Patients with certain immune‐mediated inflammatory diseases, such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD), have an increased risk of severe infectious diseases than the general population, which are mainly associated with the immunosuppressive treatments that they receive.
These treatments act on the immune system through different mechanisms, causing different degrees of immunosuppression and a variable risk depending on whether the pathogen is a virus, bacteria or fungus. This article reviews the most relevant literature on the subject, which was selected and discussed by a panel of experts. The aim of this article is to review the risk of infections in patients with IBD and RA, and the potential preventive measures.
Los pacientes con ciertas enfermedades inflamatorias mediadas inmunológicamente, como la artritis reumatoide (AR) y la enfermedad inflamatoria intestinal (EII) presentan una mayor incidencia y gravedad de enfermedades infecciosas que la población general, asociadas especialmente a los tratamientos inmunosupresores que reciben.
Dichos tratamientos actúan sobre el sistema inmunitario a través de diferentes mecanismos, causando diferentes grados de inmunosupresión y un riesgo variable dependiendo de si el patógeno es un virus, una bacteria u hongo. Este artículo es una revisión de la bibliografía más relevante sobre el tema, seleccionada y debatida por un panel de expertos. El objetivo de este artículo es revisar el riesgo de infecciones en pacientes con EII y AR y las potenciales medidas preventivas.
Rheumatoid arthritis (RA) and inflammatory bowel disease (IBD) are among the most prevalent autoimmune or chronic inflammatory diseases. Both patients with RA and patients with IBD have a higher incidence and severity of infectious diseases than the general population. The risk of infection is associated with age, the disease itself and its degree of activity, and the immunosuppressive treatments being received.1–3 Some of the infections can be prevented by treating the latent infection (e.g. tuberculosis) or by vaccination.4–7
Compliance with the vaccination recommendations for both patients with IBD and those with rheumatic diseases is very low.4,8–11 The reasons stated include fear of adverse effects, concern about the efficacy of the vaccine due to immunosuppression status, the unjustified belief that vaccination can exacerbate the disease, and specialists’ lack of knowledge about the recommended vaccines.4,8,12 It is therefore important that both doctors and patients know the risk of infection associated with each therapy and the infection prevention measures in this type of disease.13
One of the main causes of the increased risk of infections is immunosuppressive drugs. The most commonly used conventional disease-modifying antirheumatic drugs (cDMARDs) or immunosuppressive therapies include corticosteroids, thiopurines (azathioprine, mercaptopurine), ciclosporin, methotrexate (MTX), leflunomide and biologics. Small targeted oral molecules have recently been added to this therapeutic arsenal, including baricitinib and tofacitinib, both Janus kinase (JAK) inhibitors.8 These treatments act on the immune system through different mechanisms causing different degrees of immunosuppression. The risk also varies depending on whether the pathogen is a virus, bacteria or fungus.14
The aim of this article was to review the risk of infections in patients with immune-mediated diseases, specifically IBD and RA, and the potential preventive measures.
Risk of infection in patients with IBD receiving immunosuppressive therapyThe increased risk of infections in patients with IBD seems to be attributed mainly to immunosuppressive therapies.3,15 Treatment with systemic corticosteroids is associated with a very high risk of serious infection. The risk of infection also significantly increases when different immunosuppressive therapies are combined.16–18 Other associated factors include advanced age, disease activity and malnutrition.7,16–18 A recent observational, retrospective study by Zabana et al.3 using the ENEIDA registry included 6,914 patients with IBD treated with immunosuppressants (thiopurine, MTX) and/or biological therapy (anti-TNF-α) followed up for an average of 12 years. The risk of infection during the treatment period was 30 times higher than before the start of treatment and six times higher than after it was discontinued. Among the different treatments analysed, 5% of the patients suffered at least one serious infection.3
Published results on the risk of infections and the therapeutic strategy used differ according to whether the analysis is of clinical trials13 or observational studies of long-term experience in routine practice.15,16 In a meta-analysis that included 38 randomised studies and 24 different therapeutic strategies, Wheat et al.13 found no significant differences in the risk of serious infections between the immunosuppressive therapies.13
In contrast, observational studies show a different risk of serious infections depending on the treatment used and the length of exposure.3,15,16 In a recent systematic review and meta-analysis that included 15 observational studies, Singh et al.16 analysed the risk of serious infections associated with tumour necrosis factor alpha inhibitors (anti-TNF-α). Monotherapy with anti-TNF-α caused a marked increase in the risk of infection compared to monotherapy with thiopurines or MTX.16 Combined therapy with anti-TNF-α plus thiopurines or MTX was associated with an additional increase in the risk.16
