To determine the comorbidity and potential for drug–drug interactions (DDIs) among pangenotypic direct-acting-antivirals (pDAAs) and the concomitant medications associated with chronic hepatitis C (CHC) patients in routine clinical practice in Spain.
MethodsRetrospective observational study. Included patients were ≥18 years, diagnosed with CHC, on antiviral treatment and required medical attention during 2017. Two groups were differentiated according to age ranges (<50 and ≥50 years). The variables collected were: age, gender, general/specific comorbidity, concomitant medication and potential DDIs (www.hep-druginteractions.org). The pDAAs analysed were: a) Sofosbuvir/Velpatasvir (SOF/VEL), b) Glecaprevir/Pibrentasvir (GLE/PIB) and c) Sofosbuvir/Velpatasvir/Voxilaprevir (SOF/VEL/VOX). Bivariate statistical analysis, p < 0.05.
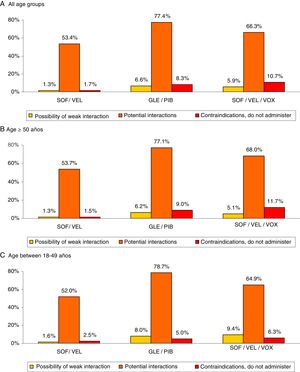
Results3430 patients with a mean age of 56.9 years and 60.3% males were enrolled. The average Charlson index was 0.8. Age range distribution: 18–49 years (28.9%) and ≥50 years (71.1%). The average number of medications per patient/year was 3.1 (SD 2.6). The total percentage of potential DDIs was: 8.6% minor DDIs, 40.5% clinically significant DDIs and 10.0% contraindicated medication. These DDIs were greater in patients ≥50 years (8.6%, 43.8% and 12.4%, respectively, p < 0.001). For all ages, SOF/VEL showed a lower percentage of: minor interactions (1.3% vs. 6.6% and 5.9%, p < 0.001); clinically significant interactions (53.4%, vs. 77.4% and 66.3%, p < 0.001) and contraindicated medication (1.7% vs. 8.3% and 10.7%, p < 0.001) compared to GLE/PIB and SOF/VEL/VOX, respectively.
ConclusionsPatients with CHC present high comorbidity and concomitant medication use, particularly elderly patients, thus implying a greater exposure to potential DDIs. Although the DDI rate was considerable with the three combinations analysed, SOF/VEL showed a lower number of clinically significant interactions.
Determinar la comorbilidad y las potenciales interacciones-farmacológicas (IFs) entre los antivirales de acción-directa pangenotípicos (AADp) y la medicación-concomitante asociada a los pacientes con hepatitis C crónica (HCC) en práctica clínica habitual en España.
MétodosDiseño observacional retrospectivo. Se incluyeron pacientes ≥18 años con diagnóstico de HCC, en tratamiento antiviral y visitados durante el año 2017. Se diferenciaron dos grupos en función de la edad (<50 y ≥50 años). Las variables recogidas fueron: edad, género, comorbilidad general/específica, medicación-concomitante y potenciales IFs [www.hep-druginteractions.org]. Los AADp analizados fueron: a) Sofosbuvir/Velpatasvir (SOF/VEL), b) Glecaprevir/Pibrentasvir (GLE/PIB) y c) Sofosbuvir/Velpatasvir/Voxilaprevir (SOF/VEL/VOX). Análisis-estadístico bivariante, p < 0,05.
ResultadosSe reclutaron 3.430 pacientes, edad-media de 56,9 años y el 60,3% varones. El promedio del índice Charlson fue 0,8 puntos. Distribución por rangos de edad: 18–49 (28,9%) y ≥50 años (71,1%). El promedio de medicamentos fue: 3,1 (DE: 2,6) por paciente. El porcentaje total de potenciales IFs fue: 8,6% débil, 40,5% clínicamente significativas y 10,0% medicación contraindicada. Estas interacciones fueron mayores en los pacientes ≥50 años (8,6%; 43,8% y 12,4%, respectivamente, p < 0,001). Para todas las edades, SOF/VEL en comparación con GLE/PIB y SOF/VEL/VOX presentó un menor porcentaje de interacciones-débiles (1,3% vs. 6,6% y 5,9%, p < 0,001); interacciones clínicamente-significativas (53,4%, vs. 77,4% y 66,3%, p < 0,001) y medicación-contraindicada (1,7% vs. 8,3% y 10,7%, p < 0,001).
ConclusionesLos sujetos con HCC presentan una elevada comorbilidad y consumo de medicación concomitante, especialmente en pacientes mayores, circunstancia que repercute en una mayor exposición a potenciales IFs. Aunque la tasa de IFs fue considerable con las 3 combinaciones analizadas, SOF/VEL mostró una menor proporción clínicamente relevante.
Chronic infection with the hepatitis C virus (HCV) is a global healthcare problem affecting 120–150 million people, with a prevalence of less than 1% in the general population.1–4 Following the natural progression of the disease, between 55 and 85% of infected patients will develop chronic hepatitis C (inflammation and/or fibrosis); approximately 15–30% of these patients will develop cirrhosis, and decompensation can then cause liver failure or hepatocellular carcinoma (3–5%).1,3–5 Cases of newly acquired disease continue to be detected, above all among young people and parenteral drug addicts. Early detection and treatment are therefore important aspects for disease prevention.1,2,6
The aim of therapy is the eradication of the HCV infection, as evidenced by the negativisation of HCV RNA at 12 weeks from the end of treatment (sustained virologic response—SVR12).5,7–10 The response to previous treatments and degree of liver fibrosis are of great importance in the choice of treatment and its efficacy.1,3,5,10
The new DAA molecules against HCV, which take advantage of the numerous therapeutic targets offered by the virus’ replication cycle, have revolutionised the treatment of chronic hepatitis C (CHC).8 Their aim is to achieve greater efficacy and a reduction in possible side effects.8,11–13 Progressive research into the virus’ replication mechanisms has enabled potential therapeutic targets to be identified. In this regard, there are three different classes/families of DAAs, with pharmacokinetic differences: NS3/4A protease inhibitors (ending in “-previr”), NS5A replication complex inhibitors (ending in “-asvir”) and NS5B polymerase inhibitors (ending in “-buvir”). With these drugs, three phases of the HCV replication process can be affected: inhibiting the viral protease, inhibiting the polymerase and inhibiting the NS5A protein. With protease inhibitors, it is essential to check for possible drug–drug interactions before recommending their use; NS5A protein inhibitors are potent and efficacious, but present a low barrier to resistance and variable toxicity profiles; meanwhile, NS5B polymerase inhibitors have a high genetic barrier (low barrier to resistance) and their metabolism does not generally depend on cytochrome P450.1,5,8
A single DAA cannot avoid HCV reproduction (mutations) alone, therefore the treatment must consist of 2/3 drugs from different families of inhibitors. The current molecules are presented in various pangenotypic combinations and in a single tablet, thereby simplifying the treatment.1,12 In addition, they have shorter treatment durations and better profiles for safety and drug–drug interactions.12 Some evidence shows that the combination of sofosbuvir (an NS5B polymerase inhibitor) generally has fewer adverse reactions than treatments based on protease inhibitors.13
In some of these patients with HCV, the presence of comorbidities is common, and they may also be receiving multiple medications, a situation that can lead to adverse effects, drug–drug interactions or errors in taking medication.4,9 Moreover, drug–drug interactions with the medications used to treat these comorbidities may interfere with or contraindicate the use of medications for the treatment of HCV (DAAs).11,13 Some studies show that 2/3 patients may have potential interaction with DAA drugs, while approximately 20% may be contraindicated. In general, careful review of theses patients’ medication is advised.9,11,13–15 Currently, there is little information about the actual risk of pangenotypic DAAs associated with the patterns of use of these medications at the population level, meaning that more data is needed to increase our knowledge of the possible interactions with pangenotypic DAAs. The objective of this study was to estimate the comorbidity and potential drug–drug interactions between pangenotypic DAAs and the concomitant medication associated with CHC patients in routine clinical practice in Spain.
