Acute liver failure is an uncommon and severe disease characterised by a rapid onset of severe hepatocellular failure in individuals without previous liver disease. Initial management of this entity determines the outcome of the patient. Initial contact with the acute liver failure patients usually occurs in the emergency department, digestology clinic or, in more severe cases, intensive care units. The management of acute liver failure patients in all these cases must be multidisciplinary, involving surgeons and hepatologists who are experts in this condition, meaning those from hospitals with active liver transplant programmes.
This article reviews the current body of evidence concerning the medical management of acute liver failure patients, from the suspected diagnosis and initial management to intensive medical treatment, including the need for an emergency liver transplantation. Moreover, we also review the use of artificial liver support systems in this setting.
La insuficiencia hepática aguda grave es una enfermedad infrecuente de etiología diversa caracterizada por la rápida aparición de insuficiencia hepatocelular grave en individuos sin enfermedad hepática previa. El manejo inicial de esta enfermedad condiciona su evolución posterior. El primer contacto con el paciente afecto de insuficiencia hepática aguda grave suele realizarse en unidades de urgencias, consultas de aparato digestivo o, en casos más graves, unidades de cuidados intensivos. En todos estos casos resulta fundamental un abordaje multidisciplinario que incluya cirujanos y hepatólogos expertos en esta enfermedad, es decir, de centros hospitalarios que dispongan de programa de trasplante hepático.
El presente artículo revisa la evidencia actual en el manejo médico de la insuficiencia hepática aguda grave, desde la sospecha diagnóstica y el manejo inicial, hasta el tratamiento médico intensivo, incluyendo la necesidad de un trasplante hepático urgente. También se revisan las evidencias sobre el uso de sistemas de soporte hepático artificial en esta enfermedad.
Acute liver failure (ALF), also known as severe acute liver injury or fulminant hepatitis, is a rare but always very serious syndrome. It is made even more dramatic by the fact that the people it affects are usually completely healthy until the onset of symptoms. The overall incidence in the West is estimated at fewer than ten cases per million population per year.1 In Spain, a 2007 study showed an approximate incidence of 1.4 cases per million population per year.2
The low incidence of the syndrome and its heterogeneity, with different patterns of presentation, severity and prognosis, mean that the evidence on management is limited and is often extrapolated from other diseases with similar effects (organ failure from other causes). Despite that, the survival of patients with ALF has improved dramatically in recent years, thanks to advances in the management of critically ill patients, and to early diagnosis and, in particular, emergency liver transplants (ELT).
These clinical guidelines from the Societat Catalana de Digestologia (SCD) [Catalan Society of Digestology] are an update of the evidence-based recommendations on the management of ALF. The grades of recommendation and evidence used follow the Grading of Recommendations Assessment Development and Evaluation (GRADE) system.3 This system reflects the quality of the scientific evidence. The grades of recommendation are divided into strong (1) or weak (2); and the grades of evidence into: randomised controlled trial (I); non-randomised controlled trial (II-1); cohort or case-control study (II-2); repeated series or uncontrolled studies (II-3); or expert opinions or observational studies (III).3
DefinitionAcute liver failure (ALF), is defined as liver injury in the context of acute, but potentially reversible, liver disease affecting a previously healthy liver. However, it also includes other aetiologies in which the condition is the acute manifestation of a chronic liver disease (Wilson's disease, reactivation of hepatitis B virus (HBV) in a non-cirrhotic liver, usually in the context of immunosuppression induced by chemotherapy, acute Budd-Chiari and autoimmune hepatitis). The definition has been subject to modification by different authors, but the accepted diagnostic criteria are essentially:
- •
Acute liver disease.
- •
Less than 28 weeks since onset (24 according to the 1999 definition from the International Association for the Study of the Liver).4
- •
Onset of hepatic encephalopathy (HE) as a clinical sign of liver failure (not considered essential in paediatric patients).
- •
Reduction of the prothrombin rate below 40% or INR≥1.5 as a biological sign of liver failure.
- •
Previously healthy liver (with the exceptions mentioned above).
It does not include diseases such as acute alcoholic hepatitis, the acute decompensation of a chronic liver disease (acute-on-chronic liver failure or ACLF), liver trauma or liver failure after hepatectomy.
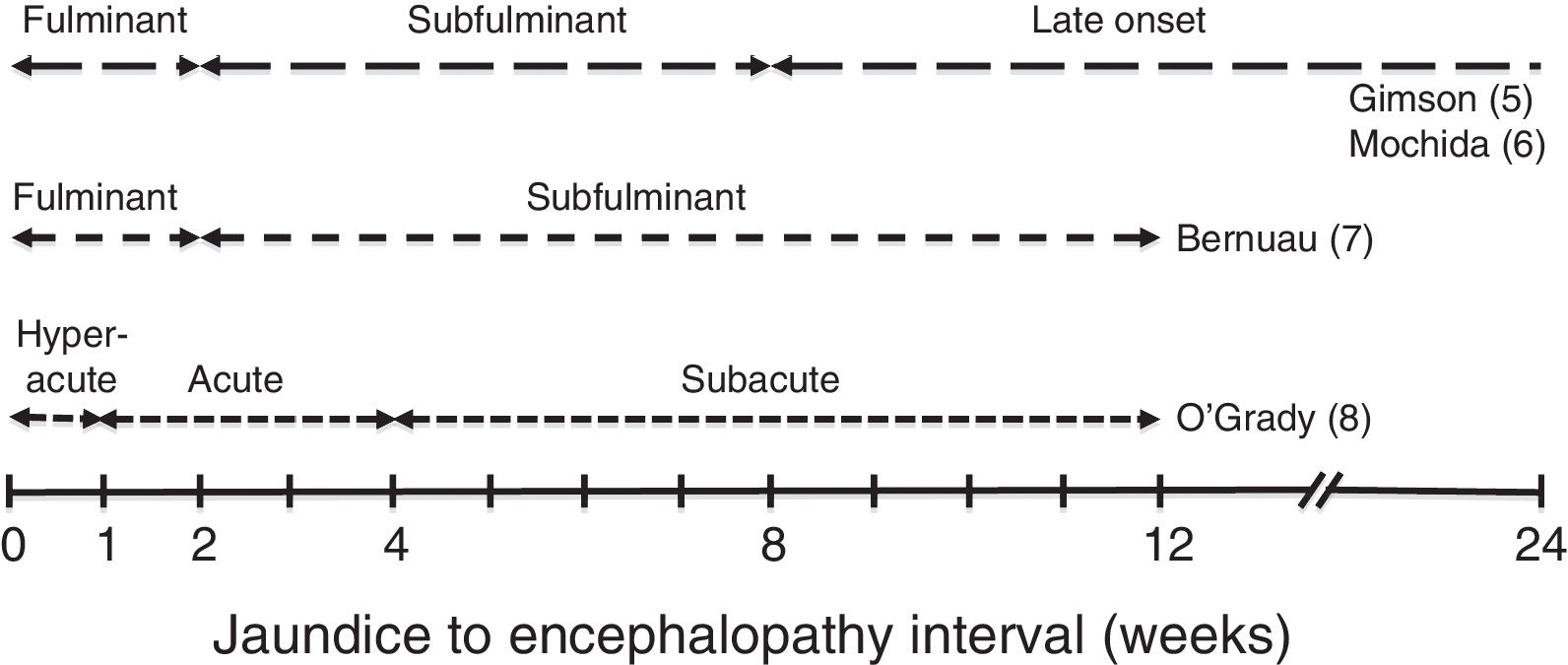
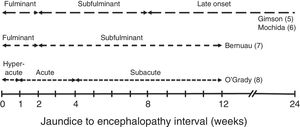
Different clinical courses have been described depending on the time between the onset of jaundice and HE (Fig. 1).5–8
These clinical courses follow the pattern of the predominant aetiology, which is what determines the progress, complications and prognosis of ALF. In general, patients with the fastest developing course (hyperacute and fulminant) tend to present more cerebral oedema, which is why brain herniation is the most common cause of death. However, it has a slightly better prognosis, as it includes cases of paracetamol-related ALF, with spontaneous survival from 20% to 40%.9 In the slower course forms, systemic complications (infections, renal failure) leading to multiple organ failure are usually the cause of death (spontaneous survival less than 10%). In these cases, moreover, there is the added risk of confusing the condition with severe decompensation of a chronic liver disease.
