflammatory cytokines in response to administration of lipopolysaccharides (LPS), as well as a decreased expression of activation markers, such as HLA-DR.24 Furthermore, a recent study showed that the circulating monocytes in patients with ACLF present with an overexpression of the MERTK receptor, a signalling inhibitor from the TLR pathway, which suppresses the ex vivo immune response to stimulation with LPS.25 These findings indicate the existence of immunosuppression, which could be explained as resulting from excessive inflammatory response.24–26 The existence of these immunosuppression mechanisms could explain the increased susceptibility to second infections of patients with ACLF.
Relationship between the precipitating factors and inflammation mechanisms in acute-on-chronic liver failureBacterial infectionPatients with cirrhosis have an excessive inflammatory response to infections, with an increased concentration of proinflammatory cytokines that is more intense than that of patients with infections but without cirrhosis.27,28 The mortality rate of patients suffering from septic shock and cirrhosis is also greater than that of patients suffering from septic shock without cirrhosis.23,29 Initial analyses of the CANONIC study have shown that any infection can cause ACLF, however, the risk is greater for spontaneous bacterial peritonitis, followed by secondary bacterial peritonitis and pneumonia. The severity of the infection also increases the risk of ACLF in such a way that sepsis or severe sepsis is more frequently associated with ACLF, compared to infections with no systemic inflammatory response syndrome (15% vs 4%).5,10 The mechanisms that predetermine an excessive systemic inflammatory response to infections in patients with cirrhosis compared to the general population are unknown. Ex vivo studies have indicated that there is a defect in the inhibition mechanisms of the TLR4 pathway of the circulating monocytes of patients with cirrhosis.30
Alcoholic hepatitisSevere alcoholic hepatitis represents 20% of all ACLF cases. It has been observed that patients with severe alcoholic hepatitis have systemic inflammation that is correlated with prognosis, which supports the role of inflammation in the development of organ failure.31 This inflammation could be linked to the high frequency of infections observed in patients with alcoholic hepatitis (approximately 20–30% of the patients).31,32 Moreover, patients with alcoholic hepatitis present with a change in the composition of intestinal microbiota and an increased intestinal permeability, which promotes the translocation of viable bacteria or bacterial products (PAMPs) which, on binding with the TLR receptors, would stimulate the production of cytokines, such as IL-8, responsible for attracting and activating neutrophils, which are the key cells in alcoholic hepatitis.33,34 Furthermore, the metabolism of acetaldehyde (alcohol metabolite) induces the production of reactive oxygen species which, in turn, leads to mitochondrial DNA stress, a type of DAMP that contributes to liver inflammation.35
Acute-on-chronic liver failure with no triggerAlthough no trigger was identified in up to 40% of the patients, these patients presented with significantly greater systemic inflammation than patients without ACLF.5 Once more, various hypotheses have been suggested to explain this inflammation: dysbiosis and production of metabolites by intestinal bacteria; translocation of the PAMPs; and the action of the DAMPs, which could all contribute to systemic inflammation. It should also be noted that the precipitating factors cannot be identified in some cases, for example, undetected bacterial infections.12,14 Future studies and new technologies – such as metabolomics, lipidomics and metagenomics – may be able to explore these hypotheses.
Despite all the advances, information on the mechanisms that promote the development of ACLF remains scarce. Thus, more studies are required to explore the inflammation, immunosuppression and tolerance mechanisms in order to understand the pathophysiology of ACLF.