Patients and methodsStudy design and populationAn retrospective, observational, longitudinal, multicentre study was conducted. The study population was obtained from the health records of healthcare providers at various centre in Spain (unified in the BIG-PAC dissociated database; Real Life Data; http://www.encepp.eu/encepp/search.htm). The data are drawn from various digitised medical records and other supplementary databases of seven autonomous regions of Spain (1.9 million patients). The population assigned to the centres was mainly urban and lower middle-class and mostly industrial.
Inclusion and exclusion criteriaPatients with a diagnosis of HCV, visited and on treatment with any combination of DAAs during 2017 were included in the study. The patients had to meet the following characteristics: a) age ≥ 18 years; b) have a diagnosis of HCV at least 12 months prior to the start of the study (active patients in the database); c) be in the chronic prescriptions program to obtain medical prescriptions (with recorded registration of the daily dose, interval and duration of each treatment, ≥2 prescriptions during the follow-up period for any medication administered); and d) that the regular follow-up of these patients during the study period can be ensured (≥2 healthcare records in the computer system). Patients transferred to other centres, or who move and/or are out of the zone were excluded from the study. Two study groups were differentiated based on age (<50 years and ≥50 years).
Screening for diagnosis and comorbiditiesThe HCV diagnosis was obtained based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-10-CM [B18.2]). In the patients included in the study, the detection of anti-HCV antibodies was verified (exposure/contact) and the presence of HCV RNA was confirmed (viraemia/active infection). The variables included in the study were: sex, body mass index (BMI = kg/m2) and time of evolution of the disease (hepatitis C), as well as the personal history detailed in Table 1: presence of cirrhosis and associated comorbidities (ICD-10-CM). As a summary variable for general comorbidity, the Charlson comorbidity index16 was used as an approximation of patient severity.
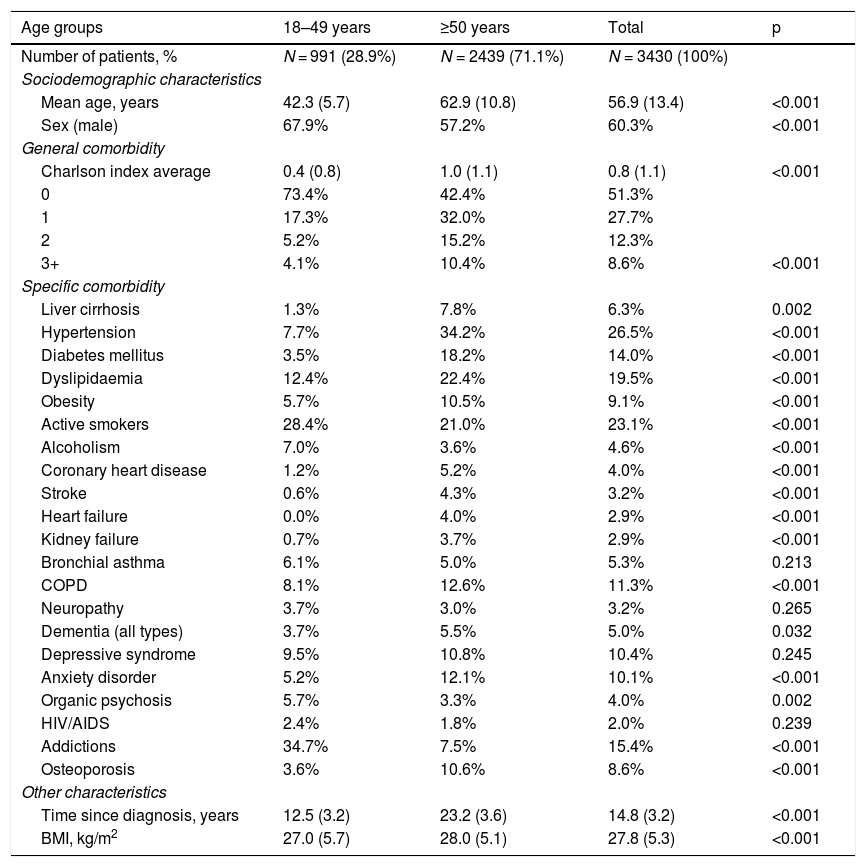
Baseline characteristics and comorbidity of the series studied by age range.
