The eradication of Helicobacter pylori infection represents a clinical challenge.
ObjectiveTo evaluate the efficacy and safety of quadruple therapy with esomeprazole plus a 3-in-1 capsule containing bismuth subcitrate, metronidazole and tetracycline, plus probiotics in patients diagnosed with H. pylori infection in routine clinical practice.
MethodsA prospective, interventional, single-centre and open-label study in consecutive patients with a confirmed indication for eradication of H. pylori infection. Patients were treated with three capsules of Pylera® four times a day (breakfast, lunch, afternoon snack and dinner), plus 40mg of esomeprazole twice daily for 10 days (30min before breakfast and dinner) and probiotics for 30 days. Eradication of H. pylori infection was confirmed by labelled urea breath test performed at least 28 days after the end of treatment.
ResultsA total of 100 patients were consecutively enrolled. Twenty-five patients (25.0%) had a prior history of treatment for their H. pylori infection. In the intention-to-treat population, eradication rates were 90.7% (68/75) and 80.0% (20/25) in patients treated with Pylera® as the first line or as rescue therapy, respectively. Eighteen patients (18%) had at least one adverse event, most of which (89%) were mild.
ConclusionTen days of treatment with a quadruple regimen of bismuth, metronidazole and tetracycline plus esomeprazole and probiotics is an effective and safe strategy in patients with H. pylori infection.
La erradicación de la infección por Helicobacter pylori representa un desafío clínico.
ObjetivoEvaluar la eficacia y seguridad de la terapia cuádruple con esomeprazol más una cápsula 3 en 1 que contiene subcitrato de bismuto, metronidazol y tetraciclina, más probióticos en pacientes diagnosticados de infección por H. pylori en la práctica clínica habitual.
MétodosEstudio prospectivo, intervencional, unicéntrico y abierto realizado en pacientes consecutivos con indicación confirmada de erradicación de infección por H. pylori. Los pacientes fueron tratados con 3 cápsulas de Pylera® 4veces al día (desayuno, comida, merienda y cena), más 40mg de esomeprazol, 2veces al día durante 10días (30min antes de desayuno y cena) y probióticos durante 30días. La erradicación de la infección por H. pylori se confirmó mediante la prueba del aliento con urea marcada realizada al menos 28días después del final del tratamiento.
ResultadosUn total de 100 pacientes fueron incluidos consecutivamente. Veinticinco (25,0%) pacientes tenían historia previa de tratamiento de su infección por H. pylori. En la población por intención de tratar, las tasas de erradicación fueron del 90,7% (68/75) y del 80,0% (20/25) en los pacientes tratados con Pylera® como primera línea o como terapia de rescate, respectivamente. Dieciocho pacientes (18%) presentaron, al menos, un acontecimiento adverso, la mayoría (89%) leves.
ConclusiónDiez días de tratamiento con un régimen cuádruple de bismuto, metronidazol y tetraciclina más esomeprazol y probióticos es una estrategia eficaz y segura en pacientes con infección por H. pylori.
