The only accepted treatment for coeliac disease is strict adherence to a gluten-free diet. This type of diet may give rise to reduced patient quality of life with economic and social repercussions. For this reason, dietary transgressions are common and may elicit intestinal damage. Several treatments aimed at different pathogenic targets of coeliac disease have been developed in recent years: modification of gluten to produce non-immunogenic gluten, endoluminal therapies to degrade gluten in the intestinal lumen, increased gluten tolerance, modulation of intestinal permeability and regulation of the adaptive immune response. This review evaluates these coeliac disease treatment lines that are being researched and the treatments that aim to control disease complications like refractory coeliac disease.
El único tratamiento aceptado para la enfermedad celiaca es el seguimiento de forma estricta de la dieta sin gluten. Este tipo de dieta puede ocasionar una disminución de la calidad de vida de los pacientes, dificultades sociales y económicas. Por lo tanto, son frecuentes las transgresiones dietéticas que pueden perpetuar el daño intestinal. En los últimos años se han desarrollado numerosos tratamientos, dirigidos hacia diferentes dianas en la patogenia de la enfermedad celiaca: modificación del gluten para conseguir un gluten no inmunogénico, terapias endoluminales que degraden el gluten en la luz intestinal, favorecer la tolerancia al gluten, modulación de la permeabilidad intestinal o regulación de la respuesta inmune adaptativa. En esta revisión se evalúan estas líneas terapéuticas que se están investigando para la enfermedad celiaca y los tratamientos enfocados al control de las complicaciones de la enfermedad, como la enfermedad celiaca refractaria.
Coeliac disease (CD) is an enteropathy triggered by the ingestion of gluten that affects genetically predisposed subjects.1,2 Gluten is a polypeptide that is insoluble in both water and dilute saline solutions, with a high prolamin and glutenin content.3 This protein causes chronic inflammation in the small intestine mediated by the HLA system, specifically HLA-DQ2 and HLA-DQ8. The intestinal inflammatory process leads to malabsorption of different nutrients. Clinical manifestations vary according to the patient's age.4 In paediatric patients, classic symptoms characterised by diarrhoea, abdominal distension and growth retardation predominate. In adults, symptoms are more atypical with oligosymptomatic manifestations,5 such as anaemia, early osteoporosis, abdominal distension or abnormal intestinal motility, which means this disease should always be considered when making a diagnosis.6
CD affects around 1% of the population in developed countries.7 However, it is estimated that a proportion of patients may not be diagnosed correctly due to the procedures commonly used, which are based on serological techniques (mainly anti-transglutaminase, anti-endomysial and anti-gliadin antibodies).8 As a result, strategies have been designed in recent years to optimise early detection of the disease in high-risk groups, such as first-degree relatives of individuals with coeliac disease.9–12
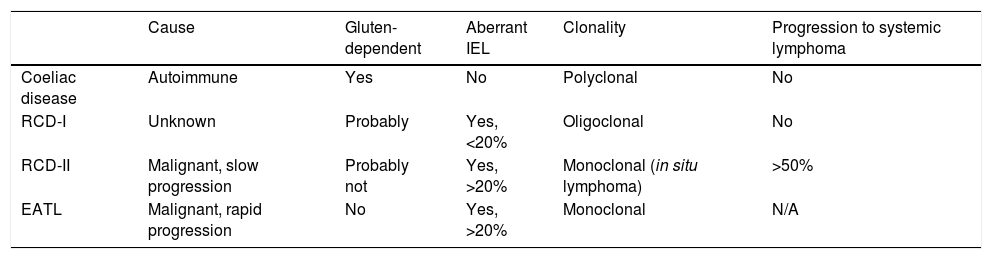
At present, the only effective treatment for CD is strict adherence to a gluten-free diet (GFD) for life. However, it has been demonstrated that mucosal recovery is not immediate and there is even a significant proportion (30–50%) of patients who have persistent intestinal lesions and recurrent symptoms despite apparently correct adherence to a gluten-free diet (known as non-responsive coeliac disease [NRCD]).13,14 Strict adherence to a GFD requires enormous personal sacrifice and has an impact on patients’ psychological and social spheres, which often makes strict adherence difficult.15 Also, a small proportion of coeliac patients (∼1%) does not respond to GFD at all (refractory CD [RCD]). These subjects are mainly diagnosed at an adult age after eating gluten for a prolonged period of time, and are at risk of developing complications such as Type II RCD (RCD-II, an in situ small bowel lymphoma) or the most severe complication, enteropathy-associated T-cell lymphoma (EATL).16,17Table 1 shows the main features that make it possible to differentiate between CD, RCD-I, RCD-II and EATL.
Features of coeliac disease, refractory coeliac disease (RCD) and enteropathy-associated T-cell lymphoma (EATL).
| Cause | Gluten-dependent | Aberrant IEL | Clonality | Progression to systemic lymphoma | |
|---|---|---|---|---|---|
| Coeliac disease | Autoimmune | Yes | No | Polyclonal | No |
| RCD-I | Unknown | Probably | Yes, <20% | Oligoclonal | No |
| RCD-II | Malignant, slow progression | Probably not | Yes, >20% | Monoclonal (in situ lymphoma) | >50% |
| EATL | Malignant, rapid progression | No | Yes, >20% | Monoclonal | N/A |
Although a strict GFD is the primary treatment for CD, various alternative therapeutic measures have been investigated in recent years. A description of the different treatments being developed and their mechanism of action in relation to the aetiopathogenesis of CD is given below.