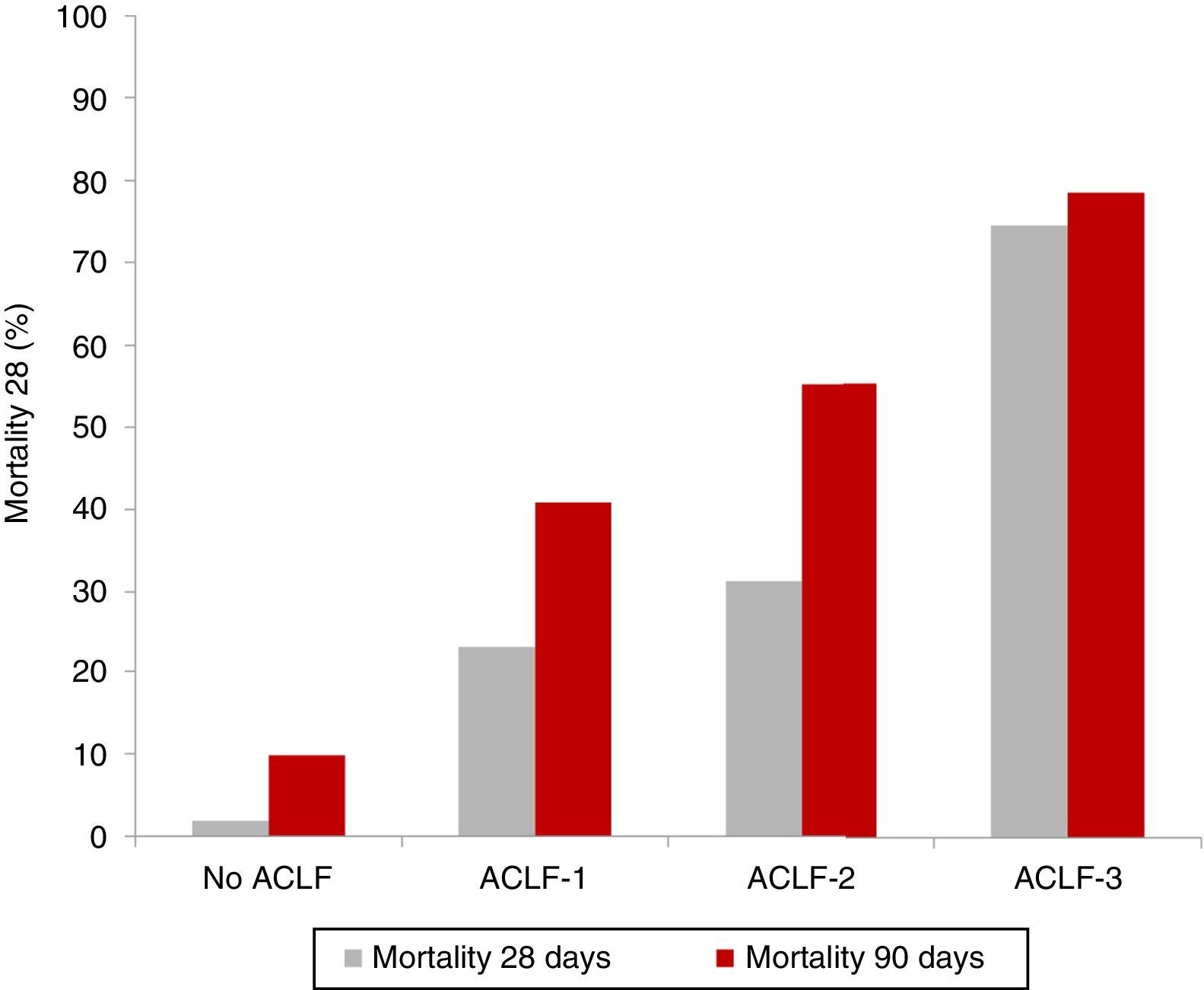
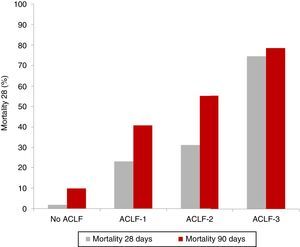
Clinical course and prognosisAs previously mentioned, ACLF is characterised by a high short-term mortality rate that ranges between 30% and 50%. As expected, patients with ACLF-3 present with the worst prognosis, compared to patients with ACLF-1 and ACLF-2.5,8 The results of the CANONIC study revealed an overall mortality rate at 28 days in patients with ACLF of 33% (23% for ACLF-1, 31% for ACLF-2 and 74% for ACLF-3), compared with only 2% in patients with decompensated cirrhosis without ACLF5 (Fig. 2).