Helicobacter pylori is one of the most prevalent pathogens in humans, affecting more than 50% of the population.1,2
H. pylori infection causes chronic inflammation of the gastric mucosa and is responsible for a significant number of gastrointestinal diseases, such as duodenal or gastric ulcers (in 1–10% of infected patients), gastric cancer (in 0.1–3%) and gastric mucosa-associated lymphoid tissue (MALT) lymphoma (in 0.01%).3–6
The treatment of choice to eradicate H. pylori infection was initially a triple-therapy regimen comprising a proton pump inhibitor (PPI) plus 2 of the following 3 antibiotics: clarithromycin, amoxicillin or metronidazole. However, over recent years, its efficacy has fallen to unacceptable levels in many countries, mostly due to increasing levels of bacterial resistance.7–10
According to recommendations of the IV Spanish Consensus Conference on treatment for H. pylori infection, an effective treatment should be able to eradicate H. pylori infection in approximately 90% of patients.11
A triple-therapy regimen with omeprazole, amoxicillin and clarithromycin (OAC) is unsuitable when local rates of clarithromycin resistance exceed 15%.11,12 In Spain, mean resistance rates of 18.3% for clarithromycin, 40.8% for metronidazole and 10.1% for both have been published.13 Furthermore, one recently published study identified resistance rates to clarithromycin of 33% in children.14
The drop in H. pylori infection eradication rate has led to the development of new therapeutic strategies.15–17
In Spain, the first-line therapy that is currently recommended is a concomitant, non-bismuth, quadruple therapy regimen (PPI, clarithromycin, amoxicillin and metronidazole).11
Recommendations of the IV Spanish Consensus Conference on treatment for H. pylori infection indicate that, after failure of a triple or quadruple therapy including clarithromycin, preferably a levofloxacin-containing quadruple therapy (PPI, amoxicillin, levofloxacin and bismuth) or, alternatively, a bismuth-containing quadruple therapy (PPI, bismuth, tetracycline and metronidazole) is recommended.11
It is possible to use doxycycline instead of tetracycline, although experience with its use is much more limited, and there are doubts regarding its therapeutic equivalence.11
Recommendations of the Maastricht V/Florence Consensus Report12 indicate that a quadruple-therapy regimen comprising bismuth, metronidazole and tetracycline (BMT) plus omeprazole (PPI) obtains a high eradication rate in patients in whom other therapeutic alternatives had previously failed.
According to scientific evidence available to date, BMT-PPI therapies achieve high eradication rates when administered as first-line therapy18–23 and when administered as rescue therapy.24–29 One of the advantages of BMT-PPI therapy is that no H. pylori resistance to bismuth has been shown to date,30 and this therapy is not affected by resistance to clarithromycin or fluoroquinolones.31
The objective of this study is to evaluate the efficacy and safety of quadruple therapy with esomeprazole plus a 3-in-1 capsule containing bismuth subcitrate, metronidazole and tetracycline in patients diagnosed with H. pylori infection in routine clinical practice.
Materials and methodsA prospective, interventional, single-centre and open-label study conducted between February 2016 and March 2017 in consecutive patients with a confirmed indication for eradication of H. pylori infection who were treated with a bismuth-based quadruple therapy under routine clinical practice conditions.
The study protocol was approved by the Hospital Universitario de la Zarzuela Ethics Committee. All patients were informed of the details of the study protocol, and patients gave written informed consent prior to starting the study. Ethical principles established in the Declaration of Helsinki and good clinical practice guidelines were followed.
Study populationPatients with confirmed H. pylori infection and indication for treatment with an eradication therapy, aged 18 years or over. H. pylori infection was confirmed by at least one of the following methods: urea breath test, histology or rapid urease test.
Those patients with contraindications/allergies to any of the drugs used in the study, major organ failure, upper gastrointestinal tract surgery, significant co-morbidities (malignancies, clotting disorders and liver, cardiorespiratory or kidney diseases), pregnancy or lactation were excluded.
Helicobacter pylori eradication therapyThe eradication therapy administered during the study consisted of a pharmaceutical preparation containing bismuth subcitrate potassium 140mg, metronidazole 125mg and tetracycline hydrochloride 125mg in each capsule (Pylera® Allergan, Inc., Irvine, CA).
Patients received 3 Pylera® capsules 4 times a day (breakfast, lunch, afternoon snack and dinner) for 10 days, plus 40mg of esomeprazole twice daily (30min before breakfast and afternoon snack) for 10 days and probiotics 1 capsule every 24h for 30 days. Patients were instructed not to drink alcohol or smoke during treatment, not to eat or drink dairy products at the same time as taking Pylera® capsules and to avoid exposure to the sun.
Eradication of H. pylori infection was confirmed by urea breath test performed at least 28 days after the end of treatment (antibiotics or PPIs were not permitted for 4 weeks).
Medication adherence was classified as good or poor according to the capsule count at each medical visit. Patients who took 80% or more of the prescribed medication were considered to show good adherence, while those who took less than 80% of the study medication were considered to show poor adherence.
Study variablesThe primary endpoint was the rate of H. pylori eradication, defined as a negative urea breath test or rapid urease test performed at least 28 days after the end of treatment.
