Colorectal cancer 5-years-survival is 57%, partway due to a low rate of participation in screening programmes. Instruments analyzing causes of low adherence are needed.
ObjectiveTo evaluate the validity and internal consistency of the Spanish version of Rawl’s Questionnaire for the screening of colorectal cancer by faecal occult blood testing.
Type of studyQuestionnaire validation methodology.
LocationThree Primary Care Centres in Valencia.
VariablesAge, sex, civil status, educational level, social class, smoking, alcohol consumption, Body Mass Index, personal and family history of cancer.
ResultsWe analyzed 408 individuals (237 cases and 171 controls). Mean age was 59.45 years (SD 5.17). Internal consistency of all variables reached a Cronbach’s alfa of 0.796. The Cronbach’s alfa benefit dimension of the screening was 0.871 and for the barrier dimension of the screening it was 0.817. Intraclass correlation coefficients of the test-retest for the benefit dimension of the screening was 0.809 (CI 95% 0.606−0.913) and 0.499 (CI 95% 0.126−0.750) for the barrier dimension.
ConclusionThe Spanish version of Rawl’s Questionnaire is valid, reliable and reproducible, so we have this validated instrument with which to identify barriers and benefits in a colorectal screening programme in Spain.
La supervivencia del cáncer colorrectal es del 57% a los 5 años, en parte debido a un diagnóstico tardío por una baja participación en los programas de cribado. Son necesarios instrumentos que analicen las causas de participación.
ObjetivoComprobar la validez y consistencia interna de la versión en castellano del cuestionario de Rawl para el cribado de cáncer colorrectal con sangre oculta en heces.
Tipo de estudioMetodología de validación de cuestionarios.
LocalizaciónTres centros de salud de Valencia.
VariablesEdad, sexo, estado civil, nivel de estudios, clase social, consumo de tabaco, alcohol, índice de masa corporal, antecedentes personales y familiares de cáncer.
ResultadosSe estudiaron 408 individuos (237 casos y 171 controles). La edad media fue de 59.45 (DE 5.17) años. La consistencia interna de todas las variables alcanzó una alfa de Cronbach de 0.796. El alfa de Cronbach de la dimensión beneficios del cribado fue de de 0.871 y para la dimensión barreras al cribado fue de 0.817. Los coeficientes de correlación intraclase del test-retest para la dimensión de los beneficios del cribado fue de 0.809 (IC 95% 0.606−0.913) y de 0.499 (IC 95% 0.126−0.750) para las barreras.
ConclusiónLa versión en castellano del cuestionario Rawl es válido, fiable y reproducible. Con lo que disponemos de un elemento validado en España con el que objetivar barreras y beneficios percibidos en un programa de cribado poblacional.
Colorectal cancer (CRC) is the second leading cause of cancer deaths in Spain, with more than 15,000 deaths per year.1 It is also the type of cancer with the second-highest incidence after prostate cancer in men and after breast cancer in women. However, if no distinction between the sexes is made, it occupies the top spot, with an estimated 44,231 new cases in 2020.2
Mean CRC five-year survival in Spain is just 57%. The survival of patients with CRC detected in a screening programme is higher than that of patients diagnosed due to symptoms.3 In screening for the average risk population (individuals over 50 years of age with no additional risk factors), the strategy used is biannual faecal occult blood testing (FOBT).4 Although screening programmes have near-universal coverage in Spain, participation in them remains below 50%, and most cases of CRC in Spain continue to be diagnosed outside of screening programmes.5
There are three groups of factors associated with adherence: those related to screening programme organisation,6 those related to social factors7 and those dependent on the outlook of the subject. In the latter group of factors, various theoretical models have been adopted in an attempt to understand the subjective elements of an individual that influence his or her participation. The most commonly studied is the Health Belief Model (HBM) described by Rosenstock.8,9
This model sets out several cognitive concepts that predict behaviour in preventive activities: perceived susceptibility, perceived severity, self-efficacy, benefits and barriers.10 With regard to CRC, Jacobs adapted an HBM-based questionnaire initially developed by Champion for breast cancer screening to colorectal cancer screening.11 In the United States, Rawl validated a specific questionnaire for each screening test: FOBT, sigmoidoscopy and colonoscopy.12 Rawl's HBM-based questionnaire was validated in several countries and various populations, and is the most widely used in the literature.13–15
At present, there are limited data on the factors that may promote or limit participation in a CRC population screening programme in Spain. It is essential to have instruments that reliably measure these variables. Therefore, the objective of this study was to confirm the validity and consistency of the Spanish version of Rawl’s questionnaire for CRC screening with immunological FOBT.