However, it must be borne in mind that ACLF is a potentially reversible dynamic process. In this respect, a recent study conducted with the cohort of patients from the CANONIC study investigated the clinical course of ACLF.36 Overall, ACLF was resolved or improved in 49% of patients, it remained stable or fluctuated in 30% and it worsened in 20%. However, these figures varied depending on the initial ACLF stage: although ACLF was resolved in 55% of patients with ACLF-1, it was only resolved in 15% of patients with ACLF-3. It should be noted that, despite the fact that the initial ACLF stage is related to prognosis, the evolution of ACLF during hospitalisation was the greatest determining factor of short-term mortality. Because most patients reach the final stage of ACLF during the first week following diagnosis, the ACLF stage 3–7 days after diagnosis was able to predict prognosis at 28 and 90 days more accurately than the ACLF stage at the time of diagnosis.36 Therefore, the sequential evaluation of prognosis during the first few days of hospitalisation is fundamental in stratifying the risk for these patients.12–14
Predictive factors for prognosisCurrent data do not show any link between precipitating factors and prognosis. Conversely, it is interesting to note that a history of previous decompensated cirrhosis has been linked to ACLF prognosis.5 It has been reported that in up to 20% of cases ACLF occurs in patients with previously compensated cirrhosis, which represents the earliest form of decompensation of the disease. In these patients without previous episodes of decompensation, ACLF has a poor prognosis, with a 28-day mortality rate of 43% compared to 30% in patients with previously decompensated cirrhosis.5
With respect to analytical data, patients with ACLF have greater C-reactive protein and leucocyte values than patients with decompensated cirrhosis without ACLF. Leucocyte values have also been linked with prognosis, in that the probability of mortality increases in line with the leucocyte value.5
Prognostic indices for risk stratification in patients with acute-on-chronic liver failureThe prognostic indices normally used to evaluate the prognosis of patients with decompensated cirrhosis, such as the Child–Pugh and MELD scores, do not take into account all the possible organ failures that can occur in patients with ACLF. The CANONIC study and subsequent analyses derived from the same cohort of patients have defined new indices that have shown greater accuracy in predicting prognosis than the Child–Pugh and MELD scores.5,6,36
The previously mentioned CLIF-C OF index is useful in diagnosing the existence of ACLF and in predicting prognosis. Its prognostic accuracy is slightly higher than that of Child–Pugh and the MELD.6 However, a new index, CLIF-C ACLIF, was subsequently determined, which has a greater prognostic capability than CLIF-C OF. This index consists of the CLIF-C OF linked to two endpoints that were selected as the baseline endpoints most associated with short-term mortality: age and leucocyte value. The index is scored from 0 to 100, with higher values signifying a worse prognosis.6 This can be easily calculated using the EF-CLIF website: www.efclif.com. The CLIF-C ACLF evaluation at 3–7 days after diagnosis was better in predicting prognosis than the calculation of this index on diagnosing ACLF.
The CLIF-C ACLF evaluation at 3–7 days after having started treatment has also been proposed as a possible futility criterion, i.e., an objective way of determining the possible limitation of the therapeutic effort in critical patients with poor short-term prognosis. In patients with ACLF who do not have access to an LT, the persistence of 4 or more organ failures, or a CLIF-C ACLF score greater than 64 at days 3–7, results in a 6-month mortality rate of 100%, in accordance with the results of the CANONIC study.36 However, these data and the use of these criteria should be validated in future studies.