| Age groups | 18–49 years | ≥50 years | Total | p |
|---|---|---|---|---|
| Number of patients, % | N = 991 (28.9%) | N = 2439 (71.1%) | N = 3430 (100%) | |
| Sociodemographic characteristics | ||||
| Mean age, years | 42.3 (5.7) | 62.9 (10.8) | 56.9 (13.4) | <0.001 |
| Sex (male) | 67.9% | 57.2% | 60.3% | <0.001 |
| General comorbidity | ||||
| Charlson index average | 0.4 (0.8) | 1.0 (1.1) | 0.8 (1.1) | <0.001 |
| 0 | 73.4% | 42.4% | 51.3% | |
| 1 | 17.3% | 32.0% | 27.7% | |
| 2 | 5.2% | 15.2% | 12.3% | |
| 3+ | 4.1% | 10.4% | 8.6% | <0.001 |
| Specific comorbidity | ||||
| Liver cirrhosis | 1.3% | 7.8% | 6.3% | 0.002 |
| Hypertension | 7.7% | 34.2% | 26.5% | <0.001 |
| Diabetes mellitus | 3.5% | 18.2% | 14.0% | <0.001 |
| Dyslipidaemia | 12.4% | 22.4% | 19.5% | <0.001 |
| Obesity | 5.7% | 10.5% | 9.1% | <0.001 |
| Active smokers | 28.4% | 21.0% | 23.1% | <0.001 |
| Alcoholism | 7.0% | 3.6% | 4.6% | <0.001 |
| Coronary heart disease | 1.2% | 5.2% | 4.0% | <0.001 |
| Stroke | 0.6% | 4.3% | 3.2% | <0.001 |
| Heart failure | 0.0% | 4.0% | 2.9% | <0.001 |
| Kidney failure | 0.7% | 3.7% | 2.9% | <0.001 |
| Bronchial asthma | 6.1% | 5.0% | 5.3% | 0.213 |
| COPD | 8.1% | 12.6% | 11.3% | <0.001 |
| Neuropathy | 3.7% | 3.0% | 3.2% | 0.265 |
| Dementia (all types) | 3.7% | 5.5% | 5.0% | 0.032 |
| Depressive syndrome | 9.5% | 10.8% | 10.4% | 0.245 |
| Anxiety disorder | 5.2% | 12.1% | 10.1% | <0.001 |
| Organic psychosis | 5.7% | 3.3% | 4.0% | 0.002 |
| HIV/AIDS | 2.4% | 1.8% | 2.0% | 0.239 |
| Addictions | 34.7% | 7.5% | 15.4% | <0.001 |
| Osteoporosis | 3.6% | 10.6% | 8.6% | <0.001 |
| Other characteristics | ||||
| Time since diagnosis, years | 12.5 (3.2) | 23.2 (3.6) | 14.8 (3.2) | <0.001 |
| BMI, kg/m2 | 27.0 (5.7) | 28.0 (5.1) | 27.8 (5.3) | <0.001 |
Values expressed as percentage or mean.
SD: standard deviation; COPD: chronic obstructive pulmonary disease; BMI: body mass index; p: statistical significance.
The description of the treatment was obtained from the Anatomical Therapeutic Chemical Classification System (ATC).17 Information was obtained on active substances and therapeutic groups (described in Table 2). The recommendations of the University of Liverpool, an international resource recommended by the European Association for the Study of the Liver1 and the HCV treatment guides18 (http://www.hep-druginteractions.org/Interactions.aspx) were used to determine the potential effect of interaction. Potential drug–drug interactions were classified in three groups: a) possibility of weak interaction (yellow); b) potential interactions (orange); and c) medication contraindicated. The information was obtained from the pharmaceutical prescription. The choice of medication for a specific patient was at the physician's discretion (clinical practice). The pangenotypic DAAs selected were: a) sofosbuvir/velpatasvir (SOF/VEL); b) glecaprevir/pibrentasvir (GLE/PIB); and c) sofosbuvir/velpatasvir/voxilaprevir (SOF/VEL/VOX). It is worth noting that the concomitant medication was analysed during the antiviral treatment period and only with the medication chronically or habitually administered to the patients.
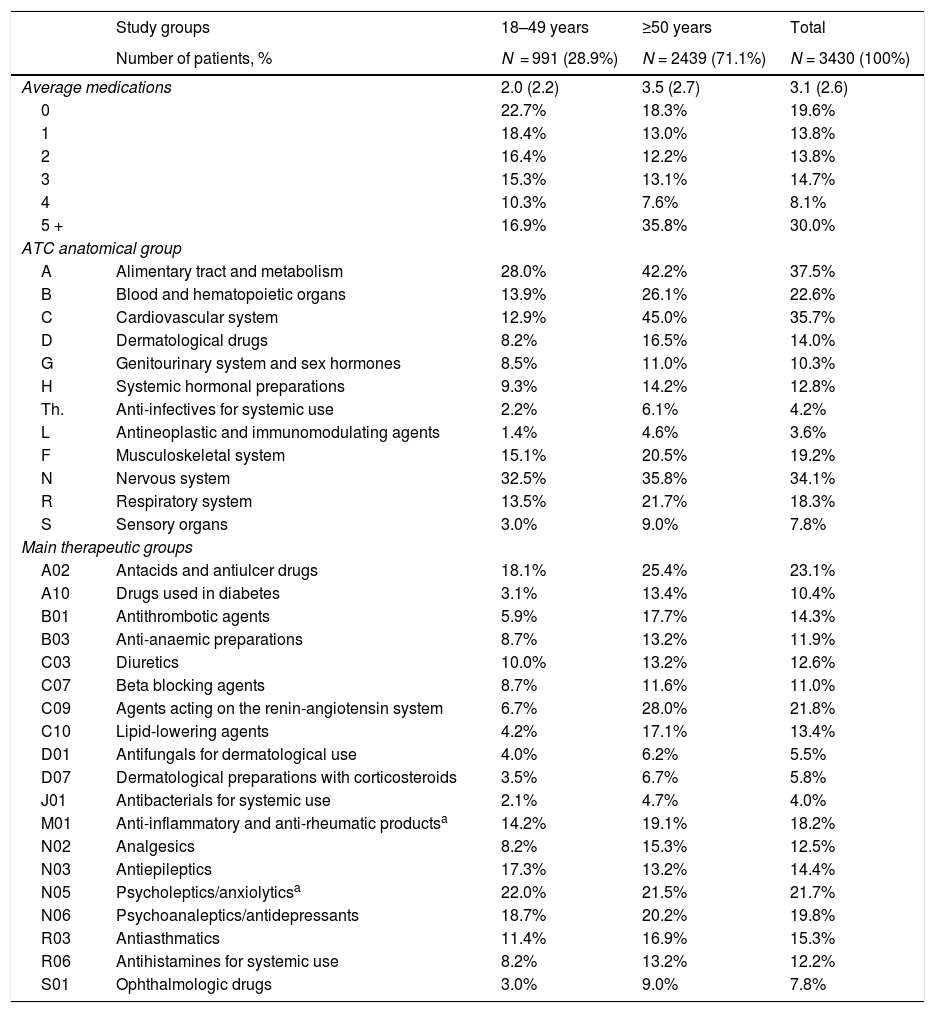
Concomitant medication administered during the follow-up period by age range.