Material and methodsThis was a questionnaire validation study within a larger case-control study. It was conducted at the Chile, República Argentina and Serrería II health centres in the city of Valencia. For an expected prevalence of low social support of 30%, a 95% confidence interval, a power of 80% and an odds ratio of 2, the required number of individuals to be included in the study was 404. Hence, simple random sampling was done, trusting that similar numbers of cases and controls would be found, given the rate of participation in these screening programmes is around 50%.
The inclusion criterion was having been invited to take part in the Colorectal Cancer Screening Programme in the Valencian Community. All individuals 50–69 years of age with no symptoms and no ongoing exclusion criteria for participating in CRC screening programmes (personal history of CRC, inflammatory bowel disease, colorectal polyposis, colorectal adenoma, history of colectomy, serious comorbidity or family history of familial adenomatous polyposis or other hereditary syndromes, hereditary colorectal cancer not associated with polyposis, two or more first-degree relatives with CRC, or one first-degree relative with CRC diagnosed before 60 years of age) are invited to participate in this programme.
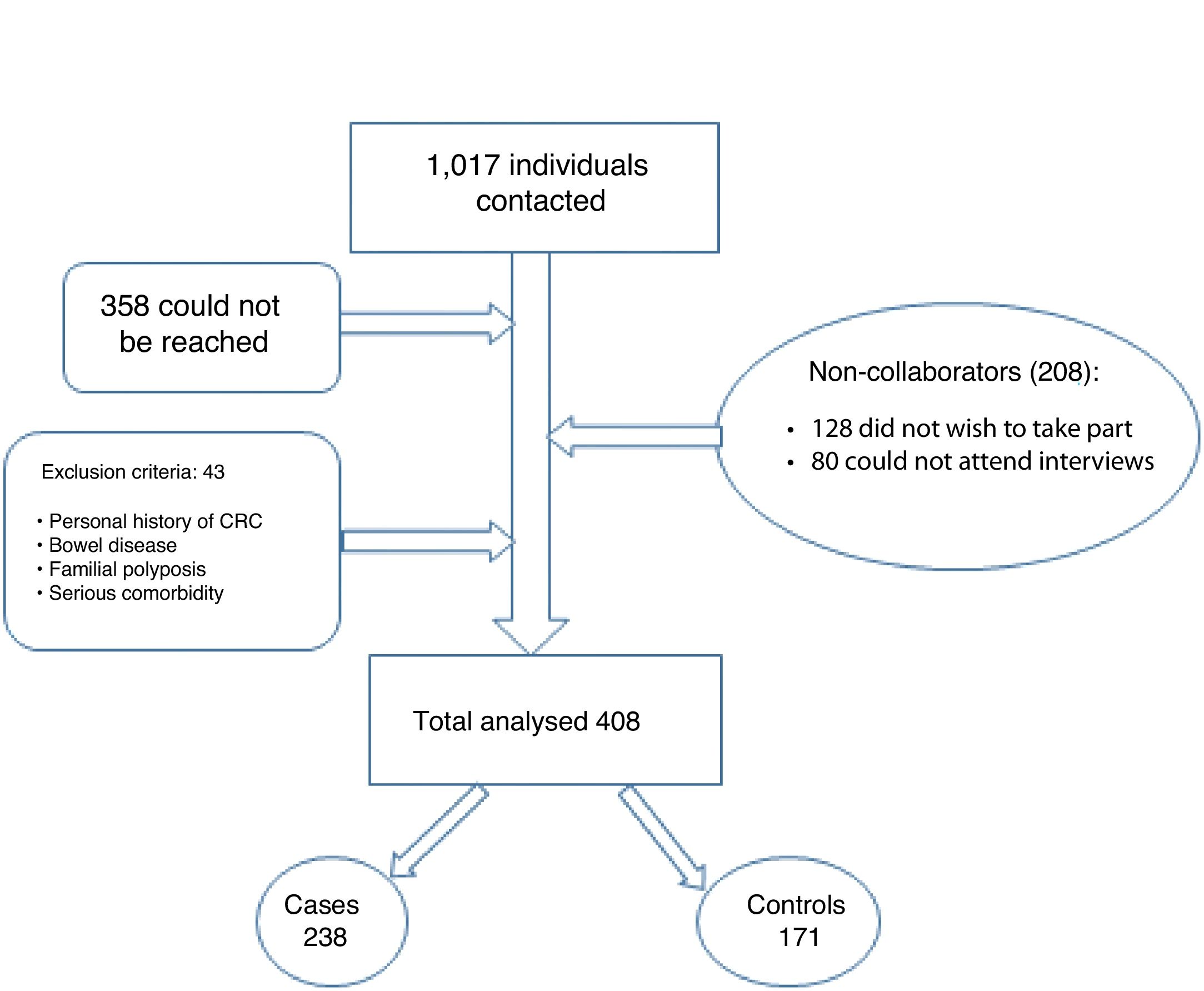
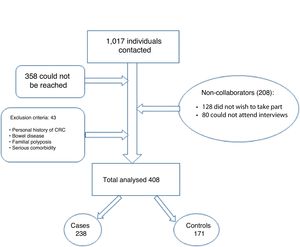
As Fig. 1 shows, between March and September 2019, we contacted 1017 patients by telephone. Of them, 358 could not be reached, 128 did not wish to participate and 80 could not participate as they could not attend interviews. Forty-three patients did not meet the inclusion criteria. In the end, we included a total of 408 patients in the study, divided into 237 cases and 171 controls. These patients were interviewed by an investigator with prior training.
“Cases” were defined as individuals who participated in at least one round of the colorectal cancer screening programme. “Controls” were defined as individuals who did not submit a faecal sample after being invited to take part.