These findings have been reproduced in studies of population databases by other authors.15 Kirchgesner et al.15 analysed the risk of serious infections in 190,694 patients with IBD (French national inter-scheme health insurance information system [SNIIRAM] database). The incidence rate of serious infections was 0.84 per 100 patient years in patients not treated with anti-TNF-α or thiopurines, increasing to rates of 1.05, 1.89 and 2.24 per 100 patient years in those on monotherapy with thiopurines, monotherapy with anti-TNF-α, or a combination of the two, respectively. Compared to monotherapy, combined therapy with anti-TNF-α and thiopurines was associated with an increased risk of serious infection, with a hazard ratio (HR) of 1.23; 95% CI: 1.05-1.45.15 Monotherapy with anti-TNF-α was associated with a significant increase in the risk of severe infection (HR: 1.72; 95% CI: 1.56-1.88), mycobacterial infections (HR: 1.98; 95% CI: 1.15-3.40) and bacterial infections (HR: 2.38; 95% CI: 1.23-4.58), compared to monotherapy with thiopurines.15
However, in a randomised study conducted in 374 patients with ulcerative colitis who received vedolizumab versus 521 with placebo, the safety analysis showed no difference regarding the incidence of serious infectious diseases between the two groups.19 The incidence rate of serious infections was 1.8 per 100 patient years.20
In a randomised study assessing ustekinumab in 961 patients, Sands et al.21 found no difference in the incidence of serious infections at week 44 (maintenance) between the treatment group and the placebo group.21 In the extension study from weeks 44 to 96, the serious infection rate was 2.33 per 100 patient years in the ustekinumab group versus 2.99 in the placebo group.22
Tofacitinib has recently been approved for the treatment of moderate to severe ulcerative colitis (UC). A meta-analysis23 compared the efficacy and safety of biological agents and tofacitinib as first-line treatments (naïve to anti-TNF-α) and second-line (previous failure to anti-TNF-α) for the treatment of moderate to severe UC.23 In maintenance trials, vedolizumab and ustekinumab were found to be the safest options in terms of risk of infections,23 serious infections generally being rare with biologics and tofacitinib.23 In the OCTAVE programme with tofacitinib, Sandborn et al.24 found the incidence rate for serious infection to be 2.0 cases per 100 patient years (95% CI: 1.4-2.8).24 The incidence remained stable over the course of the follow-up lasting 6.8 years.25
Risk of infection in patients with RA on immunosuppressive therapyPatients with RA are also more susceptible to developing infections. In the case of RA, it is believed this may be related both to immunological alterations associated with the disease and to the immunosuppressive effects of the treatments used.26 Other factors that increase the risk of infections in these patients are disease activity, the presence of extra-articular manifestations (HR: 2.07; 95% CI: 1.41-3.06), advanced age (HR: 1.30 for every 10 years, 95% CI: 1.16-1.46) and comorbidities such as chronic pulmonary disease (HR: 1.84; 95% CI: 1.40-2.41), diabetes mellitus (HR: 1.60; 95% CI: 1.20-2.30) and alcoholism (HR: 1.67; 95% CI: 1.16-2.41).26
Numerous systematic reviews and meta-analyses have assessed the risk of infections in RA according to the treatments and the stage of the disease.27–31 Bongartz et al.27 found in their meta-analysis that the use of anti-TNF-α (infliximab and adalimumab) doubled the risk of serious infections compared to placebo (OR: 2.0; 95% CI: 1.3-3.1). The risk increased with high doses (OR: 2.3; 95% CI: 1.5-3.6).27 Along the same lines, in 2015 Singh et al.31 published a systematic review including 106 randomised studies on patients with RA with data on nine biologic drugs. Compared to cDMARDs, the use of anti-TNF-α (with or without cDMARDs) was associated with an increased risk of serious infections at both standard and high doses.31 Strand et al.29 assessed the risk of serious infections associated with biologics and the JAK inhibitor tofacitinib, in a systematic review that included 66 randomised studies, 22 open-label studies, three phase 3 studies and the tofacitinib extension studies. The incidence rates of severe infection per 100 patient years estimated for abatacept, rituximab, tocilizumab and anti-TNF-α were 3.04 (95% CI: 2.49-3.72), 3.72 (95% CI: 2.99-4.62), 5.45 (95% CI: 4.26-6.96) and 4.90 (95% CI: 4.41-5.44), respectively.29 The incidence rates for tofacitinib 5 mg and 10 mg twice a day were 3.02 (95% CI: 2.25-4.05) and 3.00 (95% CI: 2.24-4.02) per 100 patient years, respectively, in the phase 3 studies, and 2.50 (95% CI: 2.05-3.04) and 3.19 (95% CI: 2.74-3.72) in the extension studies.29
In a recent systematic review and meta-analysis, which included 11 studies on tofacitinib and six on baricitinib in patients with RA, Bechman et al.32 found an incidence rate of serious infections per 100 patient years of 1.97 (95% CI: 1.41-2.68) with tofacitinib, and 3.16 (95% CI: 2.07-4.63) with baricitinib.32
Regarding the long-term risk of serious infections in patients treated with tofacitinib, the integrated safety analysis at 9.5 years,33 which included 7,061 patients, showed an incidence rate per 100 patient years of 2.5 (2.3-2.7).33