Recommendations- •
ALF is defined as a syndrome, characterised by markers of hepatocellular failure (jaundice and INR>1.5) accompanied by any grade of HE (grade of evidence II-2; grade of recommendation 1).
- •
Although they may have previous liver damage, patients with acute presentation of Wilson's disease, reactivation of HBV in a non-cirrhotic liver, acute Budd-Chiari syndrome or chronic autoimmune hepatitis are considered to be affected by ALF if they develop HE (grade of evidence II-2, grade of recommendation 1).
- •
The development of mild HE is sufficient to establish the diagnosis of ALF and to initiate the diagnostic and therapeutic protocol (grade of evidence II-2, grade of recommendation 1).
- •
The diagnosis of ALF in paediatric patients does not depend on the development of HE (grade of evidence II-3, grade of recommendation 1).
- •
It is crucial not to classify ALF with a subacute course as liver cirrhosis, as we would miss the opportunity of an ELT if it was necessary.
The causes of ALF are shown in Table 1. It is possible that they may have changed over recent years in our area. They are:
- -
Viral. Hepatotropic viruses, including HBV, the most common virus in our region,2 the A and E viruses (main cause of viral hepatitis in non-developed countries)1 and other less common viruses such as herpes simplex and herpes 6, cytomegalovirus (CMV), Epstein–Barr virus and parvovirus. Hepatitis caused by HBV can be particularly dramatic in cases of HBV reactivation in patients immunosuppressed by cancer or chemotherapy.
- -
Toxic. Liver injury caused by drugs is the most prevalent cause of ALF in Western European countries and in the United States.1 Drug-induced liver injuries can be dose-dependent and therefore predictable, as with paracetamol; or idiosyncratic, dose-independent, and therefore unpredictable and with worse prognosis. Among the toxic causes, the one that stands out by far is ALF resulting from paracetamol overdose, particularly in cases of high intake, sustained over a number of days (more than the all-at-once intake with suicidal ideation), which are not intentional (they do not consult the medical centre until obvious symptoms appear), and in malnourished patients and/or active alcoholics. Other toxic causes in our setting are ingestion of Amanita phalloides (A. phalloides), non-steroidal anti-inflammatory drugs, cocaine, ecstasy, antituberculosis drugs, amoxicillin-clavulanic acid, amphetamines, etc.
- -
Miscellaneous. Acute fatty liver of pregnancy and HELLP syndrome (haemolysis, elevated liver enzymes and low platelet count), Budd-Chiari syndrome, heat stroke, Reye's syndrome, autoimmune hepatitis, Wilson's disease (spontaneous survival 0%, so the suspected diagnosis is vital), cancer that has spread to the liver, ischaemic hepatitis, associated with sepsis±heart failure, infections (malaria, dengue, etc.), haemophagocytic syndrome, etc. Note that the last five of the above aetiologies should not be treated with an ELT.
- -
Cryptogenic. In other words, cause not clarified despite thorough investigations. This may account for 20–30% of cases and it has the worst spontaneous survival rate.2
Prevalence (%) of the different causes of severe acute liver injury in our setting.
| Aetiology | 1992–20002 | 2001–201042 |
|---|---|---|
| Viral hepatitis | 37 | 29 |
| Hepatitis A virus | 88 | 84 |
| Hepatitis B virus (± D virus) | 5 | 12 |
| Other viral aetiology | 7 | 4 |
| Toxic hepatitis | 19 | 31 |
| Paracetamol | 12 | 30 |
| Others (Amanita phalloides, NSAID, diphenylhydantoin, halogenated anaesthetics, antidepressants, amphetamines, cocaine, ecstasy, etc.) | 88 | 70 |
| Miscellaneous | 12 | 24 |
| Acute fatty liver of pregnancy | ||
| Budd-Chiari syndrome | ||
| Liver invasion | ||
| Ischaemic hepatitis | ||
| Heatstroke | ||
| Autoimmune hepatitis | ||
| Wilson's disease | ||
| Adult Reye's syndrome | ||
| Cryptogenic | 32 | 16 |
Whatever the cause, it is unknown to what extent taking paracetamol may be a cofactor in some cases, especially those in whom paracetamol adducts are detected.
Recommendations- •
The most common causes of ALF in our region are viral (especially HBV) and toxic/drug-induced. In recent years, there seems to have been an increase in the incidence of ALF due to paracetamol overdose, this being the main cause of ALF in Anglo-Saxon and Northern European countries (grade of evidence III, grade of recommendation 2).
- •
Thorough toxicological screening should be carried out in all cases and, if there is reasonable doubt, paracetamol levels should be checked. If this is not possible, N-acetyl-cysteine (NAC) should be started (grade of evidence II-2, grade of recommendation 1).
- •
Testing for possible viral infections as cause is mandatory (grade of evidence II-2, grade of recommendation 1).
- •
Many cases of suspected autoimmune hepatitis require a liver biopsy for confirmation. This needs to be performed early as corticosteroid therapy could be effective. However, if after one week of treatment with corticosteroids there is no improvement, ELT should be considered because of the high risk of infection associated with the use of corticosteroids (grade of evidence II-2, grade of recommendation 1).