Rawl's questionnaire for colorectal cancer screening is based on the theoretical human behaviour model termed HBM. The 2010 version of the questionnaire consists of 12 items that evaluate the benefits and barriers to screening with FOBT using a Likert scale.16 The questionnaire is self-administered and takes approximately 10 min to complete.
Translation/backtranslationThe instrument's author was asked for permission prior to its translation and validation. The questionnaire was translated by two bilingual individuals, yielding a first version. This version was backtranslated by two other independent bilingual individuals who produced a preliminary version. Finally, after peer reviews, the definitive version of the questionnaire was established by consensus of the entire team. This version was used to conduct a pilot with 10 patients to ensure the comprehensibility and viability of the final questionnaire (Appendix B).
Construct validityAfter it had been confirmed that the assumptions of the factorial analysis were met, the value of the Kaiser–Meyer–Olkin (KMO) statistic was 0.818 and Bartlett’s test of sphericity showed statistical significance (p < 0.001), the exploratory analysis identified two dimensions of the questionnaire that accounted for 52.25% of the total variability (19.43% and 32.82%, respectively).
The confirmatory factor analysis used maximum likelihood estimation. The score for each dimension was calculated by finding the sum of the component items, with greater perceived benefits and barriers corresponding to higher scores. The ceiling effect (number of responses with the highest possible score) and floor effect (number of responses with the lowest possible score) were calculated as well.
Reliability analysisTo analyse the internal consistency of the instrument, Cronbach’s alpha coefficient was calculated for each of the dimensions identified in the questionnaire and for all of them together. Correlation coefficients less than 0.1 were discarded, and mean and variance were calculated if the item was eliminated. Cronbach's alpha values of 0.7 and higher were also considered acceptable.