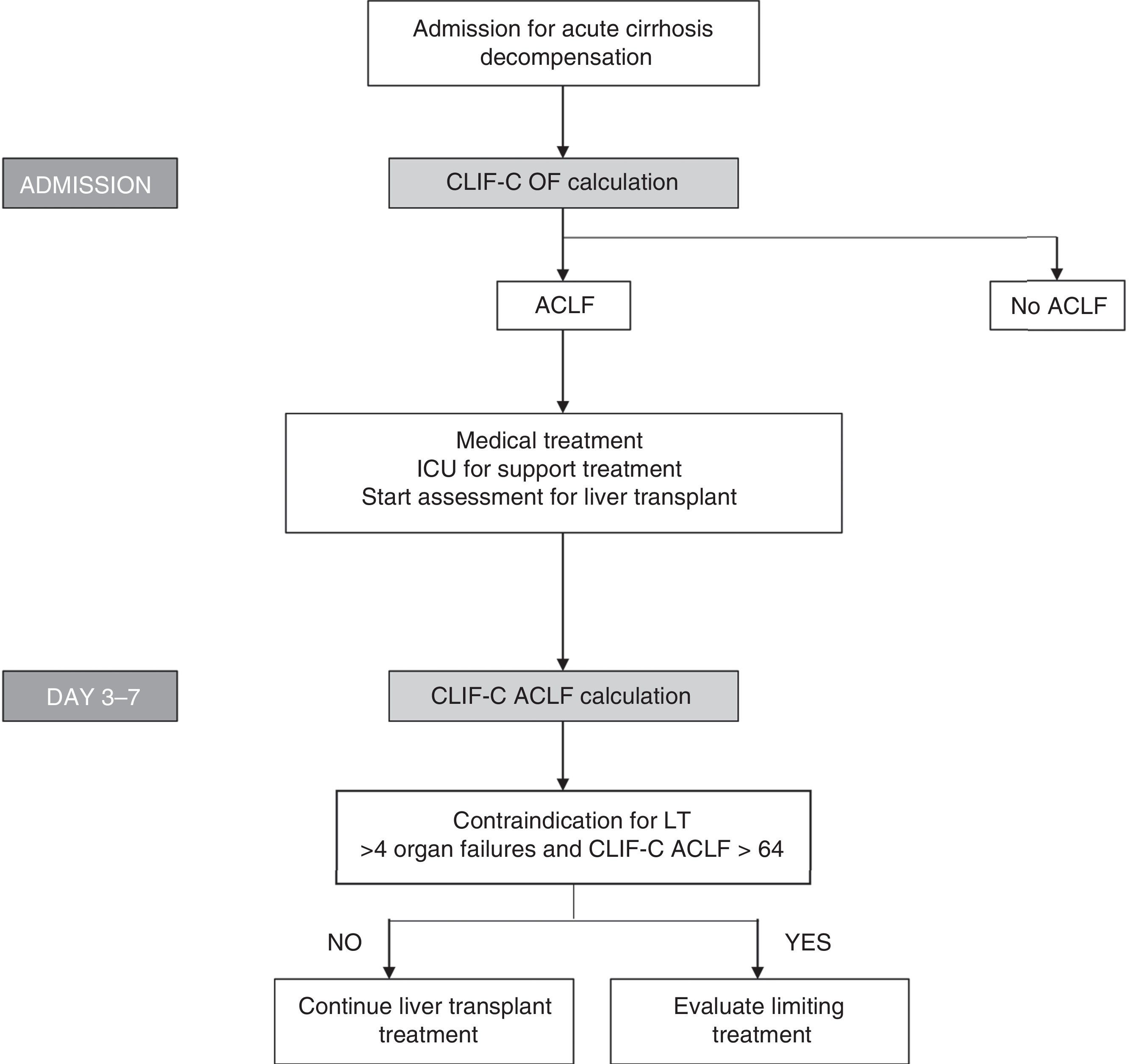
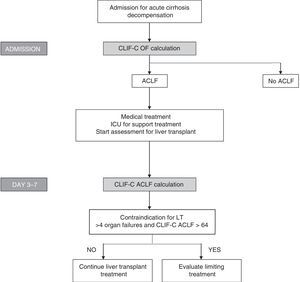
Bearing in mind the definition of these new indices, which show good diagnostic accuracy and the importance of evaluating prognosis sequentially, algorithms have been proposed to evaluate prognosis and to help in making decisions regarding patients with ACLF (Fig. 3).
Algorithm for establishing the diagnosis and sequential evaluation of prognosis in patients with ACLF. The CLIF-OF calculation on admission enables the ACLF diagnosis to be established according to the currently accepted diagnostic criteria, based on the results of the CANONIC study. The evaluation of prognosis 3–7 days after having started treatment, using the CLIF-C ACLF index, seems to be the most accurate strategy for establishing prognosis in these patients. At this point (days 3–7), the existence of 4 or more organ failures together with a CLIF-C ACLF score >64 in patients who are not able to undergo an LT has been associated with a 6-month mortality rate of 100%. Therefore, this has been suggested as a possible criterion for suggesting that the therapeutic effort is limited. However, these criteria should be validated in future studies.