The secondary endpoint was safety, which was monitored by the incidence of adverse events identified verbally via generic questions, such as: How are you? Have you experienced any new signs or symptoms since your last visit? According to severity, adverse events were classified as (1) mild: the patient is aware of the sign or symptom but it is easily tolerable. No therapy or medical intervention required; (2) moderate: inconvenience that interferes with daily activities. Medical intervention or minimal therapy required; and (3) severe: disabling and unable to work or carry out daily activities (may be life-threatening). Medical intervention or therapy required.
Statistical analysisAll statistical analyses were performed with the MedCalc software version 17.4 (MedCalc Software bvba, Ostend, Belgium).
Before starting the study, it was determined that 94 patients would need to be included in order to estimate a success rate of 87.5%, with a 95% confidence interval and 7.5% precision. Twenty per cent of patients were assumed to be lost to follow-up.
Data were expressed as a number (percentage), mean (standard deviation [SD]), mean (95% confidence interval [95% CI]) or median (95% CI), as applicable.
Categorical variables were compared using chi-square or Fisher's exact test, as applicable.
The intention-to-treat (ITT) analysis included all patients who received the study medication and took at least one dose of the study medication. Patients with no observed results were considered treatment failures. The per-protocol (PP) analysis excluded those patients who did not complete the study or who had major protocol violations.
A univariate analysis was performed to compare the baseline characteristics of patients with successful or failed H. pylori eradication using Pearson's chi-square test. Factors associated with failure in the univariate analysis with p-value ≤0.15 were included in the multivariate model. A logistic regression model was used for the multivariate analysis.
A p-value of <0.05 was considered statistically significant.
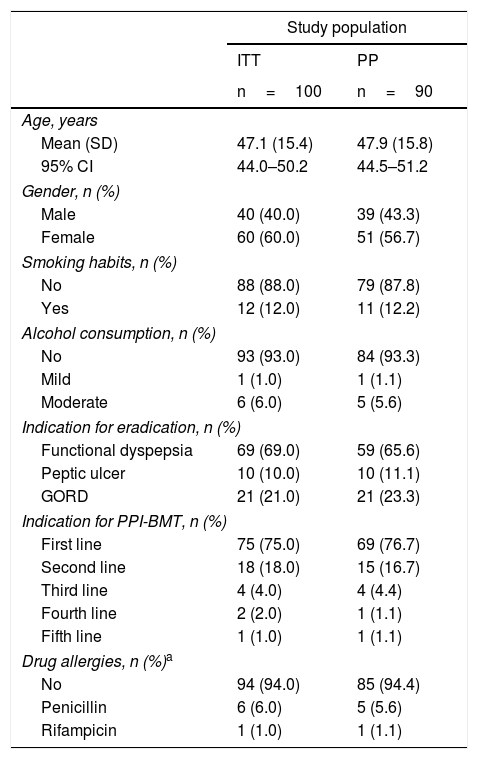
ResultsOf the 150 patients who were evaluated, 100 met the inclusion/exclusion criteria and were included in the ITT analysis. Of the 100 patients included in the ITT analysis, 10 were excluded from the PP analysis: 5 were lost to follow-up with no information provided regarding the reason for withdrawal; 3 patients were withdrawn prematurely from the study due to poor treatment tolerance (2 with vomiting and 1 with anxiety), which did not require hospital admission; and 2 patients were withdrawn due to a lack of confirmation test results. The main demographic and clinical characteristics of the ITT and PP populations are shown in Table 1.
Demographic and clinical characteristics of the study population.