Test/retestTo observe the stability of the questionnaire over time, 23 individuals were selected to repeat the test after 15 days, and the intra-class correlation coefficient was calculated for each dimension.
Descriptive analysisA descriptive analysis was performed in which categorical variables were summarised in terms of absolute frequency and percentages, and quantitative variables were summarised in terms of mean and standard deviation (SD), median, and interquartile range. All tests were performed using a bilateral approach. A value of p < 0.05 was considered significant.
The program IBM SPSS Statistics for Windows, Version 22.0, Armonk, New York: IBM Corp., and the program Epidat 4.2, Consellería de Sanidade, Xunta de Galicia [Ministry of Health, Regional Government of Galicia], in collaboration with the Pan American Health Organization (PAHO/World Health Organization [WHO]), were used to perform the statistical analysis.
Ethical considerationsThis study was approved by the Independent Ethics Committee of the Dirección General de Salud Pública [General Directorate of Public Health] and the Centro Superior de Investigación en Salud Pública [Greater Centre for Research in Public Health] (CEIDGSP-CSISP) of Valencia, with record number 20190301/04. All participants signed an informed consent form. The project was developed in compliance with the Declaration of Helsinki, the International Guidelines for Ethical Review of Epidemiological Studies, European and Spanish regulations on biomedical research, and European and Spanish regulations on personal data protection (the European General Data Protection Regulation [2016/679; GDPR-2016] and Spanish Organic Law 3/2018, of 5 December, on Personal Data Protection and Guarantee of Digital Rights [LOPDP-2018]).
The investigators signed a confidentiality commitment, and specific measures were adopted to maintain data integrity and safety and prevent access by third parties to any identified or identifiable personal data. No publication or report derived from the study will use or contain identified or identifiable data or images.
The corresponding author, on behalf of the other signatories, guarantees the accuracy, transparency and honesty of the data and information contained in the study, as well as that no relevant information has been omitted, and that all discrepancies between the authors have been adequately resolved and described.
The study was conducted with no external funding.
All authors declare that they have no conflicts of interest.
ResultsThe mean age was 59.45 (SD 5.17) years. 54.20% were women, 72.30% did not smoke and 25.40% did not drink alcohol. The mean body mass index was 26.01 (SD 4.41). A family history of CRC was reported by 20.10%, a family history of other types of cancer was reported by 55.60% and a personal history of cancer other than CRC was reported by 11%.
The questionnaire was self-administered in 87.8% of cases versus 12.20% in which it was guided; we found no statistically significant differences between the two ways of completing the questionnaire. While 58.8% had advanced studies, 22.50% had completed secondary school, 17.60% had primary studies and 1% were illiterate. Of them, 72.10% had a partner and 70.80% were married. Concerning social class, 43.90% were directors or managers, 37.20% had mid-level occupations, 10.30% were skilled workers and 8.60% were unskilled workers.
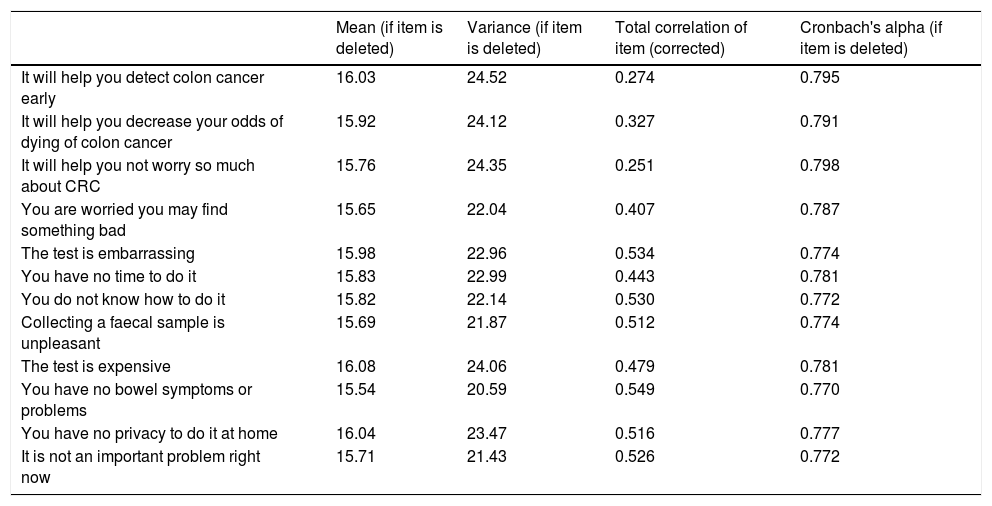
When all grouped variables were analysed, the internal consistency of the questionnaire reached a Cronbach’s alpha of 0.796. Table 1 shows the different correlations that resulted from eliminating each of the instrument’s items in alternation.