A safety analysis of baricitinib at two years by Smolen et al.,34 which included 3,492 patients (6,639 patient years) from six studies, found an incidence rate of serious infections per 100 patient years in RA, treated with baricitinib 2 mg and 4 mg per day, of 3.8 and 4.2, respectively.34 An analysis by Curtis et al.35 in patients with RA treated with tofacitinib 5 mg or 10 mg twice a day showed an incidence rate of serious infections per 100 patient years of 2.38 and 2.78, respectively, in patients aged <65 compared to 3.89 and 8.06, respectively, in patients aged >65.35 Fleischmann et al.36 assessed the safety and efficacy of baricitinib in older patients with RA and also found an increase in serious infections in over 65s (2.9% with baricitinib 4 mg vs 1.8% with placebo).36
The European Medicines Agency (EMA) recently issued a recommendation for patients aged over 65 on treatment with tofacitinib, based on an ongoing open-label post-marketing clinical study (study A3921133; NCT02092467).37,38 This study found that the incidence rate rose markedly in patients aged 60-70 and in older patients. The same trend was found with anti-TNF, although the increase was not as pronounced until over the age of 75.37
COVID-19 in patients with immune-mediated diseasesPatients on immunosuppressive therapy may be at higher risk than the general population of infection by the SARS-CoV-2 coronavirus. However, such treatment may also be associated with a decrease in adverse outcomes from SARS-CoV-2 (COVID-19), as it limits the cytokine storm characteristic of severe COVID-19.39 In patients with IBD or RA, as in the general population, the risk factors for developing severe COVID-19 have been found to be obesity, age, diabetes, cardiovascular disease, obstructive pulmonary disease and chronic kidney disease40–42; chronic comorbidities such as diabetes, obstructive pulmonary disease, coronary heart disease and hypertension affect the severity of COVID.42 Very initial data suggested that the risk of COVID-19 in rheumatic patients treated with biological agents or cDMARDs appeared to be no different from that of the general population.42 Data on 525 patients with IBD from 33 countries (IBD SECURE registry) gathered to study the natural history of COVID-19 in paediatric and adult patients with IBD showed an overall case fatality rate of 3%. Risk factors for severe COVID-19 were advanced age, number of comorbidities, use of systemic corticosteroids and use of 5-ASA/sulfasalazine. Anti-TNF-α therapy turned out not to be an independent risk factor for COVID-19 severity.43
A recent study carried out with 1,439 patients with IBD (112 [7.8%] developed severe COVID-19) from the IBD SECURE registry39 investigated the impact of different treatments associated with the risk of developing severe COVID-19. They found that compared to anti-TNF-α in monotherapy, thiopurines in monotherapy (adjusted OR [aOR]: 4.08) or in combination with anti-TNF-α (aOR: 4.01) were associated with an increased risk of developing severe COVID-19.39 The use of mesalazine/sulfasalazine was associated with an increased risk compared to not taking these drugs (aOR: 1.70). The estimated risk was increased when anti-TNF-α in monotherapy was used as the reference group (aOR: 3.52).39 There are no data to suggest that JAK inhibitors increase the risk of COVID-19 infection and its complications, but more studies are needed to give us a better understanding of any potential risk.25,44
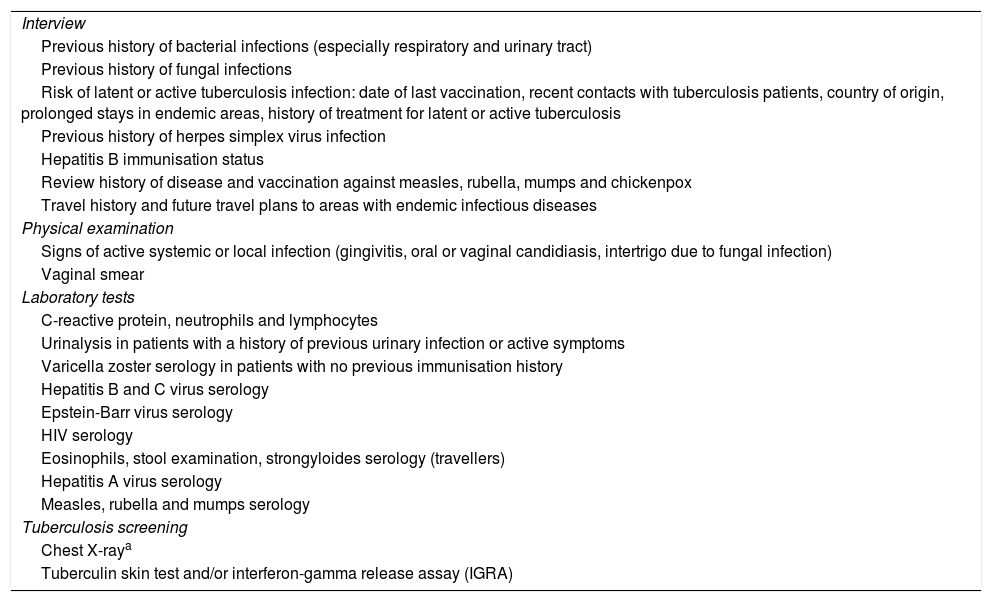
Specific prevention measures and vaccines in immune-mediated diseasesWhen IBD or RA are diagnosed, a series of key aspects need to be assessed before starting immunosuppressive therapy6,7 (Table 1). Vaccination status and screening for latent infections (Table 1) at the time of diagnosis will determine what prevention measures are necessary.6,45
Safety screening and vaccination before starting immunosuppressive therapy.
| Interview |
| Previous history of bacterial infections (especially respiratory and urinary tract) |
| Previous history of fungal infections |
| Risk of latent or active tuberculosis infection: date of last vaccination, recent contacts with tuberculosis patients, country of origin, prolonged stays in endemic areas, history of treatment for latent or active tuberculosis |
| Previous history of herpes simplex virus infection |
| Hepatitis B immunisation status |
| Review history of disease and vaccination against measles, rubella, mumps and chickenpox |
| Travel history and future travel plans to areas with endemic infectious diseases |
| Physical examination |
| Signs of active systemic or local infection (gingivitis, oral or vaginal candidiasis, intertrigo due to fungal infection) |
| Vaginal smear |
| Laboratory tests |
| C-reactive protein, neutrophils and lymphocytes |
| Urinalysis in patients with a history of previous urinary infection or active symptoms |
| Varicella zoster serology in patients with no previous immunisation history |
| Hepatitis B and C virus serology |
| Epstein-Barr virus serology |
| HIV serology |
| Eosinophils, stool examination, strongyloides serology (travellers) |
| Hepatitis A virus serology |
| Measles, rubella and mumps serology |
| Tuberculosis screening |
| Chest X-raya |
| Tuberculin skin test and/or interferon-gamma release assay (IGRA) |
Source: Rahier et al.7.
Chest X-ray will only be performed in the case of a positive screening test, either the interferon-gamma release assay or the tuberculin skin test (Riestra et al.81).