- •
If cancer spread to the liver is suspected (previous history of cancer or hepatomegaly), comprehensive imaging studies and/or liver biopsy should be performed (grade of evidence II-3, grade of recommendation 1).
Patients will often not arrive at the first consultation centre with well-established ALF. Instead, they may have the signs of acute hepatitis, with only a few non-specific symptoms, and an increase in transaminase levels which may be more or less marked. In these cases, it is essential to keep the patient under observation and refer him/her to a hospital with a liver transplant programme before the onset of HE, as that then indicates a degree of advanced hepatocellular failure.
We do not fully understand what makes a patient with severe acute hepatitis without HE evolves to ALF, but we do know that this progression is more likely to occur in certain cases. It is precisely these high-risk patients that must always be referred to a hospital capable of performing ELT or treating cases with contraindication to ELT.
Risk criteria for referral of patients with acute hepatitis10- -
- Patients with a prothrombin index from 30% to 50% and any of the following conditions:
- •
Children<15 years of age.
- •
Adults>40 with suspected aetiology with poor prognosis for spontaneous survival (e.g. liver injury caused by drugs, Wilson's disease, cryptogenic).
- •
Fever>38°C.
- •
Immediate post-operative period.
- •
Pregnancy.
- •
Comorbidities: diabetes mellitus, HIV infection, previous cancer, malaria, severe acute kidney injury, metabolic acidosis.
- •
Plasma bilirubin>250μmol/l (14mg/dl).
- •
- -
- Patients with a prothrombin index below 30%:
- •
Any patient (particularly if >40 or suspected aetiology with poor prognosis).
- •
Once the diagnosis of ALF is established, i.e. after they develop HE, regardless of the suspected aetiology, the patient should be moved to an ICU in a centre capable of performing ELT.
Recommendations- •
Early referral of a patient with severe acute hepatitis to a centre with a liver transplant programme before the onset of HE means that assessment can begin for a potential ELT, while giving the patient a greater chance of spontaneous survival or of making it to the ELT well enough to survive such complex surgery (grade of evidence III, grade of recommendation 1).
- •
Referral to a specialised centre is vital in cases of ALF with a subacute course, in view of the high incidence of associated complications, and in cases of extrahepatic involvement (grade of evidence III, grade of recommendation 1).
The patient will be admitted to the ICU while ALF persists, and be subject to conventional monitoring. Additionally, the following information is essential:
Medical historyWith special emphasis on obtaining the patient's history concerning:
- •
Family and personal history suggestive of previous liver disease.
- •
Other diseases (especially cancer and cardiorespiratory disease) and comorbidities.
- •
Potential recent exposure to viruses (enteral, parenteral or sexual), travel.
- •
Chronic carrier state (HBV).
- •
Ingestion of drugs in the previous six months (conduct a thorough assessment with the patient and companions, questioning repeatedly about the use of paracetamol in its different commercial forms, plants, complementary medicines, etc.).
- •
Exposure to toxic substances and drug addiction (including alcohol consumption).
- •
Mental health history (attempts at self-harm).
- •
Pregnancy.
- •
History of autoimmune disease.
- •
Treatment with immunotherapy or chemotherapy.
- •
Previous quality of life.
- •
Form of presentation (prodromes suggestive of acute viral liver disease, gastroenteritis in A. phalloides poisoning, etc.).
- •
Time (jaundice to encephalopathy interval).
- •
Accompanying clinical manifestations.
- •
Previous or current illnesses (especially any that may be a contraindication to a possible ELT).
Aimed at identifying diagnostic criteria and signs indicative of the aetiology, ruling out signs of chronic liver disease and detecting complications, especially infectious complications. Continuous or very frequent neurological examination is of great importance, including focal signs (which would indicate the need to perform a brain scan), degree of HE, presence of myoclonia or seizures, pupillary responses, etc. Full examination of the patient should be repeated at least every 12h. In the event of any neurological abnormality, the differential diagnosis with other disorders such as alcohol withdrawal or metabolic problems will have to be considered.
Monitoring of vital signsFrequent monitoring (at least every 4h) is required of blood pressure, heart rate, respiratory rate, temperature, diuresis and other parameters according to the case. As hypoglycaemia is a very common complication, blood glucose should be measured every 2h.
Complementary investigationsAimed at ascertaining the aetiology and severity of the condition, assessing its prognosis (ELT criteria) and identifying possible complications or the risk of developing them.
First. Blood tests- •
Hepatitis viruses: HBV (HBsAg, anti-HBc IgM, HBV DNA in all cases given the limited but real possibility of an escape mutant for HBsAg, which markedly decreases the sensitivity of diagnostic tests), HDV if HBsAg+ (delta antigen and anti-HDV), HAV (HAV IgM), HCV (HCV IgG), HEV (HEV IgM and HEV RNA as the antibodies are sometimes negative).
- •
Other less common viruses: IgM and PCR for herpes simplex, herpes 6, varicella zoster, Epstein–Barr, parvovirus and CMV.
- •
When there is well-founded suspicion: amanitins in urine, plasma levels of paracetamol or other toxicological determinations (cocaine, alcohol, etc.).
- •
Pregnancy test, both in cases of suspected acute fatty liver of pregnancy and HELLP syndrome, and in suspected pregnancy in a patient with acute hepatitis of any cause.
- •
Anti-tissue antibodies, protein electrophoresis and immunoglobulin dosage. We need to remember that autoimmunity can participate in the pathogenesis of ALF without clearly being the cause. In fact, 50% of cryptogenic ALF may have “scores” of probable autoimmune hepatitis, such as certain forms of viral hepatitis in which there is an increase in IgM. Unconfirmed cases are not usually treated with corticosteroids.11
- •
In patients aged<40 without known cause of ALF (once viral origin is ruled out), copper in blood and 24h urine (high urinary copper is highly suggestive of Wilson's disease) and serum ceruloplasmin. Ophthalmological examination with slit lamp to rule out Kayser–Fleischer corneal ring. Suspected Wilson's disease can be confirmed with a high degree of reliability in cases with alkaline phosphatase/bilirubin ratio<4 and AST/ALT>2.2.12
In order to rule out signs suggestive of chronic liver disease (key condition with which the differential diagnosis should be established), infiltrative liver disease, thrombosis of supra-hepatic veins (Budd–Chiari syndrome), and to demonstrate patency of the portal and hepatic arteries. If in doubt, particularly because of possible hepatic infiltration or vascular permeability, a CT or MRI should be requested.
Third. Liver biopsyThe need to obtain a liver biopsy is open to debate, particularly from the point of view of the risk/benefit balance considering the patient's coagulation disorder. However, the survival of patients is clearly related to how early the most appropriate treatment can be given, and that can often only be established after liver biopsy. In addition, we have techniques that minimise the risks of the liver biopsy, such as transjugular liver biopsy.13 Biopsy becomes more important in cases of uncertain aetiology or suspected chronic or infiltrative liver disease.