Internal consistency of Rawl’s questionnaire for screening with faecal occult blood testing.
| Mean (if item is deleted) | Variance (if item is deleted) | Total correlation of item (corrected) | Cronbach's alpha (if item is deleted) | |
|---|---|---|---|---|
| It will help you detect colon cancer early | 16.03 | 24.52 | 0.274 | 0.795 |
| It will help you decrease your odds of dying of colon cancer | 15.92 | 24.12 | 0.327 | 0.791 |
| It will help you not worry so much about CRC | 15.76 | 24.35 | 0.251 | 0.798 |
| You are worried you may find something bad | 15.65 | 22.04 | 0.407 | 0.787 |
| The test is embarrassing | 15.98 | 22.96 | 0.534 | 0.774 |
| You have no time to do it | 15.83 | 22.99 | 0.443 | 0.781 |
| You do not know how to do it | 15.82 | 22.14 | 0.530 | 0.772 |
| Collecting a faecal sample is unpleasant | 15.69 | 21.87 | 0.512 | 0.774 |
| The test is expensive | 16.08 | 24.06 | 0.479 | 0.781 |
| You have no bowel symptoms or problems | 15.54 | 20.59 | 0.549 | 0.770 |
| You have no privacy to do it at home | 16.04 | 23.47 | 0.516 | 0.777 |
| It is not an important problem right now | 15.71 | 21.43 | 0.526 | 0.772 |
Mean values and variance of the scale if the question is deleted, corrected homogeneity index (corrected total correlation of elements). Cronbach’s alpha if item is deleted.
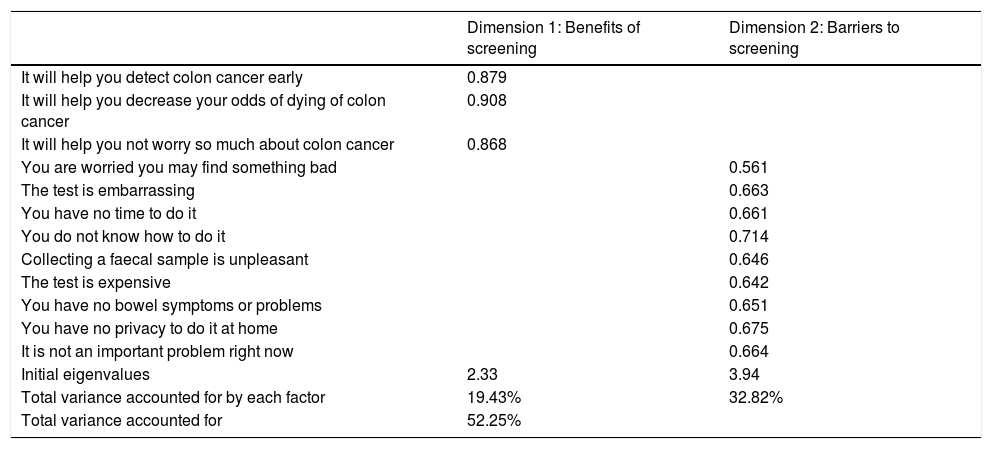
The exploratory factorial analysis identified two dimensions of the questionnaire accounting for 52.25% of the total variability (19.43% and 32.82%, respectively). The value of the KMO statistic was 0.818 and Bartlett’s test of sphericity showed statistical significance (p < 0.001).