Vaccination should be planned from diagnosis, but that is not possible, before starting immunosuppressive therapy. However, if treatment is urgent it should not be delayed in order to vaccinate.46 The recommendation is to follow an age-appropriate vaccination schedule and heed local guidelines.46 Vaccination status according to age should be checked, ensuring immunity against measles, rubella, mumps and varicella before starting treatment.46 Tetanus, diphtheria, pertussis, polio and, more recently, human papillomavirus in girls are mandatory routine vaccinations in the vaccination schedule from childhood.46
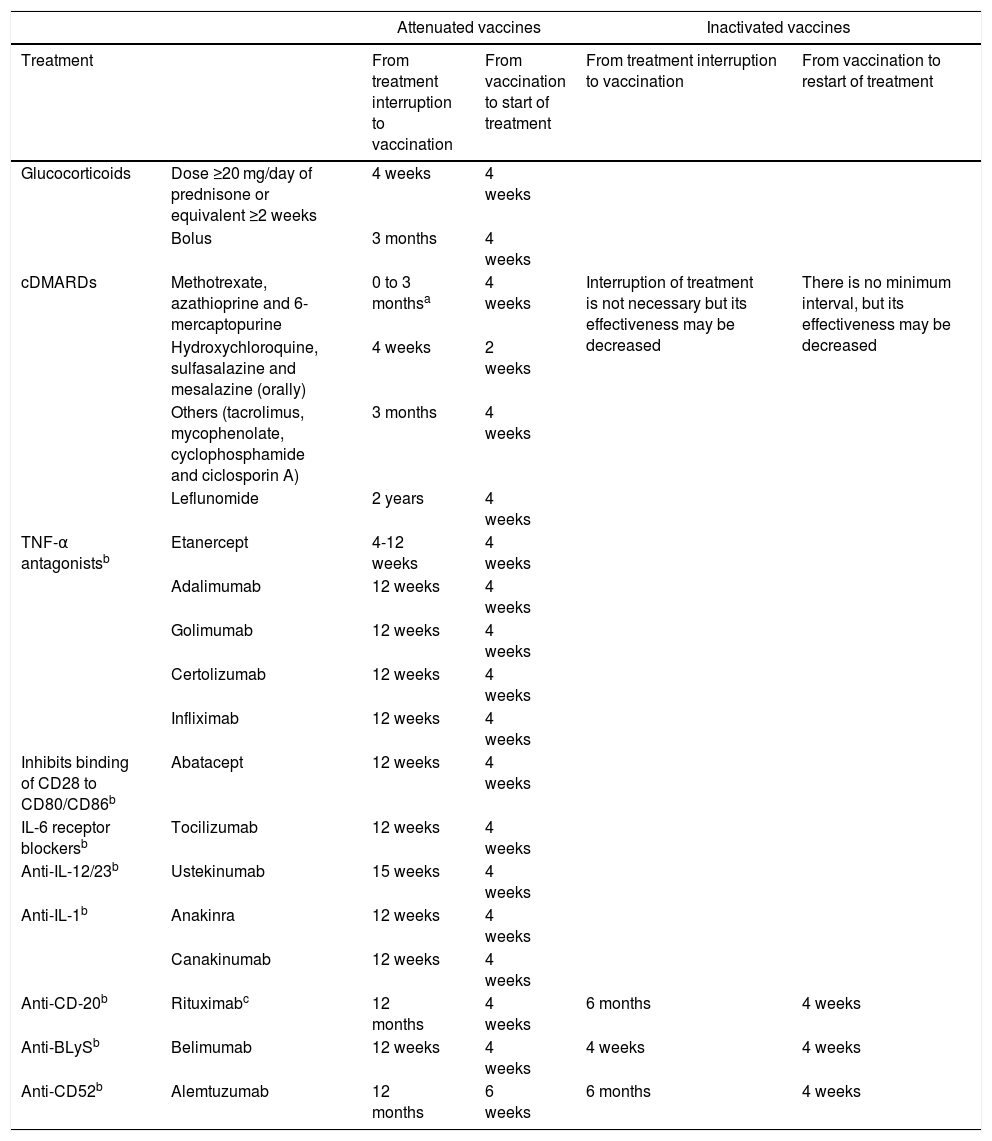
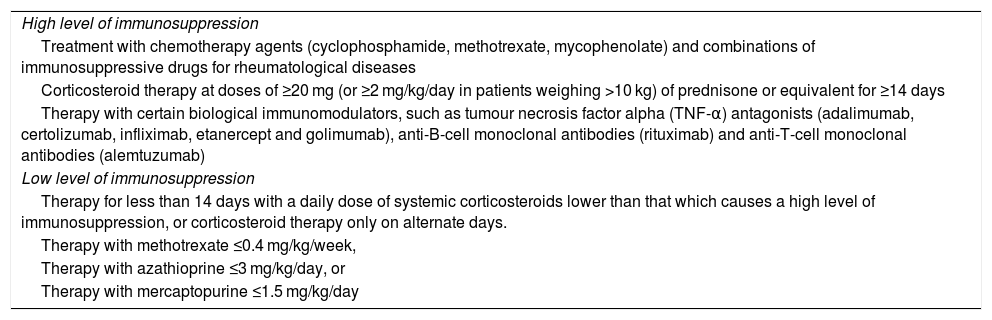
If the patient is already receiving treatment, it has to be taken into account that the level of immunosuppression may reduce the response to the vaccine; it is also important to consider the type of vaccine, whether it is inactive or attenuated4,5,46 (Table 2). Immunosuppression can be classified as high level or low level5,46 (Table 3).