Parameters with prognostic value and assessment of potential complicationsA series of parameters was recently described which can help to profile the prognosis of ALF. It should be used in combination with the classic ELT criteria that we will discuss later. These parameters are:
- •
Renal function, including acid-base balance. In fact, acidosis is a criterion for ELT in paracetamol overdose.
- •
Arterial blood ammonia. As we will discuss later, serum ammonia figures correlate with the risk of presenting cerebral oedema.14
- •
Arterial lactate. This reflects the failure of peripheral and hepatic perfusion, and impaired hepatic clearance. It has been suggested that the change in arterial lactate values after initial resuscitation or 12h later during follow-up has prognostic value.15
- •
Phosphorus. The determination of the serum phosphorus level has prognostic value in multiple models of ALF16 and in patients with ALF receiving a living-donor transplant.17 A study on ALF due to paracetamol overdose found that patients with hyperphosphataemia had the worst prognosis. In this case, hyperphosphataemia could be the result of both the absence of liver regeneration (which consumes phosphorus) and the presence of acute kidney injury.18
- •
In order to rule out potential complications, serum amylase and lipase should also be determined (possible additional pancreatitis), along with arterial blood gases (respiratory and metabolic complications), chest X-ray (infections, etc.), electro- and echocardiograms, and serial cultures.
- •
Patients with ALF require strict monitoring which, at the very least, should include: clinical evaluation every 12h; daily examination of physiological, analytical and metabolic parameters (grade of evidence III, grade of recommendation 1).
- •
Repeated assessment of the degree of HE must be carried out as routine (grade of evidence III, grade of recommendation 1).
- •
Kidney function should be monitored both analytically (serum creatinine) and clinically (hourly diuresis) (grade of evidence III, grade of recommendation 1).
- •
Liver biopsy, by the transjugular route, is a useful but not essential tool. It is of greater utility in cases of cryptogenic ALF and when there is suspected invasion of cancer, liver cirrhosis or alcoholic hepatitis (grade of evidence II-3, grade of recommendation 1).
- •
Chest X-rays should be performed with some regularity, especially in intubated patients, in order to rule out pneumonia (grade of evidence III, grade of recommendation 2).
These measures must be followed while investigating the aetiology and prognosis, regardless of the cause of the ALF. The first and important general rule is to STOP ALL DRUGS the patient is taking, except for hormone replacement therapies.
N-acetyl cysteine. The effectiveness of N-acetyl cysteine (NAC) is determined by its antioxidant effect, ability to increase nitric oxide availability, anti-inflammatory action through the nuclear factor kappa B, and vasodilation, which increases peripheral blood flow. We now know that the use of NAC increases liver transplant-free survival both in paracetamol overdose-related ALF and in ALF not caused by paracetamol.19 In non-paracetamol-induced ALF, efficacy is limited to patients with grade i-ii HE, i.e. in the early stages of the condition.19 The above data, in conjunction with its lack of adverse effects, mean that the administration of NAC, as early as possible, is considered an absolute requirement in ALF. The dose and duration of the administration regimen differ from those in paracetamol overdose (72h infusion is recommended, but no more than five days, because of uncertain effects on liver regeneration). The usual regime in non-paracetamol-induced ALF would therefore be: NAC 150mg/kg over 1h followed by 50mg/kg over 4h and lastly 6.25mg/kg over 67h (total: 72h).
Diet. Patients with ALF are in a marked hypercatabolic state. Moreover, in view of the metabolism of ammonia and the risk of infection, it is crucial to prevent the loss of muscle mass and to ensure the patient's optimum immune status. A normal-protein diet is recommended in patients with HE 0-I. In more advanced degrees of HE, oral intake is contraindicated in non-intubated patients due to the risk of pulmonary aspiration. Enteral or parenteral nutritional support has not been demonstrated to improve the prognosis.
Hydration. Enough to maintain fluid balance considering possible hypovolaemia due to vomiting, poor intake and vasodilation, but also avoiding hypervolaemia. It is recommended to avoid using hypotonic solutions, in order to prevent episodes of both hypoglycaemia (associated with development of renal failure) and hyponatraemia (with the corresponding increase in intracranial pressure, ICP). Hypoglycaemia is very common in these patients. Monitoring blood glucose every 2h and maintaining blood glucose in the range 150–200mg/dl is therefore recommended. Sodium should be maintained at 140–145mEquiv./l.
Hepatic encephalopathy. The degree of HE correlates with the prognosis of patients with ALF. In patients with subacute ALF, the presence of mild HE (grade i) already implies a poor prognosis; while in fulminant or hyperacute cases, the greater the degree of HE, the worse the prognosis. In any event, HE can develop and progress very rapidly, and the patient's neurological status therefore has to be monitored as standard and at frequent intervals. We also need to avoid and/or correct possible trigger factors for HE (sedatives, antiemetics, hypoxaemia, hypoglycaemia, hypophosphataemia, acidosis, etc.). Conventional treatment is recommended for grades I–II HE (non-absorbable disaccharides orally or by nasogastric tube or in the form of retention enemas and/or rifaximin) although there is no scientific evidence to support it. In patients with grade III–IV HE, enemas are contraindicated because of the risk of increasing ICP. In these cases, we should consider orotracheal intubation to protect the airway, with adequate sedation and analgesia, with or without mechanical ventilation.
Coagulopathy. This must not be corrected. In fact, patients with ALF have a balance between the deficiency in pro- and anticoagulant factors, which makes bleeding uncommon. Moreover, correcting the prothrombin index or INR may make it difficult to assess the ELT criteria. Therefore, we should only correct the coagulopathy in the event of clinically significant haemorrhagic manifestations or prior to invasive tests with a high risk of bleeding (placement of an ICP sensor, lumbar puncture, etc.). Fresh frozen plasma may be used or, more often, prothrombin (Factor II) in order to limit the increase in the patient's blood volume. To rule out possible nutritional deficiencies, 20–30mg of vitamin K should be administered.
The need for thromboprophylaxis should be decided on an individual basis according to each patient's risk.
The transfusion threshold is 7g/dl of haemoglobin.
Antibiotics. Administration of selective digestive decontamination regimens protects these patients from the development of infectious bacterial and fungal complications, although it does not increase survival.20 The patients at greatest risk of infections are those with grade iii or iv HE or rapidly progressive HE, renal failure and systemic inflammatory response syndrome (SIRS) parameters. A typical empirical regimen is administration of norfloxacin 400mg/day and nystatin 1MU every 8h.
Proton-pump inhibitors. They tend to be administered because of the coagulopathy and the risk of organ failure, but there is no clear evidence of their efficacy in ALF. The potential benefit is counteracted by the risk of ventilator-associated pneumonia and/or Clostridium difficile superinfection. If the patient is receiving enteral nutrition, they are not considered necessary.