| Study population | ||
|---|---|---|
| ITT | PP | |
| n=100 | n=90 | |
| Age, years | ||
| Mean (SD) | 47.1 (15.4) | 47.9 (15.8) |
| 95% CI | 44.0–50.2 | 44.5–51.2 |
| Gender, n (%) | ||
| Male | 40 (40.0) | 39 (43.3) |
| Female | 60 (60.0) | 51 (56.7) |
| Smoking habits, n (%) | ||
| No | 88 (88.0) | 79 (87.8) |
| Yes | 12 (12.0) | 11 (12.2) |
| Alcohol consumption, n (%) | ||
| No | 93 (93.0) | 84 (93.3) |
| Mild | 1 (1.0) | 1 (1.1) |
| Moderate | 6 (6.0) | 5 (5.6) |
| Indication for eradication, n (%) | ||
| Functional dyspepsia | 69 (69.0) | 59 (65.6) |
| Peptic ulcer | 10 (10.0) | 10 (11.1) |
| GORD | 21 (21.0) | 21 (23.3) |
| Indication for PPI-BMT, n (%) | ||
| First line | 75 (75.0) | 69 (76.7) |
| Second line | 18 (18.0) | 15 (16.7) |
| Third line | 4 (4.0) | 4 (4.4) |
| Fourth line | 2 (2.0) | 1 (1.1) |
| Fifth line | 1 (1.0) | 1 (1.1) |
| Drug allergies, n (%)a | ||
| No | 94 (94.0) | 85 (94.4) |
| Penicillin | 6 (6.0) | 5 (5.6) |
| Rifampicin | 1 (1.0) | 1 (1.1) |
95% CI: 95% confidence interval; GORD: gastro-oesophageal reflux disease; ITT: intention to treat; PP: per protocol; PPI: proton pump inhibitor; PPI-BMT: proton pump inhibitor-bismuth subcitrate, metronidazole and tetracycline; SD: standard deviation.
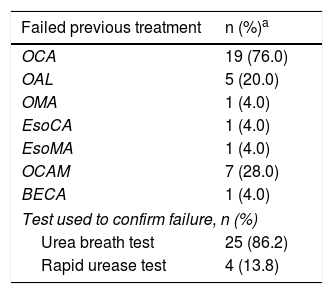
Twenty-five (25%) patients had a history of prior treatment for their H. pylori infection, primarily involving clarithromycin, amoxicillin and a PPI (Table 2).
History of Helicobacter pylori eradication regimens in patients treated with quadruple therapy based on proton pump inhibitors and bismuth (PPI-BMT) as rescue therapy (intention-to-treat population).
| Failed previous treatment | n (%)a |
|---|---|
| OCA | 19 (76.0) |
| OAL | 5 (20.0) |
| OMA | 1 (4.0) |
| EsoCA | 1 (4.0) |
| EsoMA | 1 (4.0) |
| OCAM | 7 (28.0) |
| BECA | 1 (4.0) |
| Test used to confirm failure, n (%) | |
| Urea breath test | 25 (86.2) |
| Rapid urease test | 4 (13.8) |
The total percentage may be higher than 100 because 7 patients received more than one therapy regimen.
BECA: bismuth/esomeprazole/clarithromycin/amoxicillin; 95% CI: 95% confidence interval; EsoCA: esomeprazole/clarithromycin/amoxicillin; EsoMA: esomeprazole/metronidazole/amoxicillin; OAL: omeprazole/amoxicillin/levofloxacin; OCA: omeprazole/clarithromycin/amoxicillin; OCAM: omeprazole/clarithromycin/amoxicillin/metronidazole; OMA: omeprazole/metronidazole/amoxicillin; SD: standard deviation.
The eradication rate in the ITT population was 88.0% (95% CI: 81.6–94.4%), while in the PP population the infection was eradicated in 97.8% (95% CI: 94.5–99.6%).
Based on whether patients had received treatment as first-line therapy or as rescue therapy, eradication rates in the ITT population were 90.7% (95% CI: 84.1–97.3%) (68/75) and 80.0% (95% CI: 64.3–95.7%) (20/25), respectively.
Eradication rates in the PP population were 98.6% (95% CI: 93.8–99.9%) (68/69) and 95.2% (95% CI: 90.1–100.0%) (20/21) in patients who received treatment as first-line therapy or as rescue therapy, respectively. There was no difference in eradication rate between those patients who received treatment as first-line therapy and those who received treatment as rescue therapy in the ITT (p=0.1553) and the PP (p=0.3543) populations.