Intervals for administering vaccines according to immunosuppressive therapy*.
| Attenuated vaccines | Inactivated vaccines | ||||
|---|---|---|---|---|---|
| Treatment | From treatment interruption to vaccination | From vaccination to start of treatment | From treatment interruption to vaccination | From vaccination to restart of treatment | |
| Glucocorticoids | Dose ≥20 mg/day of prednisone or equivalent ≥2 weeks | 4 weeks | 4 weeks | ||
| Bolus | 3 months | 4 weeks | |||
| cDMARDs | Methotrexate, azathioprine and 6-mercaptopurine | 0 to 3 monthsa | 4 weeks | Interruption of treatment is not necessary but its effectiveness may be decreased | There is no minimum interval, but its effectiveness may be decreased |
| Hydroxychloroquine, sulfasalazine and mesalazine (orally) | 4 weeks | 2 weeks | |||
| Others (tacrolimus, mycophenolate, cyclophosphamide and ciclosporin A) | 3 months | 4 weeks | |||
| Leflunomide | 2 years | 4 weeks | |||
| TNF-α antagonistsb | Etanercept | 4-12 weeks | 4 weeks | ||
| Adalimumab | 12 weeks | 4 weeks | |||
| Golimumab | 12 weeks | 4 weeks | |||
| Certolizumab | 12 weeks | 4 weeks | |||
| Infliximab | 12 weeks | 4 weeks | |||
| Inhibits binding of CD28 to CD80/CD86b | Abatacept | 12 weeks | 4 weeks | ||
| IL-6 receptor blockersb | Tocilizumab | 12 weeks | 4 weeks | ||
| Anti-IL-12/23b | Ustekinumab | 15 weeks | 4 weeks | ||
| Anti-IL-1b | Anakinra | 12 weeks | 4 weeks | ||
| Canakinumab | 12 weeks | 4 weeks | |||
| Anti-CD-20b | Rituximabc | 12 months | 4 weeks | 6 months | 4 weeks |
| Anti-BLySb | Belimumab | 12 weeks | 4 weeks | 4 weeks | 4 weeks |
| Anti-CD52b | Alemtuzumab | 12 months | 6 weeks | 6 months | 4 weeks |
Source: * https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/VacGruposRiesgo/docs/Inmunodeficiencias.pdf.
Tofacitinib: vaccination with live vaccines should be done at least two weeks (although four is preferable) before the start of treatment with tofacitinib, or according to the current vaccination guidelines in relation to immunomodulatory drugs**.
Vedolizumab: live vaccines, particularly oral live vaccines, should be used with caution when co-administered with vedolizumab***.
** Tofacitinib summary of product characteristics.
*** Vedolizumab summary of product characteristics.
It is not necessary to apply these intervals in patients with a low level of immunosuppression: methotrexate ≤0.4 mg/kg/week; azathioprine ≤3 mg/kg/day; 6-mercaptopurine ≤1.5 mg/kg/day; or corticosteroids at non-immunosuppressive doses.
There is insufficient evidence on the intervals between cessation of immunosuppressive therapy and vaccination. The recommendations are based on expert opinions and on the pharmacological properties of the different immunosuppressants, elimination half-life and residual effect on the immune system.
Treatments and immunosuppression.
| High level of immunosuppression |
| Treatment with chemotherapy agents (cyclophosphamide, methotrexate, mycophenolate) and combinations of immunosuppressive drugs for rheumatological diseases |
| Corticosteroid therapy at doses of ≥20 mg (or ≥2 mg/kg/day in patients weighing >10 kg) of prednisone or equivalent for ≥14 days |
| Therapy with certain biological immunomodulators, such as tumour necrosis factor alpha (TNF-α) antagonists (adalimumab, certolizumab, infliximab, etanercept and golimumab), anti-B-cell monoclonal antibodies (rituximab) and anti-T-cell monoclonal antibodies (alemtuzumab) |
| Low level of immunosuppression |
| Therapy for less than 14 days with a daily dose of systemic corticosteroids lower than that which causes a high level of immunosuppression, or corticosteroid therapy only on alternate days. |
| Therapy with methotrexate ≤0.4 mg/kg/week, |
| Therapy with azathioprine ≤3 mg/kg/day, or |
| Therapy with mercaptopurine ≤1.5 mg/kg/day |
Source: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/VacGruposRiesgo/docs/Inmunodeficiencias.pdf.
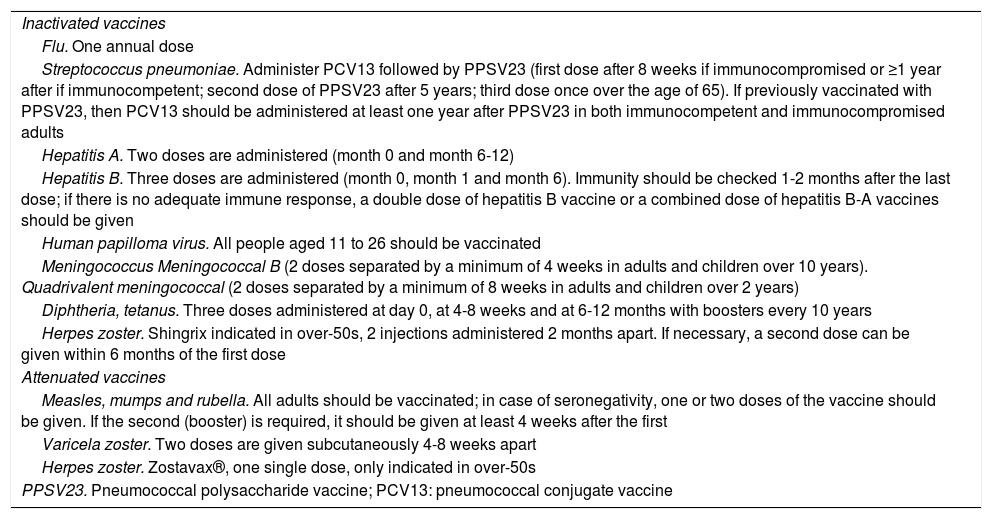
Live vaccines (Table 4) are contraindicated in patients receiving immunosuppressive therapy and should be administered at least four weeks before the start of treatment or following a period of time after the end of treatment (Table 2). In the case of tofacitinib, it may be given two weeks beforehand, but four weeks is preferable.38
Inactivated and live vaccines.