| Study groups | 18–49 years | ≥50 years | Total | |
|---|---|---|---|---|
| Number of patients, % | N = 991 (28.9%) | N = 2439 (71.1%) | N = 3430 (100%) | |
| Average medications | 2.0 (2.2) | 3.5 (2.7) | 3.1 (2.6) | |
| 0 | 22.7% | 18.3% | 19.6% | |
| 1 | 18.4% | 13.0% | 13.8% | |
| 2 | 16.4% | 12.2% | 13.8% | |
| 3 | 15.3% | 13.1% | 14.7% | |
| 4 | 10.3% | 7.6% | 8.1% | |
| 5 + | 16.9% | 35.8% | 30.0% | |
| ATC anatomical group | ||||
| A | Alimentary tract and metabolism | 28.0% | 42.2% | 37.5% |
| B | Blood and hematopoietic organs | 13.9% | 26.1% | 22.6% |
| C | Cardiovascular system | 12.9% | 45.0% | 35.7% |
| D | Dermatological drugs | 8.2% | 16.5% | 14.0% |
| G | Genitourinary system and sex hormones | 8.5% | 11.0% | 10.3% |
| H | Systemic hormonal preparations | 9.3% | 14.2% | 12.8% |
| Th. | Anti-infectives for systemic use | 2.2% | 6.1% | 4.2% |
| L | Antineoplastic and immunomodulating agents | 1.4% | 4.6% | 3.6% |
| F | Musculoskeletal system | 15.1% | 20.5% | 19.2% |
| N | Nervous system | 32.5% | 35.8% | 34.1% |
| R | Respiratory system | 13.5% | 21.7% | 18.3% |
| S | Sensory organs | 3.0% | 9.0% | 7.8% |
| Main therapeutic groups | ||||
| A02 | Antacids and antiulcer drugs | 18.1% | 25.4% | 23.1% |
| A10 | Drugs used in diabetes | 3.1% | 13.4% | 10.4% |
| B01 | Antithrombotic agents | 5.9% | 17.7% | 14.3% |
| B03 | Anti-anaemic preparations | 8.7% | 13.2% | 11.9% |
| C03 | Diuretics | 10.0% | 13.2% | 12.6% |
| C07 | Beta blocking agents | 8.7% | 11.6% | 11.0% |
| C09 | Agents acting on the renin-angiotensin system | 6.7% | 28.0% | 21.8% |
| C10 | Lipid-lowering agents | 4.2% | 17.1% | 13.4% |
| D01 | Antifungals for dermatological use | 4.0% | 6.2% | 5.5% |
| D07 | Dermatological preparations with corticosteroids | 3.5% | 6.7% | 5.8% |
| J01 | Antibacterials for systemic use | 2.1% | 4.7% | 4.0% |
| M01 | Anti-inflammatory and anti-rheumatic productsa | 14.2% | 19.1% | 18.2% |
| N02 | Analgesics | 8.2% | 15.3% | 12.5% |
| N03 | Antiepileptics | 17.3% | 13.2% | 14.4% |
| N05 | Psycholeptics/anxiolyticsa | 22.0% | 21.5% | 21.7% |
| N06 | Psychoanaleptics/antidepressants | 18.7% | 20.2% | 19.8% |
| R03 | Antiasthmatics | 11.4% | 16.9% | 15.3% |
| R06 | Antihistamines for systemic use | 8.2% | 13.2% | 12.2% |
| S01 | Ophthalmologic drugs | 3.0% | 9.0% | 7.8% |
Values expressed as percentage or mean.
SD: standard deviation; p: statistical significance.
ATC: Anatomical Therapeutic Chemical Classification System.
The confidentiality of the records (anonymous and pseudonymous) was maintained according to the Organic Data Protection Law (Law 15/1999 of 13 December). The study was classified by the Spanish Agency for Medicines and Medical Devices (EPA-OD) and was subsequently approved by the Independent Ethics Committee of the Unió Catalana Balears de Hospitales de Barcelona.