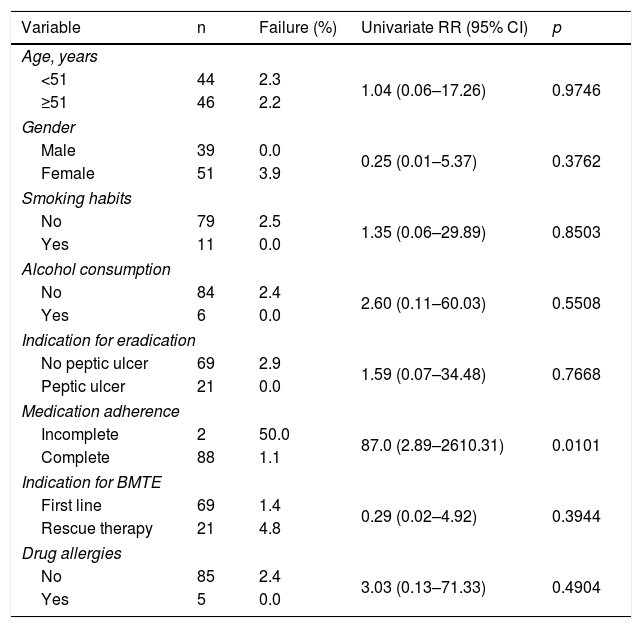
Factors associated with eradication failureIn the univariate analysis (Table 3), eradication failure was associated with incomplete treatment.
Potential risk factors for Helicobacter pylori eradication failure in a univariate analysis involving the 90 patients included in the per-protocol analysis.
| Variable | n | Failure (%) | Univariate RR (95% CI) | p |
|---|---|---|---|---|
| Age, years | ||||
| <51 | 44 | 2.3 | 1.04 (0.06–17.26) | 0.9746 |
| ≥51 | 46 | 2.2 | ||
| Gender | ||||
| Male | 39 | 0.0 | 0.25 (0.01–5.37) | 0.3762 |
| Female | 51 | 3.9 | ||
| Smoking habits | ||||
| No | 79 | 2.5 | 1.35 (0.06–29.89) | 0.8503 |
| Yes | 11 | 0.0 | ||
| Alcohol consumption | ||||
| No | 84 | 2.4 | 2.60 (0.11–60.03) | 0.5508 |
| Yes | 6 | 0.0 | ||
| Indication for eradication | ||||
| No peptic ulcer | 69 | 2.9 | 1.59 (0.07–34.48) | 0.7668 |
| Peptic ulcer | 21 | 0.0 | ||
| Medication adherence | ||||
| Incomplete | 2 | 50.0 | 87.0 (2.89–2610.31) | 0.0101 |
| Complete | 88 | 1.1 | ||
| Indication for BMTE | ||||
| First line | 69 | 1.4 | 0.29 (0.02–4.92) | 0.3944 |
| Rescue therapy | 21 | 4.8 | ||
| Drug allergies | ||||
| No | 85 | 2.4 | 3.03 (0.13–71.33) | 0.4904 |
| Yes | 5 | 0.0 | ||
A p value of <0.05 is considered statistically significant.
BMTE: bismuth, metronidazole, tetracycline and esomeprazole; 95% CI: 95% confidence interval; PPI: proton pump inhibitor; RR: risk ratio.
Given that only one of the variables obtained a level of significance equal to or less than 0.15 in the univariate analysis, the multivariate analysis was not performed.
Safety profileOf the 100 patients included in the ITT analysis, 81 (81%) patients showed good treatment tolerance. Three patients were withdrawn prematurely from the study due to poor treatment tolerance (2 with vomiting and 1 with anxiety), which did not require hospital admission or additional treatment.
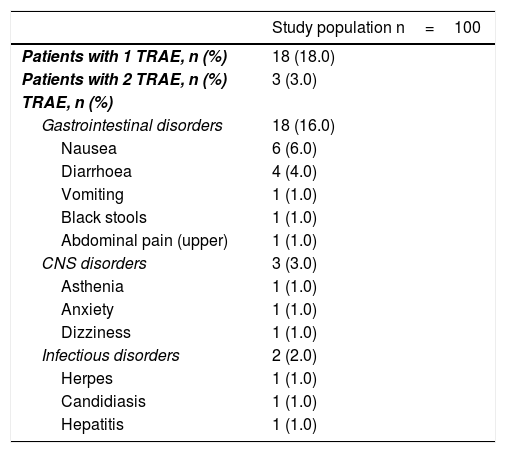
During follow-up, 18 (18%, 95% CI: 10.5–25.5%) patients reported at least one treatment-related adverse event, most of which (9%) were classified as mild, with none being classified as severe. The mean (SD) duration of adverse events was 7.4 (2.3) days.