| Inactivated vaccines |
| Flu. One annual dose |
| Streptococcus pneumoniae. Administer PCV13 followed by PPSV23 (first dose after 8 weeks if immunocompromised or ≥1 year after if immunocompetent; second dose of PPSV23 after 5 years; third dose once over the age of 65). If previously vaccinated with PPSV23, then PCV13 should be administered at least one year after PPSV23 in both immunocompetent and immunocompromised adults |
| Hepatitis A. Two doses are administered (month 0 and month 6-12) |
| Hepatitis B. Three doses are administered (month 0, month 1 and month 6). Immunity should be checked 1-2 months after the last dose; if there is no adequate immune response, a double dose of hepatitis B vaccine or a combined dose of hepatitis B-A vaccines should be given |
| Human papilloma virus. All people aged 11 to 26 should be vaccinated |
| Meningococcus Meningococcal B (2 doses separated by a minimum of 4 weeks in adults and children over 10 years). Quadrivalent meningococcal (2 doses separated by a minimum of 8 weeks in adults and children over 2 years) |
| Diphtheria, tetanus. Three doses administered at day 0, at 4-8 weeks and at 6-12 months with boosters every 10 years |
| Herpes zoster. Shingrix indicated in over-50s, 2 injections administered 2 months apart. If necessary, a second dose can be given within 6 months of the first dose |
| Attenuated vaccines |
| Measles, mumps and rubella. All adults should be vaccinated; in case of seronegativity, one or two doses of the vaccine should be given. If the second (booster) is required, it should be given at least 4 weeks after the first |
| Varicela zoster. Two doses are given subcutaneously 4-8 weeks apart |
| Herpes zoster. Zostavax®, one single dose, only indicated in over-50s |
| PPSV23. Pneumococcal polysaccharide vaccine; PCV13: pneumococcal conjugate vaccine |
Inactivated vaccines are safe and are not contraindicated in patients receiving immunosuppressive therapy (Table 4).46 These include the trivalent vaccines against influenza, pneumococcal (PCV13, PPSV23), hepatitis A, hepatitis B, Haemophilus influenzae B, human papilloma virus and tetanus.4 Inactivated flu and pneumococcal vaccines are the only ones generally recommended in people with immunodeficiency.46 In general, to increase its effectiveness it is recommended to immunise at least two weeks before treatment or three months after completion, except for rituximab, which is recommended four weeks before treatment and six months after completion.46 It is recommended that people who live with immunosuppressed IBD patients are also vaccinated against influenza, mumps, measles, rubella and chickenpox.11
Influenza virusInfluenza is a seasonal respiratory disease that causes increased morbidity in immunosuppressed patients.47 Patients with IBD or RA infected with the flu virus are more likely to suffer complications such as hospitalisation or pneumonia.4,45
The flu vaccine is an inactivated trivalent vaccine that contains particles of all three strains of the influenza virus: two A strains (H1N1 and H3N2) and one B strain.1 A quadrivalent formulation that protects against an additional B strain has recently been marketed.45 Published data indicate that the immune response to the flu vaccine is not altered by the use of anti-TNFα,48 tocilizumab49 or tofacitinib,50 while there are no conclusive data regarding MTX48 or abatacept.51 However, the response seems to be clearly decreased in patients treated with rituximab, or with combined therapy with thiopurines and anti-TNF-α.4
Despite the fact that the influenza vaccine is safe1,45 and generates an adequate humoral response, the vaccination rate among patients with IBD or immune-mediated diseases is lower than expected.4,8,9 It is therefore necessary to strongly encourage annual vaccination in patients with IBD or rheumatological diseases. The trivalent inactivated vaccine should be used following the usual immunisation schedule,1,4,45 and the inhaled live attenuated vaccine is contraindicated in patients receiving immunosuppressive therapy.4,45
Streptococcus pneumoniaeStreptococcus pneumoniae is the main cause of pneumonia and meningitis in Western countries.45 The risk of pneumococcal infection is significantly higher in patients with RA45,52 or IBD.4,53 Like influenza, the Streptococcus pneumoniae vaccination rate is suboptimal, with data from the COMORA study showing that only 17% of RA patients had been immunised.9
There are currently two variants available: the pneumococcal polysaccharide vaccine (PPSV23) and the pneumococcal conjugate vaccine (PCV13).45 The humoral response to PPSV23 is not significantly reduced by certolizumab, ustekinumab, tocilizumab, anti-TNF-α45,48 or vedolizumab,54 while the response may be decreased with tofacitinib, particularly in the group treated with tofacitinib and MTX, although the clinical impact of these findings is not known.37 In patients treated with MTX and rituximab, the response is markedly reduced.45,48 Concomitant use of thiopurines and anti-TNF-α also seems to reduce the response to the vaccine.4
In the case of adult patients receiving immunosuppressive therapy who have not previously been immunised with PPSV23 or PCV13, it is recommended to administer the two available pneumococcal vaccines sequentially and starting with the conjugate one in order to maximise serotype coverage (Table 4).4,7,55
Herpes zosterThe appearance of herpes zoster (HZ) is the result of the reactivation of the varicella zoster virus which lies latent in the dorsal root ganglia.47 Patients with IBD and RA have a higher risk of HZ infection than the general population.4,56
A recent systematic review and meta-analysis57 showed that, compared to the controls, there was a higher risk of HZ infection with tofacitinib therapy (OR: 2.16; 95% CI: 0.84-5.58), non-anti-TNF-α biologics (OR: 2.19; 95% CI: 1.20-4.02) and combined therapy with thiopurines and anti-TNF-α (OR: 3.29; 95% CI: 2.33-4.65). In patients with RA, a study based on data from routine clinical practice showed that the incidence of HZ was higher in patients treated with tofacitinib than in those receiving biologics.56