Because there is currently no specific treatment for patients with ACLF, its management relies on the early identification of the syndrome, the treatment of precipitating factors (bacterial infections, treatment for HBV, corticosteroids in alcoholic hepatitis, etc.) and support treatment for the various types of organ failure. Ideally, patients with ACLF, especially those with ACLF-2 and ACLF-3, should be treated in intensive or intermediary care units and, unless there are complications, should be transferred to hospitals with an LT programme.
Generally, these patients should be managed in accordance with current clinical guides and recent reviews on the management of critically ill cirrhotic patients.37–39 This review will therefore focus only on the possible ACLF-specific treatments.
Liver transplantAn LT is the optimal and definitive treatment in patients with ACLF.12–14 Therefore, if no absolute contraindications exist a priori, patients with ACLF should be evaluated for an LT. However, transplantation in patients with ACLF, particularly those with serious stages, is complex and controversial, especially due to the high frequency with which these patients present contraindications; the frequent development of infections or organ failures determines whether the patients are in too serious a condition to receive an LT. In this context, and due to the high short-term mortality rate, the treatment window for suggesting an LT to these patients is very narrow. Furthermore, the waitlist mortality rate for these patients is very high. In this respect, opinions have emerged suggesting that to improve the prognosis for ACLF, especially in serious stages, those on the LT waiting list should be prioritised.40
Currently, existing data on the progression and prognosis of patients with ACLF after a transplant are still scarce, and the majority of these data comes from retrospective studies and short patient series. The CANONIC study produced limited results in this regard since only 9% of the patients received a transplant. However, the results are optimistic considering that in the patients with ACLF-2 or ACLF-3, the 28-day survival without an LT was less than 20%, but increased to 80% in patients who received a transplant. In that series, the median time between ACLF diagnosis and transplantation was 11 days (1–28 days).5 These results support the idea that an early LT can improve the prognosis for these patients. The results of another study that included 144 patients with ACLF, in that case using the APASL definition, revealed a high waitlist mortality rate (greater than 50%); however, for those patients undergoing a transplant, the 5-year post-LT survival rate was greater than 80%. However, it should be noted that only 49% of patients went on the waiting list and only 23% received an LT; the others could not receive a transplant due to their advanced age, active infections, alcoholism or other contraindications.41 There are also data from studies conducted in Asia that included patients with HBV and which also provide results regarding living-donor liver transplants (LDLT). The results of one of the studies revealed that an LT from a cadaver donor vs an LDLT showed no significant differences in terms of survival, both achieving good results, with a 5-year survival rate of 74%.42 Another recent study showed that, despite the fact that LDLT in patients with ACLF produced good results, the 5-year post-transplant survival in patients with ACLF was significantly lower than that for patients without ACLF (71% vs 81%, respectively; p=0.035).43 Although LDLT is a potential option for a limited number of donors, the fact that transplantation should ideally be performed in a short period of time in the context of ACLF, makes its use for this indication complex.
In contrast with the good LT results in patients with ACLF reported until now, a recent retrospective study that included 350 patients (140 with ACLF defined according to the CANONIC study criteria) showed that the post-LT prognosis in patients with ACLF was worse than in those without ACLF who received a transplant. In that study, survival at 3 and 12 months post-LT in patients with ACLF was 79% and 70%, respectively, compared with 96% and 91% in patients without ACLF (p<0.001). Furthermore, hospital stay and stay in an intensive care unit for patients with ACLF were significantly longer.44
In summary, the data on the impact of ACLF on post-LT prognosis are still too limited to establish specific strategies aimed at these patients. Currently, the management of patients with ACLF, in terms of their evaluation and inclusion on a waiting list, follows the standard protocol used for all patients with decompensated cirrhosis. Considering the high short-term mortality rate in these patients, it is recommended that the evaluation for a potential LT be carried out early and quickly. Tests should be performed to determine the impact of ACLF on the LT and to establish objective selection and prioritisation criteria for these patients, as well as objective criteria for removing patients from the waiting list and for determining the futility of the treatment.