Recommendations- •
Early administration (HE grade II or lower) of NAC is recommended in all patients regardless of suspected aetiology (grade of evidence I, grade of recommendation 1).
- •
In view of the patient's hypercatabolic state, we have to ensure adequate nutrition (grade of evidence II-3, grade of recommendation 1).
- •
The oral or enteral route is contraindicated in patients with HE grade II or higher (grade of evidence III, grade of recommendation 1).
- •
Patients in grade III–IV HE should have orotracheal intubation to preserve the airway and we must be alert for clinical signs of increased ICP (grade of evidence III, grade of recommendation 1).
- •
Hypoglycaemia is associated with a higher mortality rate and should therefore be avoided, while also avoiding hyperglycaemia (grade of evidence II-3, grade of recommendation 1).
- •
Hyponatraemia should be avoided. Maintaining serum sodium at 140–150mEquiv./l is recommended (grade of evidence II-2, grade of recommendation 1).
- •
The routine use of fresh plasma and/or other clotting factors is contraindicated except in specific situations such as placement of intracranial sensors and presence of active bleeding (grade of evidence II-3, grade of recommendation 1).
- •
Antibiotic prophylaxis does not increase survival in ALF, but it does reduce the incidence of infections (grade of evidence II-2, grade of recommendation 1).
- •
The administration of proton pump inhibitors should be considered on an individual basis, assessing the pros and cons (grade of evidence II-3, grade of recommendation 1).
There are certain causes of ALF which are treatable, although in most cases the treatment is unlikely to modify the course of the condition, as the liver injury is already established by the time it is diagnosed. Among the treatable causes it is important to remember the following:
- •
The potentially hepatotoxic drug should be discontinued immediately. As we do not know the potential hepatotoxicity of many drugs, they should all be discontinued except for hormone replacement therapies (insulin, thyroid hormones, etc.).
- •
Infections due to herpes simplex virus, herpes 6, VZV and CMV: acyclovir and ganciclovir are the treatment options. The empirical administration of acyclovir at high doses covers both herpes infection and CMV.
- •
Both in the reactivation of HBV (positive HBV-DNA) and in primary HBV infection, often impossible to differentiate, the administration of nucleoside/nucleotide analogues has been shown to be effective.21
- •
Treatment of both A. phalloides and paracetamol poisoning can prevent the onset of hepatotoxicity. In the first few hours after ingestion of the toxin (up to 3–4h, but better within the first hour) activated charcoal should be administered orally (25g in 240ml of water every 3h) to reduce absorption of the toxin.
- -
In the event of intoxication by A. phalloides it is also advisable to induce diarrhoea (30g of sodium sulphate in the same activated charcoal water) unless the patient has diarrhoea spontaneously. To block the entry of amatoxins into the hepatocyte, penicillin G sodium (48M units/day if no renal failure) and silibinin (1400mg/day IV) are usually administered simultaneously. Specific ELT criteria have been established in this condition (Table 2).22
Table 2.Emergency liver transplant criteria in ALF.
Non-paracetamol-poisoning ALF One or more of the following criteria: • Severe hepatic encephalopathy (grade III–IV) • Prothrombin time expressed as INR>7 (or <10% expressed as index) • Factor V <20% in patients aged <30; <30% in patients aged >30, provided it is associated with any grade of hepatic encephalopathy • Lack of obvious improvement with conventional treatment in subfulminant or subacute forms ALF from paracetamol poisoning • pH <7.3 regardless of grade of encephalopathy • Or the following 3 criteria: - Grade III or IV encephalopathy - Prothrombin time >100s (INR>6.5) - Creatinine >300mmol/l (3.4mg/dl) ALF fromAmanita phalloides • Prothrombin index <10% (INR>6) 4 days after ingestion ALF from Wilson's disease • ALWAYS • Before HE develops: more than 7 points on the modified Nazer score for Wilson's disease (according to bilirubin, leucocytes and INR values) - -
In suspected paracetamol overdose, NAC should be administered at the usual doses as early as possible; the interval between the ingestion of paracetamol and the start of NAC has prognostic implications. The NAC regimen is: bolus of 150mg/kg in 250ml 5% glucose over 1h+50mg/kg in 500ml 5% glucose over 4h+100mg/kg in 500ml 5% glucose over 16h. This is a total of 21h of treatment. To determine whether or not we should treat the patient, plasma paracetamol levels are used in relation to the hours since ingestion or the difference between two separate 2h determinations. However, we often do not know the exact time or times the toxin was taken; in case of doubt, it is therefore recommended to start treatment as soon as possible.
- -
- •
The correction of haemodynamic disorders is the only treatment in cases of shock liver with or without associated stasis.
- •
Immunosuppressive treatment (corticosteroids) can be effective in cases of autoimmune origin if they are administered very early. However, it should also be remembered that they lead to an increased risk of opportunistic infections, particularly if the patient has an ELT. Therefore, initial treatment of 5–7 days is recommended and, if no improvement is observed, withdraw them and consider an ELT.9
- •
The immediate termination of pregnancy significantly improves the prognosis of patients with fatty liver of pregnancy or HELLP and ALF.
- •
ELT will always be required in ALF due to Wilson's disease. Plasma exchange is indicated when required to reduce haemolysis and renal failure caused by high plasma copper levels, and as a bridge to transplantation. d-Penicillamine is a poor therapeutic option in this phase of the disease. It is only recommended in very selected patients: young people, less than two months since disease onset, with haemoglobin levels still above 8g/dl and without HE (before developing the signs and symptoms of ALF). For its limiting effect on the intestinal absorption of copper, zinc is the maintenance treatment for Wilson's disease, but there are no data on its efficacy in the acute form of presentation. Once HE occurs, transplant is unavoidable, as there is no hope of spontaneous survival. There is a specific score for indication of ELT in ALF due to Wilson's disease (Table 2): the modified Nazer score for Wilson's disease, which is based on liver function data (INR, albumin and bilirubin), AST levels and peripheral blood leucocyte count.23 It is not therefore necessary to wait for the patient to present the classic criteria (advanced HE) for ELT to be indicated.
- •
Transjugular intrahepatic portosystemic shunt (TIPS) combined with anticoagulation and treatment of the underlying disease is the alternative of choice in Budd–Chiari syndrome or in some cases of acute veno-occlusive disease. The indication has to be early, as an ELT is usually required in more advanced cases.
- •
Chemotherapy is the only treatment in cases of cancer invasion. The utility and type of chemotherapy should be established in collaboration with oncologists and/or haematologists depending on the type of cancer and its stage of progression.
- •
Antibiotic treatment (with or without drainage or surgery) is the treatment of choice in cases of ALF due to systemic bacterial infections.
- •
The treatment of hyperthermia could limit the progression of ALF caused by this mechanism (heat stroke or ecstasy intake, often associated with hyperthermia).