The increased risk of HZ in patients treated with tofacitinib is dose-dependent.57,58 In patients with RA on tofacitinib, the risk factors related to HZ were the use of glucocorticoids, being of Asian origin (incidence more than double that observed in Europe) and being a non-smoker or ex-smoker.59 The risk was similar with baricitinib.58 However, in the clinical development programme in UC, only age (increase for every 10 years, HR: 1.28; 95% CI: 1.34-1.87; p < 0.0001) and previous failure of anti-TNF-α (p < 0.05) were found to be independent risk factors for HZ infection.60
In patients with UC treated with tofacitinib, data from the integrated safety analysis up to 6.8 years25 show an incidence rate for HZ of 3.48 cases per 100 patient years. Most of the cases were considered mild or moderate (92.1%), and there were only seven cases of disseminated HZ and four of postherpetic neuralgia.25 The condition resolved in 92.4% of the cases, and in most cases without the need to stop tofacitinib (70.1%), although it was decided to temporarily withdraw the tofacitinib in 18 patients (20.7%).25
Winthrop et al.59 analysed the risk of HZ in 6,192 RA patients (16,839 patient years) included in 19 studies with tofacitinib. A total of 636 (10%) patients developed HZ. Most of the cases (93%) were mild, and in 94% (597) only one dermatome was affected.59 In the integrated safety analysis at 9.5 years in patients treated with tofacitinib, the incidence rate for associated HZ per 100 patient years was 3.6 (95% CI: 3.4-3.9).33 Among patients with RA treated with baricitinib 4 mg, a safety analysis at two years34 found a higher rate of HZ compared to placebo (1.8% vs 0.4% of patients).34 In the systematic review and meta-analysis by Bechman et al.,32 the incidence of HZ per 100 patient years was 2.51 (95% CI: 1.87-3.30) in the case of tofacitinib and 3.16 (95% CI: 2.07-4.63) for baricitinib.32
HZ infection could be preventable through appropriate vaccination. There are two types of vaccine currently available. The first is the live attenuated vaccine, which has been available for more than a decade and is approved for use in adults over the age of 50. It is a single dose and requires a booster dose at five years. The recommendation is for it to be given prior to the start of treatment or in patients with a low level of immunosuppression.45,61 As an alternative, there is an inactivated recombinant vaccine available, consisting of glycoprotein E, which has been shown to reduce the risk of HZ and postherpetic neuralgia in adults with a higher efficacy than the live-attenuated vaccine, reaching prevention rates of 97% in over 50s.62,63 Preliminary results from phase 3 studies indicate that recombinant HZ vaccine may be immunogenic in immunocompromised adults, including patients with blood cancers, those with solid tumours receiving immunosuppressive chemotherapy, kidney transplant recipients on long-term immunosuppressive therapy, and recipients of autologous haematopoietic stem cell transplants. These results are supported by those of phase 1/2 studies in patients with HIV infection.64
According to the US and Canadian guidelines, the recombinant vaccine is not contraindicated in immunocompromised individuals, and is preferred to a live attenuated HZ vaccine in immunocompetent people.64
This vaccine is recommended by the Advisory Committee on Immunization Practices,65 and could be the best strategy in patients being given immunosuppressive therapy, especially those on treatment with JAK inhibitors.18,46 The recombinant HZ vaccine is not currently marketed here in Spain.
Hepatitis B and hepatitis AIt is considered advisable to assess individual risk and request serological determinations before proceeding with vaccination. Various studies have demonstrated the risk of reactivation of hepatitis B virus (HBV) and its serious consequences in patients with IBD and RA after treatment with immunosuppressive drugs.4,66 The risk and reactivation kinetics differ according to the immunosuppressive therapy used and the status of the infection, so it is essential to stratify the individual risk in order to adapt preventive measures.67
It is known that the greatest risk occurs during treatment with anti-TNF-α and rituximab.45 It has recently been reported that there is also a risk of reactivation in patients with positive HBV surface antigen (HBsAg) treated with abatacept, tocilizumab, tofacitinib and ustekinumab,45,68,69 but not in patients with resolved hepatitis B.70
It is recommended to screen all patients for infection status using serological markers (HBsAg, total core antibody [HBcAb] and surface antigen antibody [HBsAb]) before starting immunosuppressive therapy, and to vaccinate those without evidence of infection but who are at risk.4,7,45,67 In patients who are HBsAg-positive, the viral load should also be determined.7 Antiviral therapy (entecavir or tenofovir) is indicated in seropositive IBD patients with chronic infection (HBsAg+) who need to start biologics or JAK inhibitors, which should be introduced at least two weeks beforehand, and continued for 12 months after these have been withdrawn.7 In the case of seropositive patients with evidence of past infection (HBsAg-, HBcAb+ with or without HBsAb), HBV reactivation in IBD occurs very rarely, so routine prophylaxis is not recommended.7,67 It is recommended in the case of patients with immune-mediated diseases treated with rituximab.71 Transaminase (ALT) and HBV DNA monitoring should be performed for those who are HBsAg-positive and have occult HBV infection. In patients with HBsAb-positive resolved HBV infection, periodic monitoring of ALT levels will continue to be necessary, especially in areas endemic for HBV.67
In healthy individuals, the HBV vaccine is 95% effective.4 However, in individuals with IBD, the range of protection is reduced to 33-76%,72 which is why more intensive vaccination strategies are considered necessary.4,47 The administration of a double dose of the vaccine in three doses (0, 1 and 2 months), followed, in the event of insufficient serological response, by re-vaccination again at double dose with the same schedule, appears to be more effective than the vaccination regimen recommended for the general population.7