Statistical analysisA validation of the data was carried out to ensure the quality of the records. A descriptive-univariant statistical analysis was performed and the normality of the distribution was verified through the Kolmogorov–Smirnov test. ANOVA tests and the chi squared test were used in the bivariant analysis for independent variables. The SPSSWIN program, version 23, was used, establishing statistical significance for values of p < 0.05.
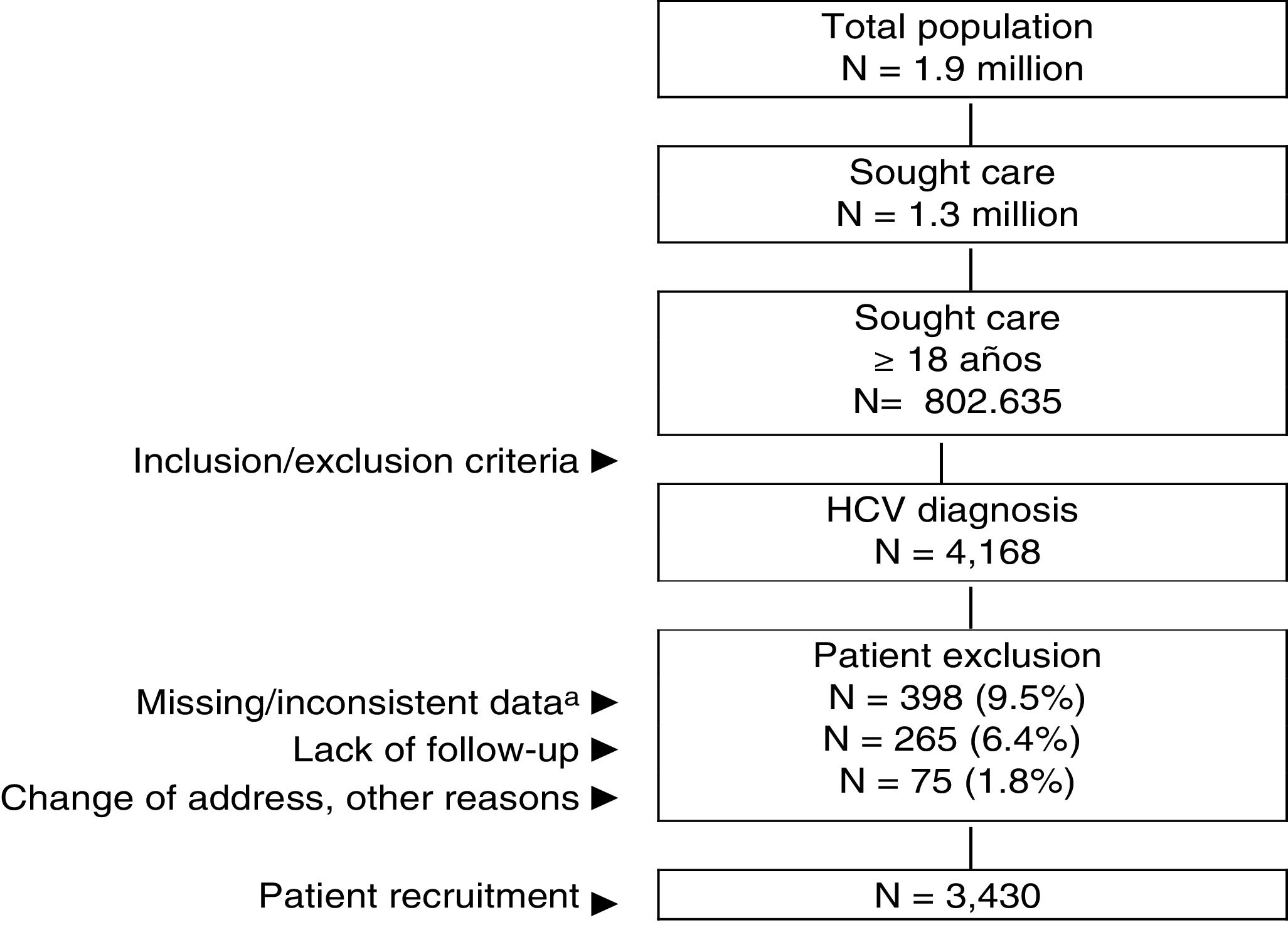
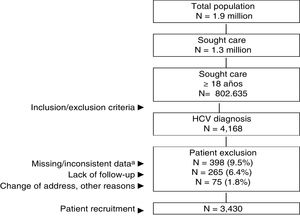
ResultsOf an initial population of 1.9 million inhabitants, 802,635 patients ≥18 years of age were treated between 01 January 2017 and 31 December 2017. Of these, 4168 were diagnosed with HCV. Ultimately, 3430 patients who met the inclusion/exclusion criteria and who could be followed up during the study period were analysed (Fig. 1). Table 1 shows the baseline characteristics of the series studied by age range. In general, the mean age was 56.9 years, 60.3% were men and the average Charlson index was 0.8 points per patient. Cardiovascular and mental illnesses were the most prevalent personal histories. The time from diagnosis was 14.8 years and BMI was 27.8 kg/m2. The distribution by study groups was as follows: a) 18–49 years (n = 991; 28.9%); and b) ≥50 years (n = 2439; 71.1%). By study group, the proportion of the male sex decreased with age (67.9% vs. 57.2%, respectively; p < 0.001) while the average Charlson index increased (0.4 vs. 1.0 points, respectively; p < 0.001). An increase in the presence of comorbidities was observed with increasing age.
General outline of the study.
HCV: chronic hepatitis C virus. An observational retrospective design was created, based on the review of medical records (computerised databases, with anonymised and disassociated data) of patients who sought care during the YEAR 2017.
aIncludes patients who did not receive antiviral treatment.
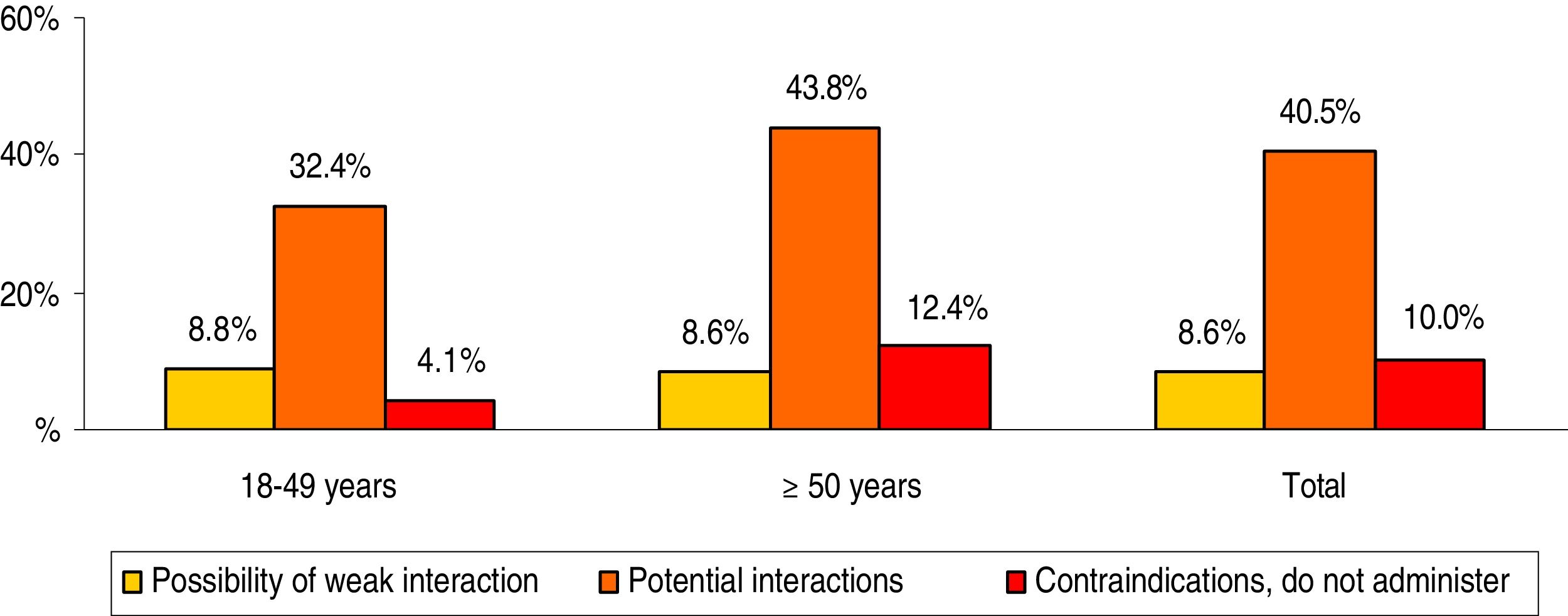
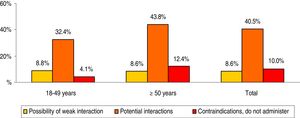
The concomitant medication prescribed to patients during the follow-up period is shown in Table 2. The mean number of drugs was 3.1 (SD: 2.6) per patient. In general, the most consumed therapeutic groups (ATC) were: alimentary tract and metabolism (37.5%), cardiovascular system (35.7%) and nervous system (34.1%). Whereas the main therapeutic groups were: A02-antacids (23.1%), C09-agents acting on the renin-angiotensin system (21.8%) and N05-psycholeptics/anxiolytics (21.7%). The prescription of drugs increased with age. The potential drug–drug interactions by age range are detailed in Fig. 2. The total percentages were as follows: 8.6% weak interaction, 40.5% potential interactions and 10.0% medication contraindicated. These interactions were greater in patients ≥50 years of age (8.6%; 43.8% and 12.4%, respectively; p < 0.001). In general, 35.6% of patients had one interaction, 19.5% had two and 2.4% had three.
