The prevalence of gastric subepithelial lesions (SEL) is 0.36–1.7%1, with gastrointestinal stromal tumor (GIST) being the most common type of SEL2. However, biopsy of SEL continues to be the subject of debate, especially if it is a GIST, due to the risk of rupture, bleeding and tumor spread3. This study aimed to look for predictive factors for gastric GIST, which would avoid biopsy.
We carried out a retrospective study with a population of patients with single gastric SEL operated on from 2014 to 2021. We compared two groups: group 1 (gastric GIST) and group 2 (non-GIST gastric SEL). Socio-personal, clinical, diagnostic, surgical and histopathological variables were all analysed. Categorical variables are expressed as frequencies and percentages, and they were compared using Pearson's Chi-square test or Fisher's exact test when appropriate. Continuous quantitative variables are expressed as mean±standard deviation, and we used the Shapiro-Wilk test to check for normal distribution. Quantitative variables were compared using the Student's t-test for independent data when they followed a normal distribution and the Mann-Whitney U test if they did not. For significant quantitative variables, we performed an analysis of the ROC curve. We calculated the sensitivity (Se), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) of the factors associated with the diagnosis of GIST. A p value <0.05 was considered statistically significant.
In group 1 (n=21) the mean age was 67.6±14.2 years and 57.1% (n=12) were male. The laparoscopic approach was used in 95.2% (n=20). One patient developed a postoperative haematoma which required reintervention. The mean size of the GIST was 5.5±3.6cm. The histological type was fusiform in 66.7% (n=14), epithelioid in 14.3% (n=3) and mixed in 19% (n=4). In the immunohistochemistry all the cases showed positivity for c-kit (CD117). There was no tumor rupture and the resection margins were free of disease in all cases. The risk of recurrence in the Fletcher and Joensuu classification was: very low risk in 9.5% (n=2); low risk in 47.6% (n=10); intermediate risk in 23.8% (n=5) and high risk in 19% (n=4). In the Miettinen classification, the risk of recurrence was: no risk in 9.5% (n=2); very low risk in 47.6% (n=10); low risk in 19% (n=4); intermediate risk in 19% (n=4); and high risk in 4.8% (n=1). According to the eighth edition of the American Joint Committee on Cancer (AJCC), the tumor stage was: IA in 57.1% (n=12); IB in 19% (n=4); II in 19% (n=4); and IIIA in 4.8% (n=1). In stages II and IIIA, analysis of the mutations of the c-kit and PDGFRA genes was performed: three cases were wild type (without mutations in the c-kit or PDGFRA genes); one case had a mutation in exon 11 of the c-kit gene, and the remaining case had a mutation in exon 18 of the PDGFRA gene. The patient with stage IIIA received adjuvant therapy with imatinib (400mg daily for three years). Over a mean follow-up period of 47.5±32.4 months no signs of disease recurrence were found.