PathogenesisCD arises in genetically predisposed individuals as an immune response to ingested gluten. This immune response comprises an innate response (direct toxic effect of the gluten on the epithelium) and an adaptive or specific response (involving CD4+ T-cells in the lamina propria or underlying tissue) and the two appear to be responsible for histological damage to the intestinal mucosa.18
Some gluten fragments, such as α-gliadin, induce an innate, toxic and immediate immune response that is not related to T-cells or HLA-DQ2/8 presentation.19,20 As a result, oxidative stress is triggered, mediated primarily by the formation of nitric oxide, which is produced by inducible nitric oxide synthase (iNOS) in enterocytes,21–24 causing expression of ligands, such as MICA, in these cells.25 Gliadin is also able to weaken intercellular bonds between the enterocytes.26,27 However, the main mechanism depends on IL-15 being released by these enterocytes in the event of stress.28 This cytokine induces NKG2D expression in intraepithelial lymphocytes, which is capable of interacting with its ligand, the MICA molecule of enterocytes, enhancing intestinal damage.29,30 The NKG2D/MICA bond induces enterocyte apoptosis, resulting in the disappearance of microvilli and flattening of the intestinal epithelium. This process activates cytotoxicity phenomena in the epithelium, which, together with the weakening of the intercellular bonds, increases intestinal permeability and the passage of gluten into the lamina propria, where the adaptive response is triggered.
The adaptive immune response is mediated by specific T-cells that have been presented with antigens by antigen-presenting cells (APC) carrying HLA-DQ2/DQ8 restriction elements. Macrophages (20%), and especially dendritic cells (DC) (80%), are the main APC of the lamina propria and accumulate in active coeliac lesions.31 These APC are also activated as a result of IL-15 induction due to the innate response.32–34 CD4+ T-cells in the lamina propria recognise gluten fragments, such as α-gliadin, presented in the context of HLA-DQ2 or DQ8 molecules,31,35,36 and after being modified by the enzyme transglutaminase 2 (tTG2).37,38 Therefore, the final effect will be mediated by CD4+ T-cells, which are responsible for a response dominated by pro-inflammatory