Potential drug–drug interactions by age range.
Paired comparisons between age groups: significant differences in all cases (p < 0.01).
Comparisons between interaction groups: possibility of weak interaction (not significant), potential interactions (p < 0.001) and contraindications (p < 0.01).
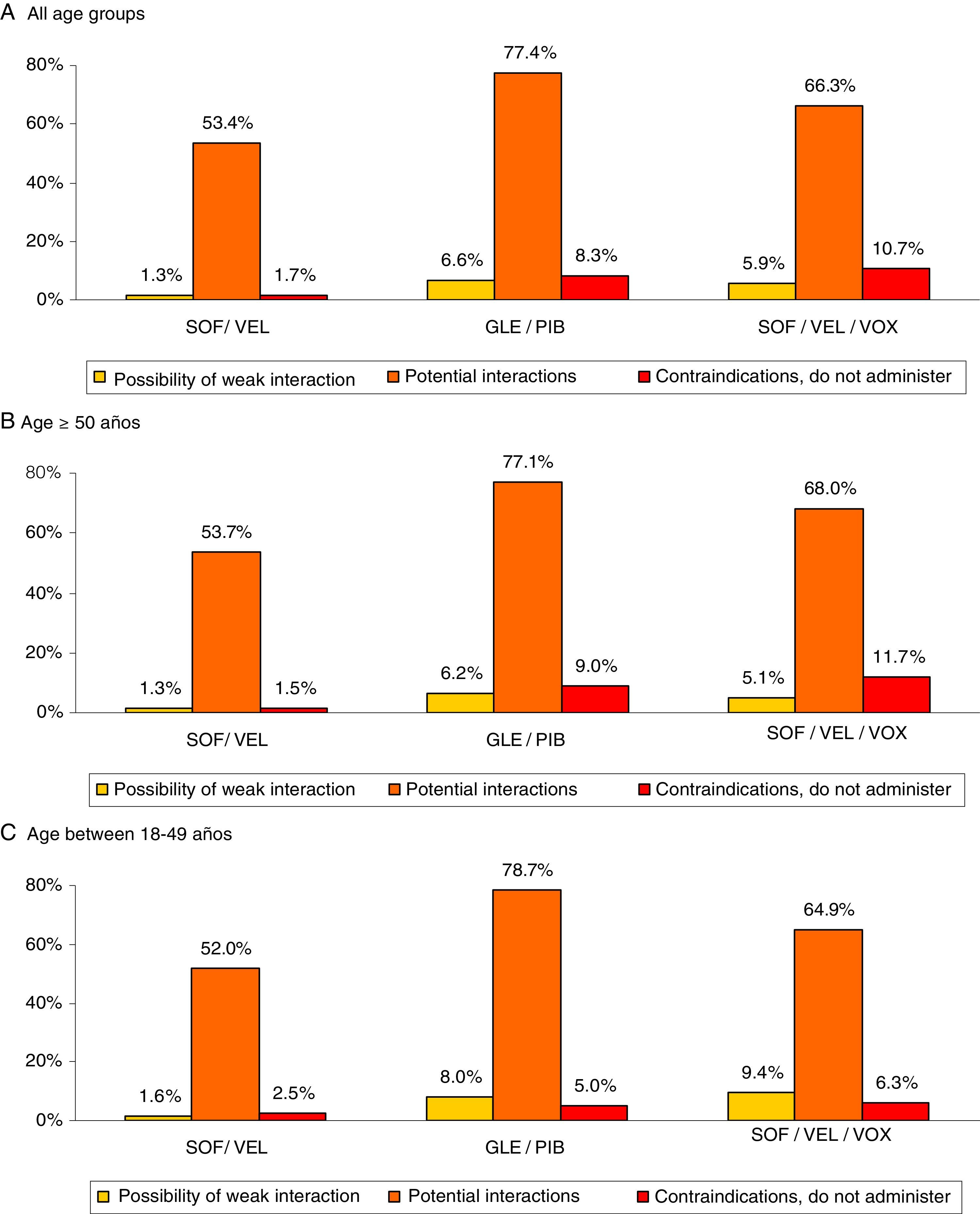
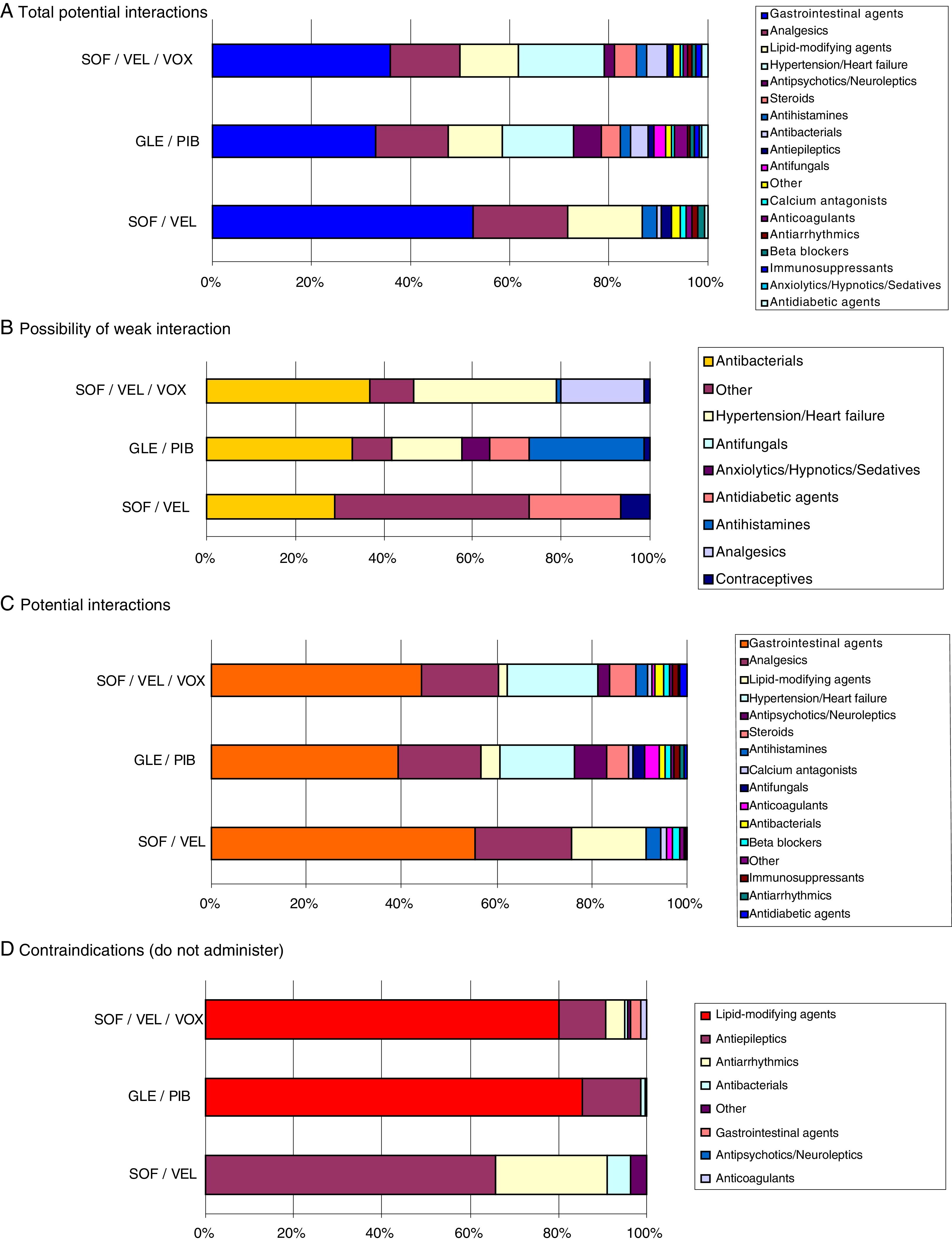
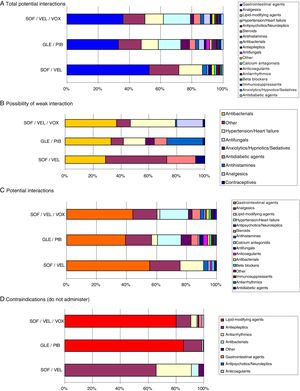
Fig. 3 shows the drug–drug interactions by the different pangenotypic DAAs studied and by age group. For all ages, SOF/VEL, when compared to GLE/PIB and SOF/VEL/VOX, presented a lower percentage of weak interactions (1.3% vs. 6.6% and 5.9%, p < 0.001), potential interactions (53.4%, vs. 77.4% and 66.3%, p < 0.001) medication contraindicated (1.7% vs. 8.3% and 10.7%; p < 0.001). Fig. 4 describes the total drug–drug interactions by pangenotypic DAA and by main therapeutic group. In general, the therapeutic groups of gastrointestinal (SOF/VEL: 52.1%; GLE/PIB: 32.7%; SOF/VEL/VOX: 35.3%; p < 0.001), analgesic (SOF/VEL: 19.0%; GLE/PIB: 14.3%; SOF/VEL/VOX: 14.2%; p < 0.001) and lipid-lowering (SOF/VEL: 14.8%; GLE/PIB: 11.0%; SOF/VEL/VOX: 11.4%; p = 0.003) drugs/medications were the most common.
Drug–drug interactions by pangenotypic DAA and age group.
Paired comparisons between age groups: significant differences in all cases (p < 0.01).
DAA: direct-acting antivirals; GLE/PIB: glecaprevir/pibrentasvir; SOF/VEL: sofosbuvir/velpatasvir; SOF/VEL/VOX: sofosbuvir/velpatasvir/voxilaprevir.
The main contraindications were anticonvulsants (61.2%) and antiarrhythmic drugs (23.5%) for SOF/VEL; lipid-lowering agents (82.3%) and anticonvulsants (13.0%) for GLE/PIB; and lipid-lowering agents (77.1%) and anticonvulsants (10.2%) for SOF/VEL/VOX. The most prescribed active substances in this group of patients with HCV that could cause a potential interactions were: omeprazole (20.5%, gastrointestinal), diazepam (11.0%, anxiolytics/sedatives), lorazepam (6.5%, anxiolytics/sedatives) and enalapril (6.4%, hypertension/cardiac). Atorvastatin (4.0%) and clonazepam (2.8%) were the most common lipid-lowering agent and anticonvulsant, respectively.