In group 2 (n=5) there were three patients with cardial leiomyoma, one with an oesophageal duplication cyst at the cardia and another with an antral heterotopic pancreas; 60% (n=3) were male, with a mean age of 47.2±10.5 years. The mean size of the lesion was 3.6±1.6cm. The lesion was rejected by laparoscopic approach in 80% (n=4). The patient with heterotopic pancreas in the antral region developed a post-surgical stenosis which had to be re-operated on.
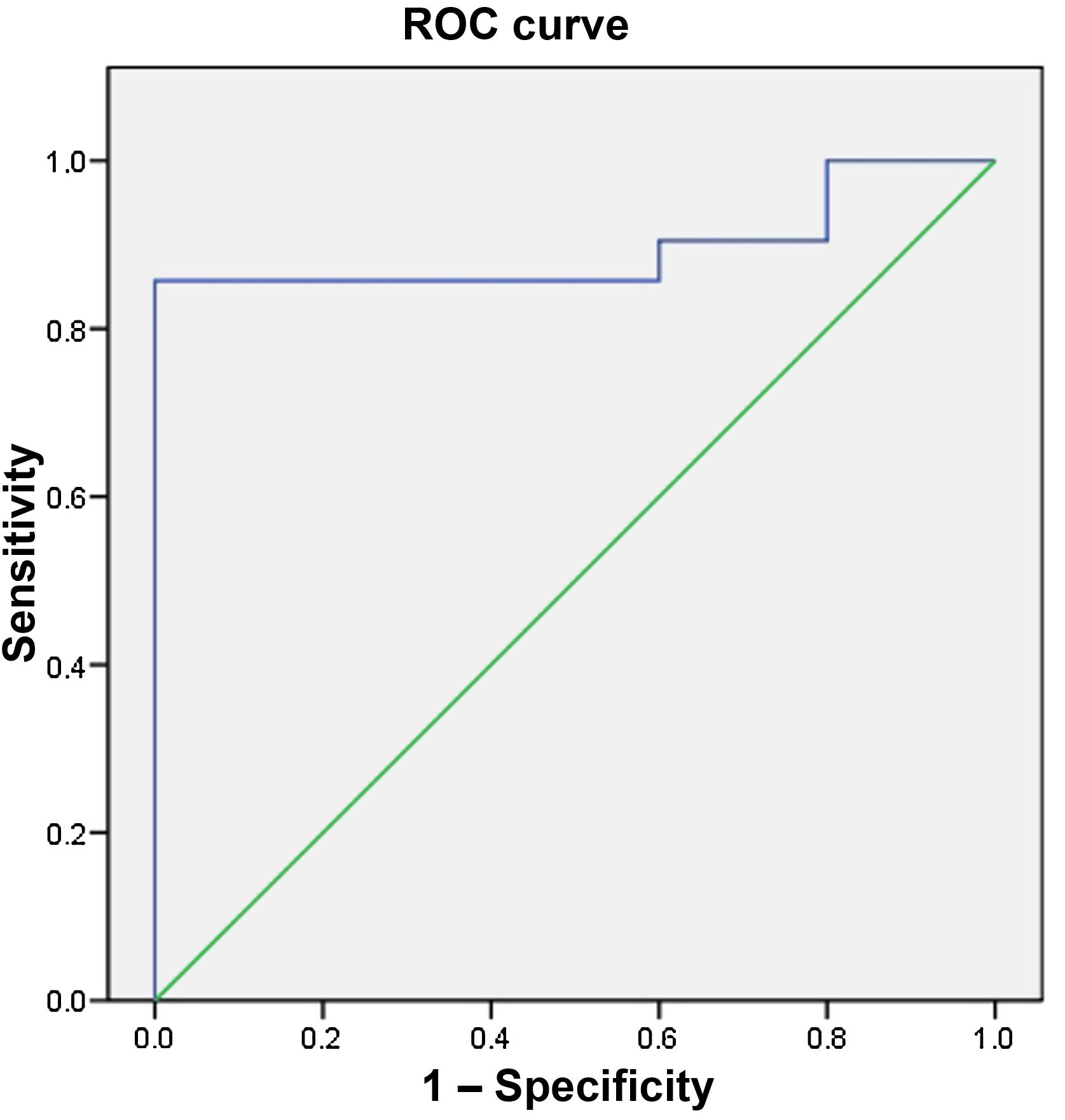
Age was significantly higher in group 1 (67.6±14.2 vs 47.2±10.5 years; p=0.006), age ≥56 in particular (85.7% vs 0%; p=0.001) had 85.7% Se, 100% Sp, PPV of 100% and NPV of 62.5% for the diagnosis of GIST (area under the curve 0.895) (Fig. 1). Location other than at the cardia (95.2% vs 20%; p=0.002) was also a factor associated with the diagnosis of GIST, with Se of 95.2%, Sp of 80%, PPV of 95.2% and NPV of 80%. Being aged ≥56 or having an SEL at a site other than the cardia had 95.2% Se, 80% Sp, PPV of 95.2% and NPV of 80% for the diagnosis of GIST, and being aged ≥56 and having an SEL at a site other than the cardia had 85.7% Se, 100% Sp, PPV of 100% and NPV of 83.3%.
The indication for biopsy of gastric SEL continues to be subject to debate. Fernández et al.3 suggest biopsy in cases of significant uncertainty about the diagnosis of GIST or if the GIST is suspected of being unresectable and might therefore benefit from neoadjuvant therapy. Akahoshi et al.2 recommend fine needle aspiration (FNA) in solid hypoechoic lesions >1cm. Meanwhile, Pih et al.1 and Cho et al.4 indicate biopsy in symptomatic lesions ≥2cm with endoscopic findings of malignancy (irregular surface, ulcerated lesion or growth during follow-up) or with high-risk factors on endoscopic ultrasound (echogenic foci >3mm, cyst-like spaces >4mm, irregular borders or regional lymphadenopathy).
In the study by Schulz et al.5 age >57, location at a site other than the cardia, positive Doppler signal and the SEL having irregular borders were factors associated with gastric GIST. In our study, age and the location of the SEL were also factors associated with gastric GIST. However, the findings of the endoscopic ultrasound were not analysed, as it was not performed in all cases.
The main limitation of this study is the low sample size, and multicentre studies are therefore needed to draw firm conclusions.
From our results, biopsy could be indicated in patients aged <56 and/or with SEL at the cardia, and all other SEL could be treated as GIST and without having to perform biopsies. From our results, a biopsy could be indicated in patients aged <56 and/or with SEL at the cardia, and all other SEL could be treated as GIST without having to perform biopsies.