cytokines, such as IFN-γ, TNF-α and IL-18, and a proportional decrease in regulatory or anti-inflammatory cytokines (IL-10 and TGFβ).39,40 This pro-inflammatory profile will finally be involved in tissue remodelling mechanisms, such as crypt hyperplasia which is typical of CD, microvillus atrophy and B-cell activation, which stimulate antibody production.41
To summarise, it can be stated that gliadin has a dual effect on the bowel of coeliac patients, with activation of the innate immune response required to trigger the adaptive response in susceptible individuals.42–47
Need for novel non-dietary therapiesCurrently, the only available treatment for CD is a strict GFD for life. Dietary restrictions have proven to be a safe and effective therapy when the patient adheres to them. However, it is not ideal or very effective in practice. One problem is that current legislation on the labelling of gluten-free products in the United States and the European Union only includes those products containing less than 20ppm gluten,48 while the gluten threshold varies greatly from one person to another.49 Furthermore, gluten-free foods are expensive and not readily available in all countries. Therefore, this may affect the nutritional value of foodstuffs, significantly impact diet adherence and affect quality of life. In the UK, one study showed that 40% of coeliac patients were dissatisfied with a gluten-free diet50 due to the imposed dietary and social restrictions, and also higher costs and sometimes lower palatability of gluten-free foods. All participants expressed an interest in other treatments, with a vaccine being the preferred choice.50 Also, since compliance with a GFD is often difficult and costly, diet adherence is poor with only limited clinical response and persistence of symptoms.51
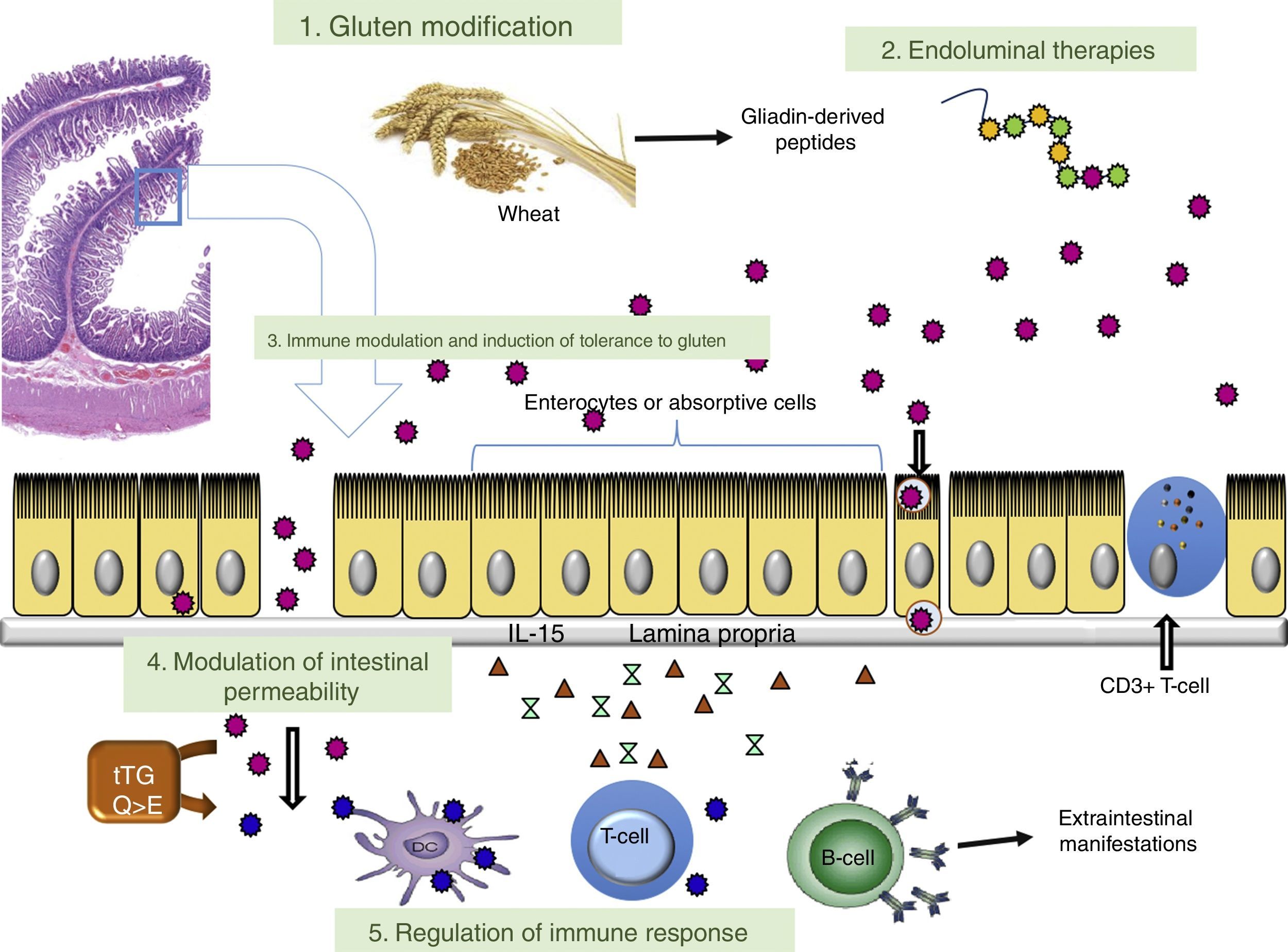
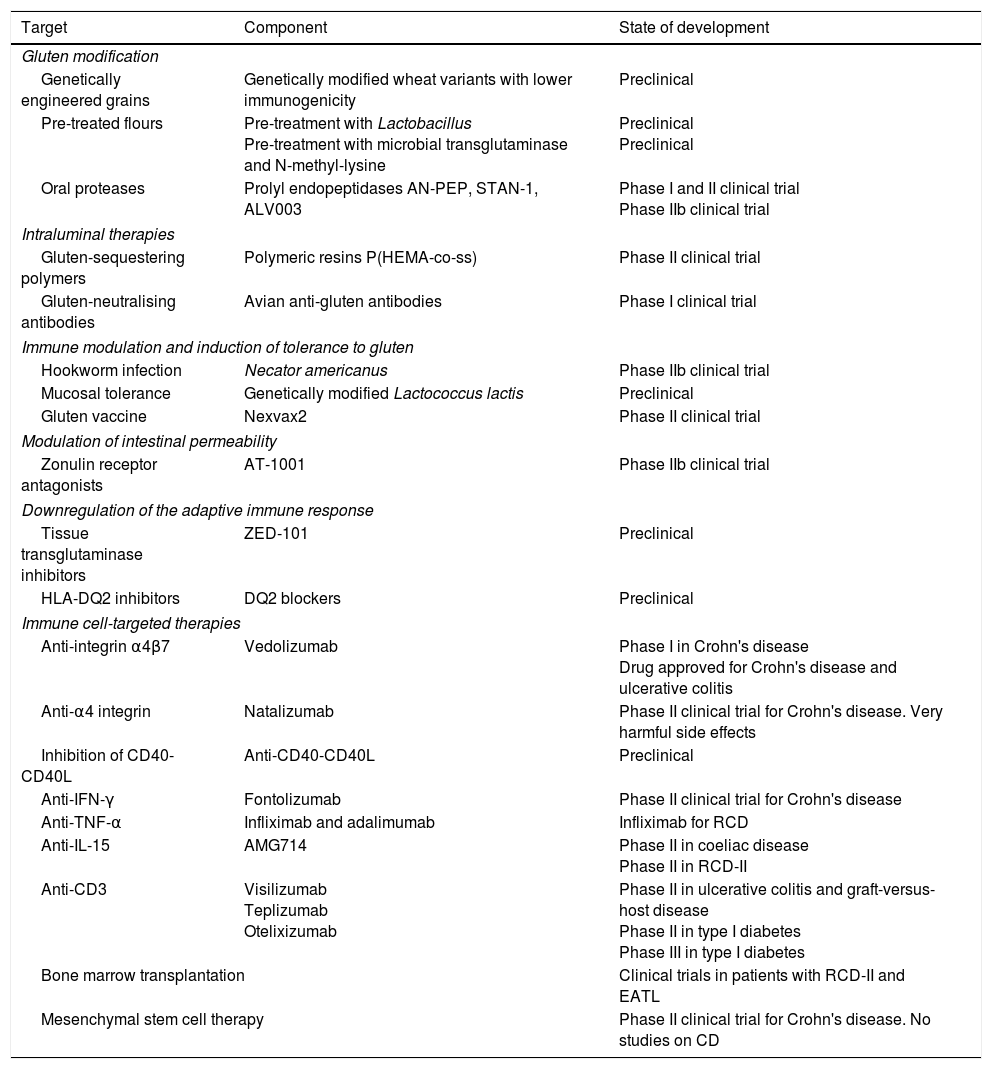
This article analyses therapeutic strategies that have been studied in pre-clinical models of CD and/or show promise in phase I and II clinical trials. Table 2 shows the different therapies grouped according to target. The main targets of the different investigational treatment options are shown in Fig. 1. Therapies targeting Type II refractory CD (bowel lymphoma) and EATL, which are the most severe complications of CD, are also analysed.