Sixteen (16.0%) patients reported upper gastrointestinal tract-related adverse events, 3 (3.0%) reported central nervous system-related adverse events and 3 (3.0%) patients had infectious disorders. The different adverse events are summarised in Table 4.
Treatment-related adverse events in the intention-to-treat population.
| Study population n=100 | |
|---|---|
| Patients with 1 TRAE, n (%) | 18 (18.0) |
| Patients with 2 TRAE, n (%) | 3 (3.0) |
| TRAE, n (%) | |
| Gastrointestinal disorders | 18 (16.0) |
| Nausea | 6 (6.0) |
| Diarrhoea | 4 (4.0) |
| Vomiting | 1 (1.0) |
| Black stools | 1 (1.0) |
| Abdominal pain (upper) | 1 (1.0) |
| CNS disorders | 3 (3.0) |
| Asthenia | 1 (1.0) |
| Anxiety | 1 (1.0) |
| Dizziness | 1 (1.0) |
| Infectious disorders | 2 (2.0) |
| Herpes | 1 (1.0) |
| Candidiasis | 1 (1.0) |
| Hepatitis | 1 (1.0) |
CNS: central nervous system; TRAE: treatment-related adverse events.
The results of this study, which was conducted under routine clinical practice conditions, showed that 10 days of quadruple therapy with bismuth, metronidazole and tetracycline plus esomeprazole gives high eradication rates, not only as first-line therapy but also as rescue therapy, with an acceptable safety profile.
The results of this study also showed that poor medication adherence was associated with significant infection eradication failure.
The eradication rates observed in this study were similar to those published earlier by other authors using the same preparation (3-in-1 capsules) 4 times a day with a PPI twice daily, whose eradication rates varied from 80% to 93.2%.18,19,22,26
In a prospective study conducted in Italy, evaluating the efficacy and safety of 3 Pylera® capsules administered 4 times a day plus omeprazole (20mg) or esomeprazole (40mg) twice daily for 10 days, eradication rates of 94.7% (95% CI: 89.3–97.8%) in the ITT population and 97.6% (95% CI: 93.3–99.2%) in the PP population were observed.32 Also, like our study, they found no significant differences in patients receiving treatment as first-line therapy or as rescue therapy, in both the ITT and PP analyses.32
A study conducted in China, involving patients with functional dyspepsia and H. pylori infection, compared the efficacy of 2 therapy regimens: clarithromycin 500mg+amoxicillin 1g+pantoprazole 40mg administered twice daily, for 7 days, versus metronidazole (400mg 3 times a day)+tetracycline (750mg 4 times a day)+bismuth subcitrate (220mg 4 times a day), for 10 days.33 Eradication rates were significantly higher in the group treated with the bismuth-based quadruple therapy than in the group receiving the triple therapy, in both the ITT population (89.4% vs 63.5%, respectively, p<0.05) and the PP population (91.6% vs 65.1%, respectively, p<0.05).33
In a randomised, controlled study conducted in China comparing the efficacy of triple therapy (CAO) (clarithromycin 500mg+amoxicillin 1g+omeprazole 20mg administered twice daily, for 10 days) and a bismuth-based quadruple therapy (CAO+bismuth subcitrate 120mg 4 times a day, for 10 days) in patients with H. pylori infection and gastrointestinal symptoms, eradication rates of 58.4% and 86%, respectively, p<0.01, were found in the ITT population.23
As rescue therapy, the results obtained in this study (PP population) are similar to those published before.