ALF is a systemic disease in which first of all the liver fails. However, if left untreated, it will progress and affect all organs, leading to multiple organ failure and sepsis. Not only can this contraindicate ELT, it can also cause the death of the patient.
Cardiorespiratory failureCirculatory abnormalities characterised by a reduction in systemic vascular resistance and effective central volume, and an increase in cardiac output are common in patients with ALF. In more advanced cases, it can cause severe hypotension associated with tissue hypoxia, which leads to anaerobic metabolism with accumulation of lactic acid (hence the importance of serial lactate determinations). This phenomenon seems to play an essential part in the development of multiple organ failure. Hyperlactacidaemia should improve with volume replacement, but the liver's inability to metabolise lactate also plays a role. The treatment with NAC could be beneficial in this aspect by increasing liver perfusion.19
In patients with haemodynamic instability or requiring ELT, an echocardiogram should be performed to assess the presence of previous or current heart disease. Remember that cardiac output can also be determined by arterial pulse wave monitors (as long as there are no heart rhythm disorders) or with a Swan–Ganz catheter.
The cardiocirculatory support required by these patients does not differ from that required in other critical diseases: replacement of blood volume with normal saline-type crystalloids and vasoactive drugs. Administration of albumin has not demonstrated any utility. The vasopressor agent of choice is noradrenaline (objective: to maintain mean arterial pressure >60mmHg, >75mmHg in individuals with previous hypertension). If noradrenaline is not enough, dobutamine can be added in the case of left ventricular dysfunction, or vasopressin or its derivative terlipressin.24 In cases refractory to vasoactive drugs, we should consider the possible presence of relative adrenal insufficiency, which is treated with hydrocortisone, although its utility in the survival of these patients has not been demonstrated.
Respiratory failure is common in ALF and is usually multifactorial (ventilatory failure associated with coma, pulmonary atelectasis in intubated patients, bronchopneumonia, adult respiratory distress syndrome, etc.). Its management, including the indications for mechanical ventilation, type of ventilation (protective, with tidal volume from 6 to 8ml/kg of ideal weight), sedation and analgesia, etc., are no different from those of other critical patients. Non-invasive ventilation is not an option due to the possible progression of HE and the risk of pulmonary aspiration.
Recommendations- •
Patients with ALF require extremely delicate management of blood volume, avoiding both hypo- and hypervolaemia (grade of evidence II-1, grade of recommendation 1).
- •
In the case of persistent hypotension, vasoactive support should be initiated. Noradrenaline is the drug of choice (grade of evidence II-3, grade of recommendation 1).
- •
The use of hydrocortisone does not reduce the mortality rates associated with the condition, but it may reduce the need for vasoactive drugs (grade of evidence II-1, grade of recommendation 1).
- •
Strategies for ventilation are as standard (protective ventilation) (grade of evidence II-3, grade of recommendation 1).
We discussed earlier the fact that the presence of HE is a key factor in the diagnosis as well as in the progress and prognostic assessment of patients with ALF. The presence of HE can mean both liver failure and the development of other serious complications, for example, a marked inflammatory response or sepsis, and this has to be actively looked for.
The most feared neurological complication in ALF is the development of cerebral oedema, with this being the cause of death in 20–25% of patients. The pathophysiology of cerebral oedema is not fully understood, but an inflammatory process that alters cerebral blood flow and increases the permeability of the blood-brain barrier to toxins, and an increase in circulating ammonia levels due to impaired hepatic metabolism of urea, are both known to be involved. Hyperammonaemia causes an increase in the intracellular osmolarity of the astrocytes, changes in the neurotransmitters, and alteration of the astrocyte mitochondrial function; this then triggers the “swelling” characteristic of these cells and finally leads to brain dysfunction.25 This situation is especially serious in fulminating, hyperacute or acute cases, where the compensatory mechanisms that prevent cerebral oedema from causing an increase in ICP (as occurs in subacute ALF or in liver cirrhosis) do not have time to take effect, with the consequence that the simple presence of cerebral oedema will lead to intracranial hypertension.
Cerebral oedema is a virtually constant complication in patients with grade iii or iv HE, although, as already mentioned, not all cases involve intracranial hypertension. Previous studies show that the risk of developing intracranial hypertension correlates closely with arterial ammonia. Patients with serum ammonia >150–200μmol/l are considered high risk for increased ICP.14 Other risk factors are the presence of hyponatraemia and hypo- or hyperglycaemia.
Continuous monitoring of ICP through placement of an epidural or subdural sensor in patients with advanced HE is the subject of debate, particularly because of the risk of cerebral haemorrhage which, although low, is not negligible.26 There are centres where continuous monitoring of ICP is the norm in patients with HE III–IV, and others where it is never performed. In order to optimise the risk/benefit, experts in the field recommend limiting ICP monitoring to very high-risk patients, which would cover those who meet ELT criteria (Table 2) and also: (1) are young with acute or hyperacute presentations; (2) cases with seizures or pupillary abnormalities; (3) presence of three or more SIRS criteria; (4) arterial blood ammonia >150μmol/l; (5) hyponatraemia <135mEquiv/l; (6) need for vasoactive drugs; and (7) indirect signs that indicate a very low or very high cerebral blood flow (if the oxygen saturation in the jugular bulb is measured, SjO2, normal 55%–70%, or a Doppler ultrasound of the middle cerebral artery is performed).27 As a precaution, before placing the sensor, we have to ensure the patient's blood is clotting. ICP should continue to be monitored until the neurological symptoms have resolved.
Treatment of cerebral oedema. Preventive measures (aimed at reducing arterial ammonia and avoiding aggravating factors) or therapeutic measures should be applied if necessary. The aims of the treatment are to maintain ICP<20mmHg and cerebral perfusion pressure (CPP, difference between mean arterial pressure and ICP≥50–60mmHg).
The general treatment measures would be: to ensure the correct cerebral venous drainage (head of the bed elevated to >30° and head kept in midline); keep the patient free of sensitive and sensory stimuli (enemas should not therefore be administered); avoid fever, hypo/hyperglycaemia and electrolyte disorders, especially hyponatraemia; delicate adjustment of fluid balance (the crystalloid of choice is 0.9% NaCl); pharmacological sedation (continuous intravenous infusion preferably of propofol, midazolam in case of hypotension or haemodynamic instability, at conventional doses); analgesia according to requirements (fentanyl or remifentanil); and, in exceptional cases, relaxation. Non-absorbable antibiotics are not usually effective and the administration of lactulose may be counterproductive. Agents acting on the urea cycle, such as l-ornithine-l-aspartate, also do not appear to have any effect on cerebral oedema.28
When despite these preventive measures, episodes of intracranial hypertension occur (ICP>20mmHg), we should proceed as follows:
First-line measures- •
Hyperosmolar therapy. Contraindicated if plasma sodium is >150mEquiv./l. By administration of 20% mannitol or hypertonic saline solution (in cases of plasma sodium <135mEquiv./l, hypotension or haemodynamic instability). The efficacy of the two treatments is similar, although hypertonic saline is associated with a lower incidence of acute renal failure or rebound ICP elevation after treatment. The efficacy of this therapy is uncertain when the ICP exceeds 60mmHg. If the patient has renal failure or no diuretic response to mannitol despite changes in plasma osmolarity, the treatment should be combined with continuous renal replacement therapy methods (haemofiltration or haemodiafiltration). The repeated need for hyperosmolar therapy (more than three administrations in 24h) to control ICP indicates that second-line measures need to be applied.