Non-dietary therapies for coeliac disease (CD) grouped according to targeted pathogenic objective.
| Target | Component | State of development |
|---|---|---|
| Gluten modification | ||
| Genetically engineered grains | Genetically modified wheat variants with lower immunogenicity | Preclinical |
| Pre-treated flours | Pre-treatment with Lactobacillus Pre-treatment with microbial transglutaminase and N-methyl-lysine | Preclinical Preclinical |
| Oral proteases | Prolyl endopeptidases AN-PEP, STAN-1, ALV003 | Phase I and II clinical trial Phase IIb clinical trial |
| Intraluminal therapies | ||
| Gluten-sequestering polymers | Polymeric resins P(HEMA-co-ss) | Phase II clinical trial |
| Gluten-neutralising antibodies | Avian anti-gluten antibodies | Phase I clinical trial |
| Immune modulation and induction of tolerance to gluten | ||
| Hookworm infection | Necator americanus | Phase IIb clinical trial |
| Mucosal tolerance | Genetically modified Lactococcus lactis | Preclinical |
| Gluten vaccine | Nexvax2 | Phase II clinical trial |
| Modulation of intestinal permeability | ||
| Zonulin receptor antagonists | AT-1001 | Phase IIb clinical trial |
| Downregulation of the adaptive immune response | ||
| Tissue transglutaminase inhibitors | ZED-101 | Preclinical |
| HLA-DQ2 inhibitors | DQ2 blockers | Preclinical |
| Immune cell-targeted therapies | ||
| Anti-integrin α4β7 | Vedolizumab | Phase I in Crohn's disease Drug approved for Crohn's disease and ulcerative colitis |
| Anti-α4 integrin | Natalizumab | Phase II clinical trial for Crohn's disease. Very harmful side effects |
| Inhibition of CD40-CD40L | Anti-CD40-CD40L | Preclinical |
| Anti-IFN-γ | Fontolizumab | Phase II clinical trial for Crohn's disease |
| Anti-TNF-α | Infliximab and adalimumab | Infliximab for RCD |
| Anti-IL-15 | AMG714 | Phase II in coeliac disease Phase II in RCD-II |
| Anti-CD3 | Visilizumab Teplizumab Otelixizumab | Phase II in ulcerative colitis and graft-versus-host disease Phase II in type I diabetes Phase III in type I diabetes |
| Bone marrow transplantation | Clinical trials in patients with RCD-II and EATL | |
| Mesenchymal stem cell therapy | Phase II clinical trial for Crohn's disease. No studies on CD | |
The current hypothesis suggests that reduced exposure to gluten-containing cereals may decrease triggering of CD.52 The wheat variants currently used are considered more immunogenic than ancient or wild variants such as tritordeum or triticum.53 There are genetically modified diploid wheat species that completely lack or have a limited amount of gluten immunogenic peptides, but these species are difficult to grow and develop. One of the strategies used by genetic engineering is the application of RNA interference to silence gluten genes containing CD-toxic epitopes,54 or to engineer wheat variants with reduced immunogenic epitope content, such as hexaploid wheat strains generated from diploid and tetraploid wheat species that are thousands of years old. These wheat strains have been shown to confer less immunogenicity and also have lower proportions of α, β, γ and ω gliadins. Psyllium55 has recently been studied as a possible replacement for gluten because it has minimal effects on wheat odour or texture while retaining suitable baking properties.56 Bread made with psyllium was rated highly both by patients with coeliac disease and non-coeliac controls for its texture and taste. Other studies have also evaluated the genetic modification of wheat through the deletion of key gliadin genes. Specifically, the deletion of the α-gliadin locus on chromosome 6 in the hexaploid modern wheat strain Triticum aestivum led to a decrease in T-cell stimulatory epitopes without significant alterations in baking properties.57 The deleted gliadin genes will probably need to be replaced by non-immunogenic gliadin variants or avenins to obtain adequate elasticity.
The use of different probiotics, such as Lactobacillus, is also being considered as a therapeutic measure. Lactobacilli possess peptidases that, when added to sourdough for fermentation, are able to hydrolyse glutamine and proline-rich gluten peptides, including the highly immunogenic 33-mer gliadin peptide.58 A double-blind study involving 17 patients with CD evaluated symptomatic response to two types of bread containing 2g of gluten: one made with traditional yeast and the other made with Lactobacillus. Patients who ate the bread made with yeast developed enhanced intestinal permeability, as measured by the increased excretion of the carbohydrates lactulose and l-rhamnose, whereas the patients who ate the bread pre-treated with Lactobacillus did not show any changes in intestinal permeability. However, the study duration was only 2 days, which is not enough time to evaluate possible peptidase inactivation and, therefore, firm conclusions cannot be drawn.59 Other authors have used gluten-degrading proteases during wheat fermentation.60 Bread made from flour pre-treated with these proteases eliminates the major glutamine and gliadin proteins, resulting in unpalatable and crumbly bread. Another therapeutic approach that is currently being tested in preclinical studies is to incubate gliadin with anti-transglutaminase antibodies and lysine methyl ester. As a result, lysine-modified gliadins lose their affinity for HLA-DQ2, leading to decreased activation of intestinal T-cells. Pre-treatment of wholemeal flour with lysine methyl ester and microbial transglutaminase derived from Streptomyces mobaraensis greatly decreases the stimulatory effect of the flour on the T-cells.61 The product obtained following pre-treatment with microbial transglutaminase improves the texture and volume of the bread. However, the use of microbial transglutaminase in food preparation remains a matter of debate. Further studies are required to develop and validate non-immunogenic flours and to produce bread and other baked products with good consistency and adequate nutritional value.