Liver support systemsLiver support systems have been proposed as therapeutic options that could act as a bridge between the transplant and patients with ACLF.45 These systems are based on replacing liver function and eliminating various substances from systemic circulation that are thought to be involved in the pathophysiology of ACLF (for example, nitric oxide, PAMPs) with the aim of improving the clinical and biological parameters in these patients.
There are two randomised studies, one with MARS (molecular adsorbent recirculating system) and another with Prometheus (fractionated plasma separation and absorption system), which have evaluated their use in patients with ACLF. Although both studies showed some improvement in relation to liver and kidney function and systemic haemodynamics, neither of them showed a significant improvement in survival.46,47 However, the lack of a widely accepted definition for ACLF means that these studies have not included a homogeneous population of patients, but rather patients with decompensated cirrhosis involving varying numbers and levels of organ failure.
On the other hand, there are non-randomised studies that have evaluated the use of plasma exchange in patients with ACLF linked to HBV, which did show an improvement in survival compared to patients treated with the standard treatment.48 Another randomised study also revealed an improved survival rate in patients with acute liver failure.49 However, there are currently no studies that evaluate the usefulness of plasma exchange in patients with ACLF with aetiologies other than HBV.
To summarise, more studies are required that include a homogeneous population of patients with ACLF linked to different aetiologies in order to establish whether liver support systems can really play a role in managing these patients.
Immunomodulatory and regenerative therapyThe hypothesis on the pathophysiology of ACLF and the high susceptibility of developing infections presented by these patients is currently focused on an excessive inflammatory response and the possible existence of varying degrees of immunoparesis. In this regard, the research efforts on ACLF-specific treatments is partly focused on treatments that limit excessive inflammatory response or that modulate immunological response. Although limited, there are data on the potential role of treatments such as the administration of G-CSF (granulocyte colony-stimulating factor) or stem cells in patients with ACLF.
G-CSF is a cytokine that activates the mobilisation of bone marrow stem cells, as well as the proliferation and mobilisation of granulocytes. Two randomised studies have evaluated the role of G-CSF as a treatment for patients with ACLF. In one of these, 47 patients were randomised to receive 5μg/kg of G-CSF administered subcutaneously vs the standard treatment, and they showed an improvement in survival at two months of 66% vs 26%, respectively. An improvement in the Child–Pugh and SOFA scores was detected in the patients who received G-CSF, as well as a lower risk of developing hepatorenal syndrome, hepatic encephalopathy and sepsis.50 In the other study, 55 patients with ACLF linked to HBV were randomised to receive G-CSF vs the standard treatment. Similar to what happened in the first study, the patients who received G-CSF presented with improved survival at 90 days (48% vs 21%, respectively), as well as a reduction in the MELD score.51 Although these studies produce promising results, it should be noted that, overall, the data come from a limited number of patients. Furthermore, because in both studies the ACLF was defined according to the APASL criteria, patients with initial stages of ACLF were included whereas patients with more advanced stages were excluded, as currently defined by the CANONIC study criteria.
Stem cell therapy has also been proposed as a potential treatment, with the aim of being a regenerative therapy that can, at the same time, be associated with an immunomodulatory effect. However, there are currently only results of one study in a short patient series. That study included 43 patients with ACLF linked to HBV who received umbilical cord stem cells vs placebo associated with the standard treatment. Survival at 90 days was 79% in patients who received stem cells vs 52% in the control group (p=0.015).52
Therefore, although treatments based on an immunomodulatory or regenerative effect produce interesting results, they are still in an experimental stage and so future studies should be conducted to clarify their potential benefit.
FundingElsa Solà received a Joan Rodés grant from the Spanish Association for the Study of the Liver (Asociación Española para el Estudio del Hígado, AEEH) and a grant from the Catalan Society of Digestology (Societat Catalana de Digestologia).
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Solé C, Solà E. Actualización en la insuficiencia hepática aguda sobre crónica. Gastroenterol Hepatol. 2018;41:43–53.