- •
Moderate hyperventilation (PaCO2=30–35mmHg). We should never allow sustained PaCO2<25mmHg or pH>7.60. It causes a transient response (of 4h) due to vasoconstriction and reduction in cerebral blood flow. It should NOT be withdrawn abruptly as this causes rebound vasodilation. Side effects include coronary vasospasm and seizures in susceptible patients, hypocalcaemia and hypokalaemia, increased risk of barotrauma and nosocomial infection.
- •
Indomethacin 0.5mg/kg body weight if cerebral hyperaemia is detected (increase in SjO2).
- •
Barbiturate-induced coma. This is the most common measure, although there are authors who suggest the possibility of using propofol as an alternative. An induced coma leads to a reduction in ICP but also in arterial pressure, with which the CPP may decrease. As a general rule, the minimum dose that enables control of the ICP should be used.
- •
Moderate hypothermia. Hypothermia acts by reducing the systemic metabolism, which decreases the production of ammonia and its reuptake in the brain, leading to a decrease in brain metabolism of ammonia and in cerebral blood flow. It may also improve the patient's haemodynamic stability.29 Results on the efficacy of moderate hypothermia (32–34°C) in the control of refractory intracranial hypertension are inconsistent. In fact, a multicentre study in patients with advanced HE who were kept at 34°C, found no benefit on ICP.30 For hypothermia to be applied, the patient has to be sedated, paralysed and on mechanical ventilation; be given broad-spectrum antibiotics to prevent infections; and maintain CPP>50mmHg. This situation has to be maintained until the ICP returns to normal and, if possible, until the ELT. Potential adverse effects of hypothermia: coagulopathy, thrombocytopenia and platelet dysfunction, increased incidence of pneumonia and other infections, bradycardia, severe tachyarrhythmias (if below 32°C). Reheating of patients should be done slowly and gradually to avoid both a rebound effect on ICP and potential adverse effects, particularly on heart rate. One practical strategy could be to keep the patient at normothermia (35–36°C) avoiding febrile episodes.1
- •
Non-invasive techniques, such as transcranial Doppler, are not useful in the assessment of ICP (grade of evidence II-3, grade of recommendation 1).
- •
Invasive monitoring of ICP should be considered in patients at high risk of intracranial hypertension, i.e. in grade III-IV HE, intubated and ventilated patients, and with at least one of the following: young with hyperacute presentation; presence of seizures; three or more SIRS criteria; serum ammonia >150μmol/l; serum sodium<135mEquiv./l; renal failure; need for vasoactive support (grade of evidence II-3; grade of recommendation 1).
- •
Measures to prevent increase in ICP should be applied in all cases. Hyperosmolar therapy (with mannitol or hypertonic saline) should be the first option in cases of intracranial hypertension (grade of evidence II-2, grade of recommendation 1).
Renal failure occurs in 40–80% of patients with ALF, especially those of advanced age, and it worsens the prognosis. In half of cases renal failure has functional characteristics, while in the rest it is due to acute tubular necrosis, sometimes resulting from a direct nephrotoxic effect of the same agent that caused the ALF (e.g. 80% of patients with paracetamol overdose-related ALF have acute kidney injury, from which they gradually recover).31 The diagnostic criteria and the pathophysiology of functional renal failure in ALF are the same as in the hepatorenal syndrome of cirrhosis, and it occurs mainly in subacute cases.
The treatment of renal failure, in general, requires delicate adjustment of the patient's blood volume, withdrawal or dose reduction of nephrotoxic drugs (including mannitol) and conventional management of associated disorders. The use of diuretics in oliguric states should be limited to cases of hypervolaemia, since they could aggravate renal failure, particularly when it is functional. The use of terlipressin may be considered. Although there were initial reports suggesting that terlipressin might increase ICP, subsequent studies showed that not only did it not increase ICP or intracerebral lactate, but that it did increase CPP.24
The indications for renal replacement therapy are the conventional indications, but they must be established at an early stage. In patients with cerebral oedema, acute hypo-osmolar changes induced by dialysis can precipitate episodes of intracranial hypertension, so continuous renal replacement therapy techniques are advisable. Anticoagulation is not usually required, but, if necessary, heparin or citrate may be used. However, citrate should be used with caution due to the lack of hepatic metabolism.
Recommendations- •
The occurrence of acidosis, metabolic (hyponatraemia, etc.) and haemodynamic (hypervolaemia) abnormalities in the context of renal failure require the early introduction of renal replacement therapy (grade of evidence III, grade of recommendation 1).
- •
Anticoagulation of the renal replacement therapy circuits is subject to debate and should be assessed on an individual basis (grade of evidence II-2, grade of recommendation 1).
- •
The continuous renal replacement techniques are those of choice (grade of evidence III, grade of recommendation 1).
Both bacterial and fungal infections are common in ALF due to the functional immunosuppression or immunoparesis associated with the condition32; 80% of patients with ALF develop bacterial infections and 33% fungal infections. When infection is suspected, the treatment of choice is broad-spectrum antibiotics that cover aerobic Gram-negative bacilli, especially enterobacteria, and aerobic Gram-positive cocci, especially streptococci, until the results of the microbiological cultures are known.
In prolonged stays in ICU, beyond two weeks, the possibility of a fungal infection should be considered.
In the absence of a reliable reference for adjusting the doses of drugs metabolised in the liver to hepatocellular function, it is not advisable, at least systematically, to reduce the conventional doses of antibiotics in patients with ALF.
Recommendations- •
Serial cultures should be performed to monitor for the presence of infections or colonisation (grade of evidence III, grade of recommendation 1).
- •
Identification of clinical criteria for SIRS or sepsis or the progression of HE requires prompt introduction of antibiotic therapy (grade of evidence II-3, grade of recommendation 1).
- •
Administration of antifungals should be considered in patients with prolonged stays in ICU (grade of evidence II-3, grade of recommendation 1).
The early identification of patients who will not spontaneously survive the ALF episode enables an ELT to be proposed and this, in fact, is the only therapeutic alternative shown to increase overall survival in ALF.