Gluten detoxification using oral proteasesGluten proteins are difficult to digest due to their high proline and glutamine content. This feature makes them resistant to proteolysis by gastric, pancreatic and intestinal brush-border endopeptidases and exopeptidases.62 This resistance results in the accumulation of long oligopeptides, such as the 33-mer or 26-mer peptides, with high affinity for HLA-DQ2 or DQ8 molecules. These HLA-DQ-deamidated peptide complexes can activate T-cells in patients with coeliac disease. One strategy to prevent these peptides from reaching the lamina propria is to use prolyl endopeptidases (PEP). PEPs are a group of proteases that are able to hydrolyse peptide bonds involving the carboxyl group of an internal proline residue that have not been detected by the body's digestive secretions.63 Therefore, the addition of PEP to the gastrointestinal tract is being considered as a therapeutic system that would complement the action of pancreatic proteases, degrading and preventing the accumulation of these glutamine and proline-rich peptides. Several PEPs have been identified as candidates for the treatment of CD that can be administered orally. Some of these proteases are of fungal origin, such as the PEP from Aspergillus niger, which efficiently digests gluten proteins in vitro.63,64 Bacterial PEPs, such as the PEPs from Flavobacterium meningosepticum, Sphingomonas capsulata and Myxoxoxxus xanthus, have also been studied. These proteases are active at the pH of the duodenum, are moderately resistant to acidic pH and retain enzymatic activity at least in the small intestine of rats.65 In a randomised, double-blind, cross-over study involving 20 asymptomatic patients with coeliac disease who were following a GFD, patients were administered 5g of gluten for 14 days and were subsequently crossed over to consume gluten pre-treated with PEP derived from F. meningosepticum for an additional 14 days. The study showed that pre-treatment with PEP reduced carbohydrate and fat malabsorption after 2 weeks.66
Combination therapy has also been used that involves two proteases acting on complementary amino acid residues: PEP from S. capsulata, which recognises proline residues, and a cysteine protease, from germinating barley seeds, that recognises glutamine residues. The combined action fully detoxifies gluten.67 This two-enzyme system is considered glutenasic activity and is called ALV003.68 In one study, 20 patients with coeliac disease were randomised to receive 16g of gluten for 3 days pre-treated with ALV003 or placebo. No significant differences in clinical response were observed between the two groups. However, patients in the group that received ALV003 had no small intestinal mucosal injury, compared with the group that received placebo which showed increased intraepithelial lymphocytes. A subsequent phase IIb clinical trial with ALV003 has been conducted for which the goal was to establish its efficacy and safety in preventing gluten-related symptoms and immune response in patients with coeliac disease. In this study, adult patients with coeliac disease were randomised to receive ALV003 (n=20) or placebo (n=21), and were then administered 2g of gluten daily for 2 months. Those patients with coeliac disease who received the probiotic showed less histological injury in duodenal biopsies than those patients from the placebo group. No statistical differences in symptoms were observed between the two groups.69 Oral enzyme therapy therefore poses several problems. Firstly, the apparent lack of efficacy may be the main limitation for use in conventional clinical practice. And, secondly, it is necessary to determine how much enzyme supplement should be administered and how much time is required to degrade immunotoxic gluten peptides.
Intraluminal therapiesGluten-sequestering polymersUse of an oral polymeric resin to sequestrate intraluminal gliadin was suggested as a strategy to block access of gluten immunotoxic peptides into the intestinal mucosa. This resin is called P(HEMA-co-ss) (poly(hydroxyethyl methacrylate-co-styrene sulfonate)).70In vitro studies showed that this polymer bound gliadin in a specific manner and prevented its digestion to immunogenic peptides. In vivo studies involving HLA-DQ8 transgenic mice pre-sensitised with gliadin showed that use of these resins reduces intestinal injury.71 This procedure is a therapeutic measure that requires further studies to answer multiple questions, such as potential problems due to a lack of specificity for gliadin and potential binding to other nutrients, or knowing what dose of gluten can be effectively sequestrated in vivo by a given amount of this resin. The phase I study conducted showed that use of such resins is safe, but with side effects such as diarrhoea.
Gluten-neutralising antibodiesOral IgG antibodies that specifically bind to and inactivate intraluminal antigens can be administered.72 The role of cows was initially evaluated as a readily available source of colostrum with a high concentration of antibodies. However, these types of antibodies did not pass phase I due to a lack of clinical efficacy. In contrast, the large-scale production of gluten-neutralising IgY antibodies from chicken egg yolk has been proposed as a safe and effective therapeutic option for patients with coeliac disease following a GFD who have mild to moderate symptoms related to occasional transgressions and who have no allergy to eggs.73 One clinical trial has shown the usefulness of this therapeutic measure (NCT01765647). These antibodies could be labelled as food additives and used as adjunctive therapy to the consumption of gluten-containing foods during travel, meetings, social, family or business events, etc. However, this would not replace the daily intake of a GFD, but would allow ingestion of trace amounts of gluten. Phase I clinical trials to evaluate this type of therapy are underway in the United States.
Immune modulation and induction of tolerance to glutenHookworm infectionGluten desensitisation induced by immunotherapy has been evaluated using different strategies. One possible hypothesis suggests that excessive hygiene causes inflammatory autoimmune processes. One of the measures used to control immunoregulatory activity is infection with helminths, especially whipworm from pigs (Trichuris suis) and hookworms from humans (Necator americanus), with mixed success.74,75 It has been proposed that chronic helminth infection, such as hookworm infection, may alter Th1-weighted immune responses and control different diseases, such as inflammatory bowel disease and coeliac disease. However, the use of hookworms in Crohn's disease does not control symptoms to such an extent to be considered a therapeutic strategy. A double-blind, placebo-controlled study was conducted over 21 weeks in patients with coeliac disease on a strict GFD who were randomised to be infected with N. americanus and then exposed to gluten. No differences in symptoms were observed between the two groups, but subjects with hookworm infection had reduced inflammation of the intestinal mucosa with decreased INF-γ, IL-17, IL-23 and increased IL-10 and TGF-β.75 In a study conducted over 52 weeks in 12 patients with coeliac disease who were infected with N. americanus followed by the introduction of gluten into their diet, no notable histological injuries were observed and there was even a reduction in anti-transglutaminase antibody levels over time.76 These findings suggest that long-term intestinal helminth infection helps prevent immune response to gluten with no major adverse effects, although these results need to be confirmed in further prospective studies.
Induction of mucosal toleranceInduction of intestinal mucosal tolerance has been studied in rodent models of CD.77 In HLA-DQ8 transgenic mice, intranasal administration of α-gliadin after peripheral immunisation decreased INF-γ levels and T-cell proliferation and increased IL-10 levels.78,79 The mechanisms for inducing gluten-specific tolerance are based on the administration of immunogenic gliadin peptides pre-treated with Lactococcus lactis. This results in a deamidated, HLA-DQ8-restricted immunodominant gliadin epitope. This strategy poses multiple questions, such as whether it is possible to also suppress T-cell responses to other gluten epitopes. It is also important to remember that this approach has only been used in mice models, and has never been validated in humans.