Dimension 1 corresponded to the first three questions of the questionnaire, and dimension 2 corresponded to questions 4−12. The internal consistency of each dimension found was calculated. Cronbach's alpha for dimension 1 was 0.871, pointing to the benefits of screening with FOBT. For dimension 2, which examined barriers to screening, Cronbach’s alpha was 0.817 (Table 2).
Validity of Rawl’s questionnaire for colorectal cancer screening: exploratory and confirmatory factorial analysis.
| Dimension 1: Benefits of screening | Dimension 2: Barriers to screening | |
|---|---|---|
| It will help you detect colon cancer early | 0.879 | |
| It will help you decrease your odds of dying of colon cancer | 0.908 | |
| It will help you not worry so much about colon cancer | 0.868 | |
| You are worried you may find something bad | 0.561 | |
| The test is embarrassing | 0.663 | |
| You have no time to do it | 0.661 | |
| You do not know how to do it | 0.714 | |
| Collecting a faecal sample is unpleasant | 0.646 | |
| The test is expensive | 0.642 | |
| You have no bowel symptoms or problems | 0.651 | |
| You have no privacy to do it at home | 0.675 | |
| It is not an important problem right now | 0.664 | |
| Initial eigenvalues | 2.33 | 3.94 |
| Total variance accounted for by each factor | 19.43% | 32.82% |
| Total variance accounted for | 52.25% |
Factors identified, eigenvalues, total variability accounted for.
When the frequency of responses to each question on the questionnaire was analysed according to the dimensions defined, it was found that, for the benefits dimension, the floor effect rate was 3.5% and the ceiling effect rate was 2.5%. For the barriers dimension, 0.2% of the participants had the lowest possible score (floor effect) and 0.2% had the highest possible score (ceiling effect).
To examine the stability of the questionnaire, a test/retest analysis was performed in which the questionnaire was re-administered to 23 patients after 15 days. We found intra-class correlation coefficients of 0.809 (0.606−0.913) for the benefits of screening dimension and 0.499 (0.126−0.750) for the barriers to screening dimension.
DiscussionOur study validated Rawl’s HBM-based scale in Spanish for evaluating patient-perceived benefits and barriers to a CRC screening programme based on immunological FOBT. Determining this is very important for achieving the objective of a higher participation rate of 65% in screening programmes, breaking down barriers and enhancing the benefits perceived by the population.
The first questionnaire that adapted the theoretical model of the HBM to CRC screening was carried out by Jacobs.11 In 2001, Rawl validated it in African American patients16 and subsequently validated a new version in 2010. Most validations published to date have been in populations with low income and low socioeconomic status.16–18 There are some validated studies in manual workers and others in automotive factory employees.19 Validations have been performed in Turkish,13 Chinese14 and Iranian17 populations. Adaptations of the HBM in Korean immigrants in the United States and in African Americans have also been published.18,20,21
Rawl found a 74% rate of family history of CRC in her validation on African American patients;16 in our sample, just 20% of patients had a family history of CRC. We believe that this difference may be due to racial factors, as the black population has the highest incidence of CRC,20 and because Rawl sampled patients who had not participated in the screening programme.
Our sample population was urban and middle- or upper-class, whereas most published studies have been conducted in populations with low income and lower socioeconomic status.16–18 There are also some studies conducted in manual workers.22 The questionnaire has proven to be a good instrument for objectively assessing benefits and barriers to individuals who undergo CRC screening, as it has been validated in populations of different social, cultural and economic backgrounds.