Although there are other criteria (Clichy, etc.), most centres follow the King's College Criteria from King's College Hospital in London. In Catalonia, OCATT (Organització Catalana de Trasplantaments [Catalan Transplant Organisation]) have agreed on these criteria (Table 2).33
There are diseases with specific ELT criteria, such as ALF due to paracetamol overdose or due to A. phalloides poisoning and Wilson's disease (Table 2).
In recent years, we have identified the need to improve the sensitivity and specificity of these criteria, but the low incidence of the disease means that has not been possible. What is clear is that all the prognostic scales have common elements: age, the degree of HE, and impaired coagulation. They differ on whether or not to consider the aetiology and bilirubin values. A recent meta-analysis34 showed that the King's College Criteria and the Model for End-Stage Liver Disease (MELD) score have a similar prognostic value in patients with paracetamol overdose. Some authors consider that adding other prognostic variables, such as phosphorus, to MELD (MELD-p), could increase its predictive value.18
Recommendations- •
The assessment of the patient's prognosis must be carried out continuously in order to decide on the best therapeutic option at all stages (grade of evidence III, grade of recommendation 1).
- •
Factors of poor prognosis are: severe hepatic involvement, extrahepatic involvement, and subacute presentation (grade of evidence II-3, grade of recommendation 1).
- •
ELT should be considered in patients who meet the criteria (grade of evidence II-2, grade of recommendation 1).
- •
The decision to perform an ELT must be taken by a multidisciplinary team who are experts in this procedure (grade of evidence III, grade of recommendation 1).
ELT is the only treatment option in some cases of ALF. However, transplant is not universally applicable and, in fact, less than 10% of liver transplants are performed in patients with ALF (7.6% in adults in Catalonia in 2015).35 Patients with ALF and ELT criteria are put on an emergency transplant list and have priority at a state-wide level (alert 0, which can be iso-group, compatible or incompatible, depending on the patient's blood group and their severity).
The general and specific contraindications to ELT are the same as for non-urgent transplantation, and are set out in the OCATT consensus document.33 We also have to take into account the specific contraindications linked to ALF which involve an unacceptable surgical risk (active or uncontrolled infections, uncontrolled bleeding, intracranial hypertension with impossibility of normalising the ICP with the medical measures discussed above and with signs of neurological damage, multiple organ failure, etc.).
Post-ELT survival in ALF is close to 80% at one year, and <75% at five years.35 Death usually occurs in the first three months post-ELT, mainly as a consequence of septic complications, and is more likely to occur in older patients and those with poorer quality grafts or incompatible ABO matching.36
Early liver graft dysfunction leads to an increased risk of intracranial hypertension and sepsis.
Recommendation- •
Patients with ALF requiring an ELT should be put on a priority transplant list (grade of evidence III, grade of recommendation 1).
We start from the basis that the hepatocellular dysfunction in ALF is reversible in many cases given the regenerative capacity of the liver. Hence the reason liver support systems as a bridge to regeneration, or ELT if that is not feasible, are so important in this disease. These support systems should provide all the liver functions: synthesis, excretion and metabolism. Unfortunately, we do not yet have such complete systems. The largest amount of available clinical evidence is on artificial liver support systems. These systems are based on the removal of toxic substances, both water soluble and albumin-bound, which accumulate as a result of the loss or “death” of hepatocytes and can lead to systemic and hepatic inflammatory processes.
The most widely used artificial liver support system is the Molecular Adsorbent Recirculating System or “MARS”. Initially, there were a number of reports of short series of patients or isolated cases of ALF treated favourably with MARS. In 2013, Saliba et al. published the results of a multicentre, randomised, controlled study using MARS in patients with ALF and ELT criteria.37 The study included 110 patients, 102 of whom were randomised to conventional treatment (49 patients) vs. conventional treatment+MARS (53 patients). In view of the prognostic implications, it is important to mention that half of the cases in both groups were associated with paracetamol overdose. There were no significant differences in the actuarial probability of survival in the two treatment groups. No differences were observed when the patients were categorised according to the aetiology of the ALF; paracetamol vs. non-paracetamol. One interesting point was the fact that the waiting time on the liver transplant list was less than 24h in 75% of the cases. Moreover, the patients who had received three or more sessions of MARS (14 in total) were found to have significantly longer survival than the rest of the patients in the MARS group (57% vs. 8%; p=0.0004) and the rest of the patients overall (57% vs. 18%, p=0.004). In other words, it is possible that the patients in the MARS group did not receive the necessary number of sessions, or did not receive them early enough, to obtain a favourable outcome. Unfortunately, this hypothesis will have to be explored in future studies.
Pillukat et al.38 treated nine cases of A. phalloides poisoning with MARS, four of whom had ELT criteria, achieving transplant-free survival in all nine patients.
At Hospital Clínic in Barcelona, a pilot study was conducted with MARS (minimum three treatments) for patients with ALF, ELT criteria and formal contraindications for ELT. Compared to previous findings, the outcomes were better than expected (in-hospital mortality in previous series: 82%; pilot study mortality rate: 59%).39
Larsen et al. recently published a study, which included 182 patients recruited over 10 years and randomised to receive conventional treatment or conventional treatment+three days of high-volume plasma exchange sessions (8–15% of the patient's ideal weight).40 This study showed an increase in transplant-free survival in patients treated with plasma exchange, probably because this treatment attenuates the inflammatory and immune response, thereby reducing the incidence of multiple organ failure. The plasma exchanges did not increase post-transplant survival if the patient ended up having a transplant. If further studies confirm these data, high-volume plasma exchange should be considered as a “bridge”, or even an alternative, to ELT.
As far as the bioartificial methods, i.e. those using liver cells, are concerned, results of a multicentre study were published in which the use of an extracorporeal circuit with live liver cells did not reduce mortality in the series of patients overall, but it did in the subgroup of patients with fulminant ALF of viral aetiology or caused by paracetamol overdose.41
Other systems are currently under study for use as a “bridge” to transplantation. The use of these methods is only recommended in the context of controlled studies.
Recommendations- •
The artificial and bioartificial liver support systems should be used in the context of controlled clinical trials (grade of evidence II-1, grade of recommendation 1).
- •
High-volume plasma exchange has been associated with an increase in transplant-free survival in a randomised controlled trial (grade of evidence I, grade of recommendation 1).
- •
The efficacy of plasma exchange is higher when it is used early and in patients who do not subsequently require an ELT (grade of evidence I, grade of recommendation 2).
The authors declare that they have no conflicts of interest.
Please cite this article as: Escorsell À, Castellote J, Sánchez-Delgado J, Charco R, Crespo G, Fernández J. Manejo de la insuficiencia hepática aguda grave. Documento de posicionamiento de la Societat Catalana de Digestologia. Gastroenterol Hepatol. 2019;42:51–64.