Gluten vaccineThe aim of vaccination against autoimmune diseases is to induce regulatory T-cells to suppress T-cell-mediated inflammation. In CD, the aim of this therapy is to shift the T-cell response from pro-inflammatory to regulatory when gluten is ingested. In other words, the aim is to promote “gluten tolerance”.48 Vaccination is based on the administration of 16-mer peptides derived from α-gliadin, ω-gliadin and hordein, which account for 60% of gluten. This gluten vaccine has been studied in murine models involving the subcutaneous injection of these three peptides. The vaccine was observed to reduce proliferation of T-cells exposed to the three peptides in question and IL-2 and IFN-γ levels through increased expression of regulatory T-cells.80 The vaccine is called ImmusanT, Nexvax2 (Cambridge, MA) and was initially developed in Australia. It has passed phase I clinical trials and is currently being tested in phase II clinical trials in volunteers to evaluate its clinical efficacy. The frequency of vaccine administration has not been established, but a weekly or monthly subcutaneous injection may be required to allow patients with coeliac disease to eat foods containing gluten without experiencing adverse effects. However, during the phase I trial, patients who received the formulation at high doses experienced mild abdominal pain. The vaccine is only suitable for patients with the HLA-DQ2 haplotype (most patients with coeliac disease) because the peptides used for sensitisation bind only to the HLA-DQ2 molecule on the antigen-presenting cell. A separate vaccine would therefore have to be investigated for HLA-DQ8–positive patients. The vaccine was chosen as the preferred option among alternative treatments to GFD by patients with CD when compared to genetically modified wheat, peptidases or treatments that decrease intestinal permeability. This could be explained by the prophylactic aspect of immunisation, which requires less frequent administration than other therapeutic procedures requiring daily intake, and may give rise to issues of compliance. Nevertheless, despite the attractive aspects of self-management of coeliac disease and increased food independence, the vaccine may be associated with a higher risk of autoimmune system activation, causing potential progression of the disease to refractory forms or the development of other autoimmune diseases. This potential drawback must be assessed in ongoing studies.
Modulation of intestinal permeabilityModulation of enterocyte tight junctions. Zonulin receptor antagonistsIn healthy individuals, the tight junctions between intestinal epithelial cells regulate the passage and exposure of subepithelial tissues to different macromolecules and bacterial components that could elicit an immune response. One of the molecules affected is gluten-derived peptides, which would stimulate T-cells through the antigen-presenting cells. Patients with active CD have a defect in these tight junctions, which may increase intestinal permeability for immunodominant gluten peptides to reach the lamina propria and trigger the immune response.81,82 Zonulin is a precursor of pre-haptoglobin-2 that is considered a regulator of epithelial permeability and is overexpressed in the intestinal tissue of patients with coeliac disease compared to healthy subjects.83 This protein has a similar effect to the Zonula Occludens Toxin (ZOT) expressed by Vibrio cholerae that alters epithelial permeability secondary to tight junction damage.84 Lammers et al.85 have shown that gliadin binds to the receptor CXCR3 eliciting the release of zonulin, thereby increasing intestinal permeability. Larazotide, or AT1001, is an octopeptide derived from ZOT that antagonises zonulin action by blocking the receptor CXCR3, thereby preventing epithelial damage.86 One of the phase I studies conducted evaluated the safety and efficacy of this orally administered medicinal product in a randomised, double-blind clinical trial.87 In this study, a dose of gluten was administered in association with AT1001 for 4 consecutive days to 14 patients with coeliac disease and the results were compared to a group of 7 patients from the placebo control group (no gluten). Intestinal permeability in both groups was measured by calculating the fractional excretion of lactulose and mannitol. Following gluten stimulation, intestinal permeability remained intact in those subjects who received the treatment, while adverse events, gastrointestinal symptoms, inflammatory markers and cytokine levels were not more frequent than in the placebo group. In a phase IIb dose-escalation study, AT1001 was administered to 184 patients with CD in remission who were challenged to 3g of gluten daily for 42 days. Although the 61 patients administered AT1001 showed a significant improvement in gastrointestinal symptoms and lower antibody titres, the primary endpoint to evaluate intestinal permeability determined as a reduction in the lactulose to mannitol ratio in faeces was not reached.88 Other phase IIb studies have evaluated the efficacy of different doses of larazotide.89–91 The first clinical trial (CLIN1001-004)90 evaluated different doses of AT1001 (0.25, 1, 4 or 8mg) in 86 patients with coeliac disease in remission who had followed a GFD for the previous 6 months. Patients were randomised to receive larazotide or placebo. Patients who received the drug at a dose of 1mg showed less change in fractional excretion of lactulose and mannitol without reaching statistical significance, better clinical response and the IgA anti-transglutaminase antibody titre was less than 10IU/ml. The second clinical trial (CLIN1001-006)89 randomised 186 patients with coeliac disease who maintained a GFD for 6 months prior to receiving larazotide (1, 4, 8mg) or placebo. Excretions of lactulose and mannitol and clinical manifestations were lower in the group receiving 1mg of AT1001, but were not statistically significant. The largest study conducted (CLIN1001-012) evaluated the efficacy of larazotide in 342 patients with coeliac disease who had been on a GFD for more than one year with persistent symptoms.91 This study did not include a gluten challenge and participants were randomised to receive larazotide (0.5, 1 and 2mg) or placebo. Treatment with 0.5mg significantly decreased intestinal and extra-intestinal symptoms (mainly headache and asthenia) compared to placebo. From these studies, it is clear that there is an inverse dose-response relationship. The lowest doses used were more effective than higher doses. Some explanations for this dose-response relationship may be receptor desensitisation or possibly peptide aggregation at higher doses resulting in a loss of effect. Larazotide could therefore be a novel treatment for managing symptoms of patients with CD who are following a GFD and improving quality of life.88
Downregulation of the adaptive immune responseTissue transglutaminase inhibitorsThe tissue transglutaminase enzyme plays a fundamental role in CD pathogenesis. Gluten-derived peptides are bound to HLA-DQ2/8 molecules on the surface of the antigen-presenting cells and are then recognised by the T-cells. In this situation, gliadin peptides need to be deaminated by tissue transglutaminase to have higher affinity for the HLA molecules, obtaining an antigen with a more effective presentation and the ability to develop a more effective immune response. Inhibition of gliadin peptide deamination using tissue transglutaminase 2 inhibitors reduces the peptides’ binding affinity for antigen-presenting cells.43 Various types of competitive, reversible and irreversible transglutaminase inhibitors have been suggested as potential compounds for the treatment of CD, neurological disorders and some types of cancer.92 Cystamine is a competitive transglutaminase inhibitor that has been evaluated in cultures of duodenal tissue from patients with coeliac disease, where it has been found to block the proliferative capacity of T-cells.93 One dihydroisoxazole derivative is an irreversible tissue transglutaminase 2 inhibitor which has been studied in rodents. Following administration to mice, no adverse events were observed and good oral bioavailability, a short half-life and transglutaminase inhibition in intestinal tissue were shown.94 However, despite their efficacy in preclinical trials, transglutaminase inhibitors should be used with caution because of the ubiquitous expression of transglutaminase, causing possible biological deficiencies. A new generation of selective inhibitors of transglutaminase 2 based on a high affinity thiol binding group has recently been studied that increases specificity for the enzyme. They are administered orally, and act by inhibiting tissue transglutaminase 2 in intestinal tissue, and can neutralise the immunogenicity of ingested gluten peptides.18 However, this novel therapy is in the early stages of research.