DiscussionThe study’s results reveal that HCV carriers are associated with significant comorbidity and elevated medication use. primarily in patients ≥50 years of age, the repercussions of which are increased exposure to potential drug–drug interactions when receiving antiviral treatment. It is worth noting that the data are based on results obtained in a routine clinical practice situation, which is far from the ideal conditions of clinical trials or specific patient groups. In the coming years, it is foreseeable that the downward trend in the number of new hepatitis C cases will continue, although there remain many patients diagnosed with hepatitis C who for various reasons have not received antiviral treatment.1,3 Knowledge of drug–drug interactions represents a challenge for the treatment of HCV infection.1,11,13 Our study is one of the first published works on pangenotypic DAAs, and moreover, was conducted in a large number of patients; these two aspects could be considered as its strengths.
In our study the overall disease burden (Charlson index, RUB) related to carriers of HCV was high. Cardiovascular, metabolic, mental and osteomuscular diseases were the most prevalent. These results are consistent with the literature reviewed.19,20 By way of example, Basseri,21 in a cross-sectional study, described renal disease, diabetes and obesity as being more prevalent in patients with HCV than in the general population of the United States. Chen22 detected a 28.8% level of obesity in a cohort study of 1118 patients with HCV; the independent factors associated with obesity were age and viral load. In two published studies, McKibben23 and Serres24 concluded that HCV may increase the risk of cardiovascular disease, though it should be determined whether the duration of the infection or the treatment administered may influence the development of the atheromatous plaque. Our results are similar to those reported in the literature consulted.