Knowing that the minimum acceptable Cronbach's alpha value is above 0.7, we found an internal consistency of 0.796 when we grouped all variables. We also determined a Cronbach’s alpha of 0.871 and 0.817 in each dimension found (benefits and barriers, respectively). This confers a great deal of reliability on the questionnaire. These values are even better than the values from the initial validation in 2001 (with Cronbach’s alpha values of 0.65 and 0.72) and the 2010 update (with Cronbach’s alpha values of 0.76 and 0.82) which also identified the two dimensions. In our case, these two dimensions accounted for 52.5% of the total variability versus 34% of the variance accounted for in Rawl’s original validation.12
Rawl’s questionnaire was validated in people of different races. Ozsoy et al. conducted a validation in a Turkish population, yielding a Cronbach’s alpha of 0.58–0.88.13 Leung adapted the questionnaire to a Chinese population and found a Cronbach’s alpha of 0.74–0.88.14 There is another validation in Taiwan.23 Adaptations in Korean immigrants in the United States and in African Americans have also been published.21 There is a Persian version with good reliability data.17 Recently, Tahmasebi et al. found a Cronbach’s alpha of 0.78 when they adapted the scale to an Iranian population.15 In Spain, we did not find any HBM-based questionnaires validated for CRC screening, although we did find some for breast cancer screening.24 There is a questionnaire in Spanish applied to CRC screening, but it is based on the Social Determinants of Health theoretical model and consists of 23 items obtained following qualitative studies, and we did not find any evidence of its validation in the literature.25,26
There are other models of validated questionnaires on CRC screening to determine peoples' attitudes and knowledge, but they are less widely used than those based on the theoretical model of the HBM.27
To interpret questionnaire reproducibility, intra-class correlation coefficient values above 0.4 are considered appropriate;28 in our case, the values for the two dimensions exceeded that threshold. The reproducibility data from the validations in Turkey,13 Iran17 and Taiwan23 were similar. Hence, it can be affirmed that the measurement instrument is stable over time.
Our study contributes new knowledge and a transcultural perspective, in a seldom-studied sector of the population, to objectively measure patient-perceived barriers and benefits to CRC screening. This is key to personalising and prioritising measures on the part of the health authorities in order to achieve increased participation in screening programmes.
The limitations of our study include the fact that we analysed the behaviour of patients at average risk of CRC only. The results of our study cannot be extrapolated to institutionalised or hospitalised populations, as it was conducted in primary care. They also cannot be extrapolated to patients at high risk of CRC or to patients screened using techniques other than FOBT. We did not include the income levels of the patients as other authors have done,14,16,20 as income levels have important implications in health systems in which the patients must cover the cost of the test, but not in the Spanish health system. In addition, some authors have developed more specific instruments for investigating determining factors such as privacy, community beliefs and embarrassment by the test that are not included in the instrument that we validated.29,30 In addition, the population in our study that could not be reached must be borne in mind, as it could represent selection bias, although we did not find any differences with respect to age or sex in the population included. Therefore, further studies that analyse these variables and take these limitations into account are needed.
We did not find any validations of Rawl's questionnaire in Europe, apart from a validation in the Balearic Islands for breast cancer, which had negative results due to low correlation. We did find literature in the United States, Turkey, Iran, China, Taiwan and Korea. The corresponding studies were largely conducted in occupational settings, war veterans, primary care, outpatient settings and shopping centres and through telephone interviews.
In conclusion, we wish to stress the need for an instrument validated in our setting that objectively measures the barriers and benefits perceived by the population when deciding whether or not to take part in a screening programme. With this, information strategies could be designed to enhance perceived benefits and adapt screening techniques to decrease barriers sensed by patients. All this will bring us closer to the goal of a participation rate of at least 65% which would achieve decreased CRC mortality.
With this study, we have validated the Spanish version of Rawl’s questionnaire. It is a reliable, reproducible questionnaire that will determine subjective factors associated with participation in population CRC screening programmes in Spain.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Menéndez Rodríguez M, Garau Ramírez J, Traver Salvador A, Hervás Jiménez Y, García Morales N, Seoane Pillado T, et al. Validación al castellano del cuestionario Rawl de cribado de cáncer colorrectal con sangre oculta en heces. Gastroenterol Hepatol. 2022;45:106–113.