HLA-DQ2 inhibitorsBlocking HLA-DQ2 and DQ8 molecules is an attractive therapeutic target for preventing activation of the immune response by gluten. This approach has been explored before for other HLA system-associated diseases, but has not been effective, mainly due to the difficulty of delivering blocking drugs to the affected organs. However, oral administration makes it possible to reach the intestinal epithelium in patients with coeliac disease.95 Several peptides with high affinity for HLA-DQ2 have been designed by amino acid substitution and dimerisation or introduction of aldehyde groups.18 These peptide antagonists have shown moderate efficacy by inhibiting IFN-γ production in cultures of cells from patients with coeliac disease, which shows a potential for decreasing gluten-induced T-cell activation. The main drawback of these inhibitors is the ability to retain partial agonist effects on gliadin-stimulated T-cells, causing an exacerbated immune response. Moreover, binding affinity for most antagonist peptides is not high enough to completely block the access of stimulatory gliadin peptides to HLA-DQ2. As a result, efforts have been intensified on identifying optimal HLA-DQ2 antagonists, based primarily on peptides, with a 50-fold higher binding affinity for HLA-DQ2 than immunodominant gluten peptides. However, it is not known whether this type of HLA-DQ2 blocker is able to reach its target in the lamina propria or if they are able to compete with luminal gluten-derived peptides. It is also not known what side effects they may have, such as hypersensitivity reactions or potential immunosuppression, causing secondary infections. Therefore, studies investigating HLA-DQ2 inhibition are currently being conducted in order to identify a highly specific, high-HLA-DQ2-affinity, non-toxic and non-immunogenic antagonist to assess its efficacy and usefulness.
Integrin α4β7 antagonistCirculating leukocytes are selectively recruited to intestinal tissue via cytokines and tissue-specific adhesion molecules. Intestinal T-cells express integrin α4β7⋅. T-cells reach the intestines by binding to integrin α4β7, which facilitates attachment to the intestinal mucosa via the mucosal vascular addressing MAdCAM-1.96 Inhibition of MAdCAM-1 is also a potential therapeutic target. One humanised monoclonal antibody whose target is integrin α4β7 is vedolizumab, a drug that is currently used in inflammatory bowel disease with fewer adverse effects than other biological treatments. A clinical trial is currently being carried out to evaluate the benefit of blocking migration and adhesion to the intestinal mucosa (NCT02929316).97–99
CXCL10 is another cytokine which is primarily expressed by Th1 cells that binds to its receptor CXCR3, strengthening the immune response of CD. Different studies have determined that stimulation of monocytes by gliadin increases CXCL10 expression.100 This finding suggests that CXCL10 may play a role in T-cell recruitment as part of the innate immune response. The ability to block CXCL10 may represent a future therapeutic target in coeliac disease.
Immune cell-targeted therapies: refractory coeliac disease and enteropathy-associated T-cell lymphomaThis section reviews novel therapies that target two of the most serious complications of CD: type II and II refractory CD (RCD) and enteropathy-associated T-cell lymphoma (EATL).
Inhibition of the CD40-CD40L interactionThe interaction between CD40 molecules, located on antigen-presenting cells, and its ligand, CD40L, located on T-cells, is a fundamental signal in T-cell activation. The effect and activity of the CD40-CD40L interaction has been studied using duodenal biopsies from patients with coeliac disease with and without gluten and in healthy subjects. Expression was higher in patients with coeliac disease following a gluten diet and much lower in healthy subjects. Also, when the anti-CD40L antibody was added to biopsies from patients with coeliac disease, decreased production of IFN-γ and other cytokines involved in CD was observed, which finally inhibited the immune response.101 These findings suggest that blocking the CD40-CD40L interaction may be a promising therapeutic option in the future in RCD.