In a European multinational study including patients from France, Germany, Italy and Spain, eradication rates ranged from 93.2% to 93.8%.26
In a prospective, multi-centre study, it was observed that, in patients with H. pylori infection who were allergic to penicillin, first-line therapy with a bismuth-containing quadruple therapy (PPI, bismuth, tetracycline and metronidazole) was a better option than the triple-therapy regimen containing PPI, clarithromycin and metronidazole.34
A prospective study evaluating the efficacy and safety of quadruple therapy with omeprazole plus a 3-in-1 capsule containing bismuth subcitrate, metronidazole and tetracycline in patients diagnosed with H. pylori infection, in the Seville area, under routine clinical practice conditions,35 has recently been published. The results of this study showed eradication rates of 97.6% and 82.4% in patients treated with Pylera® as first-line therapy or as rescue therapy, respectively, in the ITT population.35
In a study conducted in France, Müller et al.36 found eradication rates ranged from 83% to 87% with Pylera® as rescue therapy.
Several factors have been suggested as the cause of eradication failure, including age, gender, smoking, alcohol and specific drug history (e.g. acetylsalicylic acid).36,37
Medication adherence was associated, significantly, with H. pylori eradication failure.
Medication adherence is a very important parameter when assessing the results of a H. pylori eradication regimen, and must be taken into account when evaluating reasons for treatment failure. In this study, poor medication adherence was identified as an independent risk factor for treatment failure.
The results of this study coincide with those published by Müller et al., who observed that poor medication adherence was associated with H. pylori eradication failure.36
A study conducted in children treated for H. pylori infection was recently published. This study showed that, in the population that received at least 90% of the prescribed medication, the eradication rate was 89.9%, while in patients with poor medication adherence, the eradication rate was just 36.6%.38
With regard to the safety profile, 18 patients reported adverse events, with the most common being gastrointestinal disorders. Three patients reported central nervous system-related adverse events and 2 reported infectious disorders. One of the patients had hepatitis, which was diagnosed due to the presence of jaundice and elevated liver enzymes in blood tests. This patient did not require treatment discontinuation.
Symptoms were generally well tolerated and no severe adverse events were observed. These data coincide with available scientific evidence, which considers bismuth-based quadruple therapy for H. pylori eradication to be safe and well tolerated.18–38
Esomeprazole was used in this study instead of omeprazole. Although the use of esomeprazole may increase treatment costs, it has been published that esomeprazole showed better H. pylori eradication rates than omeprazole.39–41
Regarding the use of probiotics, according to recommendations of the IV Spanish Consensus Conference on treatment for H. pylori infection, generalised use of probiotics with eradication therapy was not recommended.11 However, current scientific evidence shows that, in comparison with placebo, most therapies with probiotics have been deemed useful since they improve H. pylori eradication rates and reduce therapy-related side effects.42–44 Nevertheless, these results should be interpreted with caution. Subgroup analyses examining therapy regimens and duration of eradication therapy showed that probiotic supplementation increased eradication rates in the triple-therapy,42–44 7-day eradication therapy42–44 and 14-day eradication therapy42,44 subgroups. These positive effects were not confirmed for patients who received a quadruple or sequential therapy.42
This study has limitations that must be taken into consideration. Its limitations include the fact that it is a non-randomised, non-controlled, open-label study.
Another limitation is the fact that it is a single-centre study, which means that it is only possible to include a limited number of patients. Nevertheless, before starting the study, the required sample size was calculated.
Since the study was conducted in a specific geographical area, caution should be used when generalising the results of this study.
To summarise, 10 days of treatment with a quadruple regimen comprising bismuth, metronidazole and tetracycline plus esomeprazole and probiotics is an effective and safe strategy in patients with confirmed H. pylori infection.
Comparative and especially prospective, randomised studies in Spain are necessary to compare concomitant non-bismuth quadruple therapy and bismuth-containing quadruple therapy.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank Laboratorios Allergan for its support in the form of medical writing.
It must be noted that Allergan S.A. did not help prepare the document, and the company had no influence on the conclusions reached.
Editorial services to help with the preparation of this manuscript were provided by Dr Antonio Martínez from Ciencia y Deporte S.L. This support was funded by Allergan S.A.
Please cite this article as: Pérez-Arellano E, Rodriguez-Garcia MI, Galera Rodenas AB, de la Morena-Madrigal E. Erradicación de la infección por Helicobacter pylori con una nueva terapia cuádruple basada en bismuto en la práctica clínica. Gastroenterol Hepatol. 2018;41:145–152.