The growing rates of comorbidities observed are related to the ageing of the populations (polymedicated elderly patients) and therefore have implications in the complexity of assessing potential interactions; the percentage of these was 40.5%, while 10.0% were contraindicated medications.25 Maasoumy26 investigated the risk of potential interaction in subjects treated with protease inhibitors (telaprevir, boceprevir) in a German hospital, determining that half of the patients were exposed to a drug with a potential interaction. In a study of similar design to ours, it was observed that, of the 40 main outpatient medications, 62% of patients were exposed to at least one of the 22 medications with potential interactions.15 In our case, we looked at all medications prescribed chronically, with no limits on the active substances, and analysed both outpatient and inpatient medications.27 Smolders28 (n = 461; sofosbuvir/velpatasvir, sofosbuvir/simeprevir, sofosbuvir/ledipasvir, sofosbuvir/ daclatasvir, elbasvir/grazoprevir, paritaprevir/ritonavir/ombitasvir/dasabuvir) observed that antidepressants (7.4%), proton pump inhibitors (7.1%) and benzodiazepines (7.1%) were used most often. Many patients were at risk of presenting a clinically relevant drug–drug interaction with at least one DAA. The DAAs that have recently come onto the market are associated with a lower risk of drug interactions. Langness29 identified that hypertensive agents, analgesics and psychiatric medications cause frequent interactions with DAAs (sofosbuvir/simeprevir, sofosbuvir/ledipasvir, sofosbuvir/ribavirin, paritaprevir/ritonavir/ombitasvir/dasabuvir). The authors conclude that drug–drug interactions are common (1.2 per patients) and that treatment with DAAs may require adjustments to concomitant medications. Kondili30 (PITER study, n = 449; sofosbuvir/ribavirin, sofosbuvir/simeprevir, sofosbuvir/daclatasvir, sofosbuvir/ledipasvir, paritaprevir/ritonavir/ombitasvir/dasabuvir) highlights that we can estimate that 30–44% of patients on DAAs have a risk of clinically significant interactions. The authors highlight the need to further increase awareness in the administration of these medications, especially in patients with moderate/severe liver disease. Our results are in line with these authors, although we have observed a lower proportion of clinically relevant drug–drug interactions. This is because the study was performed with pangenotypic DAAs, with more recently marketed medications, although there is a scarcity of literature available for their comparison.
In this regard, SOF/VEL presented a lower proportion of drug–drug interactions. SOF is an NS5B polymerase inhibitor, while VEL is an NS5A replication complex inhibitor. GLE is a pangenotypic inhibitor of the HCV NS3/4A protease, which is essential for viral replication. Meanwhile, PIB is a pangenotypic inhibitor of HCV NS5A; concomitant administration of GLE/PIB can increase exposure to certain medications (digoxin, dabigatran, statins, ethinylestradiol, CYP3A). SOF’s intracellular metabolic activation pathway is mediated by generally low affinity and high capacity nucleotide and hydrolase phosphorylation pathways, so it is unlikely that they would be affected by concomitant medications.31 Recent reviews state that drug combinations with SOF generally have fewer interactions than protease inhibitor-based regimes. However, the analysis of each interaction is theoretical and further interaction studies would be needed to confirm the real effect.31 It appears that the key to interpreting drug–drug interactions is based on knowledge of the pharmacokinetic profiles of the medications and their capacity to inhibit CYP450-3A4 and the transporters (hepatic and intestinal) in relation to their potential clinical consequences.12,13
At a practical level, it is worth noting that the potential drug–drug interactions of contraindicated medications (red) and potential interactions (orange) are of greatest clinical relevance, and therefore those that require greater attention. In this regard, when introducing DAAs, some concomitant medications could be substituted or the dose administered reduced, given the short period of administration of DAAs. This could be the case for statins (simvastatin, atorvastatin, etc.) and proton pump inhibitors (lansoprazole, pantoprazole, etc.), which were widely prescribed medications in our study (Fig. 4). In other cases, such as patients with HIV/HCV co-infections (2.0% of the population), another type of intervention would perhaps be preferable, such as more carefully selecting the type of DAA. Moreover, it will always be necessary to ask the patient about their use of other drugs, as unfunded medications (homoeopathic products, supplements, vitamins, etc.) or through purchased without a prescription.
The article displays the limitations typical of retrospective studies such as the under-reporting of the disease or possible variability between professionals and patients due to its observational design. It should be noted that this type of design is not without biases (factors not taken into account), such as socioeconomic, cultural or education level, pharmacological doses consumed, duration of treatment, adherence to the same or therapeutic adequacy. In addition, over-the-counter medications, patient self-medication and prescriptions issued by other healthcare institutions (public or private) were not taken into account in the study. The main objection to the study is its undoubted selection bias by the lead physician when administering one or another drug, and the external validity, so the results must be interpreted with caution.
Potential interactions may be a problem in clinical practice, although may of them can be avoided by adjusting the pharmacological dose or selecting a safer alternative, but only if practitioners have sufficient knowledge and experience to manage these pharmacokinetic problems.11,13 New care models, including, among others, electronic prescription assistance tools or clinical decision-making assistance systems, may offer clinicians considerable aid. The success of care for HCV carriers lies in prevention,2,10 i.e. reducing the risk of exposure to the virus in healthcare settings in high risk population groups; strategies should also be proposed to slow the progression of the disease.1,2,5,9 Knowledge of the consequences of HCV can be useful to policy makers to determine the value of early detection and treatment (DAAs) in the advanced stages of the disease.
In conclusion, carriers of HCV are associated with a high rate of comorbidities and use of concomitant medication, especially in older patients, the repercussion of which is greater exposure to potential drug–drug interactions. The availability of pangenotypic DAAs offers an opportunity to expand treatment and maximise cure levels in HCV patients. Although the rate of drug–drug interactions was considerable with the three combinations analysed, SOF/VEL demonstrated a lower proportion of clinically relevant interactions. The absence of a protease inhibitor may be the reason for this different profile of potential interactions.
AuthorshipThe conception and design of the manuscript were done by A. Sicras and R. Navarro, data collection by I. Hernández and the statistical analysis by A. Sicras and data interpretation, drafting, review and approval of the submitted manuscript, by all authors.
FundingThe study was sponsored by Gilead Sciences.
Conflicts of interestA. Sicras is an independent consultant in relation to the development of this manuscript. The other authors declare that they have no conflicts of interest.
Please cite this article as: Sicras Mainar A, Navarro Artieda R, Hernández I, Morillo R. Prevalencia de las potenciales interacciones medicamentosas entre los antivirales de acción directa pangenotípicos y la medicación concomitante asociada a los pacientes con infección del virus de la hepatitis C crónica en España. Gastroenterol Hepatol. 2019;42:465–475.