Anti-IFN-γ and anti-TNF-α therapiesThe pro-inflammatory cytokines IFN-γ and TNF-α are important molecules involved in CD pathogenesis secreted by T-cells in response to gluten. These cytokines stimulate matrix metalloproteinase (MMP) secretion by intestinal myofibroblasts, causing remodelling of the intestinal architecture and promoting villous atrophy.102 Intestinal blocking of these pro-inflammatory molecules may help control the inflammatory cascade and prevent proteolytic activation of MMP. The anti-INF-γ monoclonal antibody, fontolizumab, was initially developed for the treatment of inflammatory bowel disease (IBD). However, although it was well tolerated by patients, its development appears to have been currently halted and no clinical trials have been planned for its use in CD.103 Anti-TNF-α monoclonal antibodies (infliximab and adalimumab) are used in clinical practice in IBD, and various publications establish that they may be useful in RCD.104
IL-15 antagonistsThe IL-15 molecule is one of the main pro-inflammatory cytokines involved in the development of CD and its progression to RCD or EATL. Anti-IL-15 monoclonal antibodies have been shown to reverse intestinal damage in animal models with transgenic mice that had the immune enteropathy caused by the over-expression of IL-15.105 Neutralisation of IL-15 is a potential therapeutic option for CD and for RCD-II given that the spreading of premalignant and malignant lymphocytes is conditioned by IL-15. One phase II clinical trial has evaluated the usefulness of an anti-IL-15 monoclonal antibody (AMG714) in patients with rheumatoid arthritis (with great success) and psoriasis.62 Phase IIa clinical trials with AMG714 for CD (NCT02637141) and RCD-II (NCT02633020) have recently concluded, and results supporting the use of an IL-15 antagonist as treatment for RCD or EATL are expected in the near future. Both studies compared the effect of IL-15 and placebo for 12 weeks in CD and for 2 weeks in RCD-II.
IL-10 agonistsIL-10 inhibits the secretion of pro-inflammatory cytokines secreted by Th1 cells. Therefore, in theory, an IL-10 agonist may be used to treat Th1-mediated autoimmune disorders, such as CD or IBD. A phase I clinical trial in patients with Crohn's disease evaluated the use of bacteria that overexpress IL-10, with no clinical benefit observed.106 Another study showed that the adhesion of IL-10 to cultured biopsies from patients with coeliac disease inhibited T-cell activation.107 However, when evaluating the efficacy of subcutaneous IL-10 in patients with RCD, no improvement in intestinal injuries was observed.108 IL-10 agonists are a potential treatment to consider, but further studies are required to determine their potential therapeutic role.109
CladribineCladribine (2-CdA) is a synthetic purine nucleoside. It is cytotoxic and useful in lymphoproliferative disorders. When metabolised, cladribine triphosphate is obtained, a metabolite that induces apoptosis.110 Cladribine is used as treatment for haematological malignancies and autoimmune disorders, such as multiple sclerosis. It is also a potential treatment for RCD-II, together with azathioprine and corticosteroids. A prospective study in a cohort of 32 patients with RCD-II evaluated survival rate, EATL occurrence, clinical course and histological and immunological response rates after administration of cladribine and a median follow-up of 31 months.111 Eighteen of the 32 patients responded well with increased survival. However, 16 patients progressed to EATL and all of these patients died. Another limitation of this study is the fact that there was no control group to increase the internal validity of the study. Cladribine appears to be a promising therapeutic option, but further studies are required to determine its efficacy in CD.
Bone marrow transplantationThe classic treatment of lymphoproliferative disorders with doxorubicin, cyclophosphamide, vincristine and prednisone (CHOP) gives poor results in EATL and low survival rates. It is therefore essential to develop new strategies to improve the results obtained. Autologous haematopoietic stem cell transplantation has been used in RCD-II and EATL.112 Eighteen patients with RCD-II who did not respond to cladribine received an autologous haematopoietic stem cell transplantation following administration of high doses of fludarabine and melphalan. Thirteen patients were transplanted successfully, with a 4-year survival rate of 66%. One patient developed EATL after 4 years of follow-up. Autologous transplantation preceded by conditioning with high doses of chemotherapy in patients with RCD-II after a lack of response to cladribine may be a promising therapeutic strategy. Unfortunately, relapses are common due to the presence of residual cells in the bone marrow.113,114 Therefore, allogeneic stem cell transplantation may potentially be a better therapy, but it also has a higher risk of complications. Studies are currently being conducted to evaluate the use of allogeneic bone marrow transplantation for the treatment of RCD-II or EATL.
Mesenchymal stem cell therapyMesenchymal stem cells have a low immunogenic potential because they lack major histocompatibility complex class I or II and co-stimulatory molecules. This therapy is therefore a safe and promising option for patients who do not respond to autologous bone marrow transplantation.115 Mesenchymal stem cell transplantation has been studied in various inflammatory gastrointestinal diseases, such as IBD that is refractory to conventional medical therapy and liver fibrosis. This therapeutic approach has potential applications for the management and treatment of patients with RCD-II and EATL. However, no clinical trials are currently investigating this therapy.
ConclusionsFig. 1 shows, in diagrammatic form, the different points of aetiopathogenesis of CD which are targeted by the current investigational lines of therapy. The limitations caused by dietary restrictions and lack of efficacy in 30–50% of patients following a GFD poses the need for other therapeutic approaches. Different drugs or non-dietary therapeutic approaches are currently being developed that may be a medium- or long-term useful option in CD. This review describes the main lines of research being developed, highlighting the fact that none of the clinical trials are yet in phase III. Most agents currently being studied are also intended to complement a GFD. Therapies that induce immune tolerance to gluten have the potential to allow gluten to be added to diets again. However, until further studies are available on the use of novel therapies in routine clinical practice, strict compliance with a GFD is still the basic treatment.
FundingThis study has been partly funded with projects by the Instituto de Salud Carlos III, Fondo de Investigación Sanitaria [Health Research Fund] (Ref. PI13/01133 and PI16/01574) and co-funded by the ERDF (European Regional Development Fund).
Conflicts of interestThe authors declare that they have no conflicts of interest regarding this article.
Please cite this article as: Vaquero L, Rodríguez-Martín L, León F, Jorquera F, Vivas S. Nuevas terapias en la enfermedad celiaca y sus complicaciones. Gastroenterol Hepatol. 2018;41:191–204.