Data on the impact of treatments on the HBV vaccine are limited, but seem to indicate that anti-TNF-α and rituximab may reduce the humoral response,45 while vedolizumab does not affect its efficacy.73
TuberculosisTuberculosis (TB) is an infection caused by Mycobacterium tuberculosis which, after primary infection, can persist in an inactive latent state (latent TB infection, LTBI). In this state, the infection is asymptomatic, but there is a risk of reactivation depending on the immune status of the patient.74 In Spain, over 10% of patients who are candidates for treatment with biologics have LTBI.75,76 Anti-TNF-α therapy is associated with a two-to-four-fold increase in the risk of reactivation of a latent TB, although this risk is lower with etanercept.77 In a pooled analysis of clinical trials and registration studies, Zhang et al.78 found an incidence rate for tuberculosis per 100 person years of 0.08 for tocilizumab, 0.09 for abatacept, 0.2 for tofacitinib and 0.0-0.47 for anti-TNF. Baricitinib had an incidence rate of 0.15.34 The incidence rate with vedolizumab in clinical trials was also low, at 0.1.79 Experience with ustekinumab is more limited, but so far few cases of TB have been reported with this treatment.80
Recommendations for screening for and treatment of LTBI before administering immunosuppressive therapy have reduced the risk of active tuberculosis by 78-90%.76 In Spain,74 two techniques are recommended for the diagnosis of LTBI: the interferon-gamma release assay (IGRA) and the tuberculin skin test (TST). A positive result in either is considered indicative of LTBI.74 Any history of TB should also be assessed.7,81 Chest X-ray will only be performed in the case of a positive screening test, be it the interferon-gamma release assay or the tuberculin skin test.81 A recent study in Spain revealed a low degree of adherence to these recommendations (56%), where only 36% of those surveyed requested the recommended diagnostic tests.76 More widespread adherence to the recommendations would most likely contribute to further reduce the incidence of tuberculosis in patients who are candidates for biological therapies and JAK inhibitors.76
In the case of LTBI, it is recommended to delay biological treatment for at least three weeks after starting anti-tuberculosis treatment, except in cases of clinical emergency and on specialist advice.7 Adequate treatment of active TB is defined as six months or more of first-line drug therapy, and includes two or more months with the combination of rifampicin + isoniazid + pyrazinamide + ethambutol.74 Latent infection can be adequately treated with nine months of isoniazid (most common regimen), three months of isoniazid + rifampicin, or four months of rifampicin alone.74 It should be noted that rifampicin is an inducer of CYP450 3A4 and could reduce exposure to various different drugs, including tofacitinib, and decrease their efficacy.78
ConclusionsPatients with IBD or RA are at increased risk of developing infections. The risk of serious and opportunistic infections is especially increased during immunosuppressive therapy. In our setting, some of the strategies in place for these patients, such as the vaccination rate and screening for latent tuberculosis, have room for improvement. It is therefore recommended that vaccination status be assessed at diagnosis and the necessary vaccines administered, and that patients be screened for infections and latent tuberculosis treated before starting immunosuppressive therapy.
Conflicts of interestRosario García-Vicuña has received training and research grants from Abbvie, BMS, Janssen Lilly, Novartis, MSD, Roche and Sanofi; fees for participating in expert committees from Abbvie, Biogen, BMS, Celltrion, Mylan, Pfizer, Roche, Sandoz and Sanofi; has given presentations sponsored by BMS, Lilly, Pfizer, Sandoz and Sanofi; and has received non-financial aid from Abbvie, BMS, Lilly, MSD, Novartis, Pfizer and Sanofi.
Xavier Calvet has received research grants from Abott, MSD and Vifor; fees for participating in expert committees from Abott, MSD, Takeda, Pfizer, Janssen and Vifor; and speaker fees from Abbott, MSD, Janssen, Pfizer, Takeda and Allergan.
Jordi Gratacós has received research grants from Pfizer and fees for participating in expert committees or giving presentations from Pfizer, Novartis, Janssen, Amgen, MSD, Abbvie and Lilly.
Daniel Carpio has received fees for giving talks and presentations and preparing training material from Abbvie, Amgen, Ferring, Janssen, Kern, MSD, Pfizer and Takeda; for participating in expert committees from Abbvie, Amgen, Celltrion, Dr Falk, Janssen, MSD, Pfizer, Takeda and Tillots; research grants from Janssen, and to support the Department/Hospital/Institution from Abbvie and MSD. The other authors have no conflicts of interest to declare.
FundingThis manuscript has been funded by Pfizer España.
The authors would like to thank Dr Guillermo Bastida Paz of Hospital Universitario la Fe, Dr Elisa Trujillo Martin of Hospital Universitario de Canarias, Dr Marta Carrillo Palau of Hospital Universitario de Canarias, Dr Eva Pérez Pampin of Hospital Clínico Universitario de Santiago de Compostela, Dr M. Luz García Vivar of Hospital de Basurto, Dr Luis Alberto Menchen Viso of Hospital Gregorio Marañon and Dr Dolores Martin Arranz of Hospital La Paz for their participation on the panel of experts; Mónica Valderrama, M. del Pilar Fortes, Ana Cábez and Susana Gómez from Pfizer for carrying out the literature search and reviewing the manuscript; and Esther Tapia for her help in writing the manuscript.
Please cite this article as: Calvet X, Carpio D, Rodríguez-Lago I, García-Vicuña R, Barreiro-de-Acosta M, Juanola X, et al. Riesgo de infección asociado a los inhibidores de las quinasas Janus (JAK) y las terapias biológicas en enfermedad inflamatoria intestinal y artritis reumatoide. Estrategias de prevención. Gastroenterología y Hepatología. 2021;44:587–598.