Ustekinumab, a monoclonal antibody that blocks interleukins 12/23, has proven in clinical trials its efficacy in inducing and maintaining clinical remission of Crohn's disease (CD). Its effectiveness and safety in actual clinical practice is less known and may differ from trials.
ObjectiveTo evaluate its effectiveness and safety in clinical practice (intravenous induction pattern essentially), such as induction and over the long term, in patients with CD refractory to biological treatment.
Material and methodsMulticentre retrospective analysis (6 hospitals in Aragón), which includes all patients (N=69) with CD undergoing treatment with Ustekinumab (either with intravenous or subcutaneous induction), who had at least 16 weeks of follow-up. The clinical response or remission has been evaluated at weeks 16, 24, 32 and 48 using the Harvey-Bradshaw index.
ResultsA total of 69 patients have been included, mean age 42 years, 54% men. 89.86% (95% CI [0.805, 0.949]) of the patients presented clinical improvement at week 16 (15.95% remission, 73.92% response). In the subsequent follow-up, this response has been maintained. Age (OR 0.95, p=0.028) and smoking habits (OR 0.19, p=0.027) have been identified by an ordinal regression model as predictors of poor treatment response while the need for biological change due to adverse effect (OR 96, p=0.00017) and due to loss of secondary response (OR 7.07, p=0.034) have been predictors of good response. No serious adverse effects have been reported that forced them to stop taking Ustekinumab.
ConclusionUstekinumab is effective and safe in real clinical practice to achieve induction and maintenance of the response in patients with refractory CD. Tobacco and age have been shown to be predictors of poor response, while the indication for adverse effect to previous biological and for loss of secondary response has been shown to be predictors of good response.
Ustekinumab, anticuerpo monoclonal que bloquea las interleukinas 12/23, ha demostrado en ensayos clínicos su eficacia para inducir y mantener la remisión clínica en la enfermedad de Crohn (EC). Su efectividad y su seguridad en la práctica clínica real es menos conocida, y podría diferir respecto a los ensayos.
ObjetivoEvaluar en práctica clínica sobre su efectividad y seguridad (pauta de inducción intravenosa esencialmente), como inducción y a largo plazo, en pacientes con EC refractarios a tratamiento biológico.
Material y métodosAnálisis retrospectivo multicéntrico (6 hospitales aragoneses), que incluye a todos los pacientes (N=69) con EC en tratamiento con ustekinumab (fuese con inducción intravenosa o subcutánea), que hubiesen alcanzado al menos 16 semanas de seguimiento. La respuesta o remisión clínica se ha evaluado en las semanas 16, 24, 32 y 48 mediante el índice de Harvey-Bradshaw.
ResultadosSe han incluido un total de 69 pacientes, edad media 42 años, 54% hombres. Un 89.86% (IC 95% [0.805, 0.949]) de los pacientes ha presentado mejoría clínica en la semana 16(15.95% remisión, 73.92% respuesta). En el seguimiento posterior dicha respuesta se ha mantenido. Se han identificado mediante un modelo de regresión ordinal la edad (OR 0.95, p=0.028) y el hábito tabáquico (OR 0.19, p=0.027) como predictores de mala respuesta al tratamiento mientras que la necesidad de cambio de biológico por efecto adverso(OR 96, p=0.00017) y por pérdida de respuesta secundaria (OR 7.07, p=0.034) han sido factores predictores de buena respuesta. No se han reportado efectos adversos graves que obligasen a interrumpir el tratamiento con ustekinumab.
ConclusiónUstekinumab es efectivo y seguro en práctica clínica real para lograr la inducción y el mantenimiento de la respuesta en pacientes con EC refractaria. El tabaco y la edad han mostrado ser predictores de mala respuesta, mientras que la indicación por efecto adverso a biológico previo y por pérdida de respuesta secundaria han mostrado ser predictores de buena respuesta.
Crohn's disease (CD) is part of the group of inflammatory bowel diseases. This group of conditions, CD, ulcerative colitis and indeterminate colitis, is characterised by their chronicity and course in the form of outbreaks. Its aetiology is unknown, probably multifactorial, and involves genetic, environmental factors related to the microbiota, etc.1–3
Within the therapeutic arsenal available for CD there are multiple treatments available: oral topical corticosteroids; systemic corticosteroids; immunomodulators such as azathioprine, mercaptopurine and methotrexate; and biologic drugs, where we find anti-TNF drugs (infliximab, adalimumab) and, more recently, vedolizumab and ustekinumab.4
In addition to medical treatment, it may sometimes be necessary to resort to surgery. It does not cure CD, but it may be necessary both to treat complications and in the face of refractoriness to medical treatment. Also in perianal disease.
Ustekinumab has been one of the last drugs to be available for the treatment of patients with CD (in Spain, since July 2017). It is a fully human monoclonal IgG1 antibody that binds to the p40 subunit, which is found in cytokines IL-12 and IL-23.5 Ustekinumab is indicated in patients with active luminal CD, in a severe or moderate outbreak refractory to conventional or anti-TNF treatment. The efficacy and safety of this drug was demonstrated in the UNITI studies.6
Studies in real practice are scarce, in many cases following highly variable administration guidelines (usually clinical criteria), used before drug approval by regulatory agencies. Many of these studies were carried out with subcutaneous induction, as was done before intravenous induction was approved in the summary of product characteristics. Nowadays, this induction is carried out by an intravenous dose adjusted to the patient's weight and, subsequently, the maintenance treatment is performed subcutaneously, every 8 or 12 weeks. However, despite the limitations, these studies seem to confirm that ustekinumab is highly effective in real clinical practice.7–17
Regarding its safety, ustekinumab presented a very good profile in clinical trials of approval in CD, as well as in the three large studies conducted in psoriasis.18 In the long term, the PSOLAR study did not demonstrate an increased risk of infections in patients treated with ustekinumab.19 However, patients with CD usually have other causes of immunosuppression and the doses in inflammatory bowel diseases are higher than those used in psoriasis, so these data should be taken with caution.
Our work proposes to expand the knowledge we currently have about the effectiveness of treatment with ustekinumab in patients with CD refractory to biological treatments, as there are few studies that evaluate the effect of ustekinumab since intravenous induction has been used. This would also allow us to analyse the safety of the drug, as well as evaluate the response in the longer term, not only in the induction period.
The main objective of this study is to evaluate its clinical efficacy in induction: proportion of patients who achieve clinical response or remission at week 16 of treatment. Other objectives have been to evaluate the long-term response (weeks 24, 32 and 48), the response and corticosteroid-free remission and the safety of the drug. Possible factors associated with the clinical response have also been examined.
Material and methodsThis is an observational, non-interventional, retrospective and multicentre study that includes patients with CD treated with ustekinumab in Aragonese hospitals where follow-up is done in monographic digestive tract consultations of inflammatory bowel diseases. The data of patients from a total of six Aragonese hospitals have been collected.
The sample is made up of all patients with CD diagnosed according to the usual criteria,2 who have started treatment with ustekinumab according to clinical practice in accordance with the criteria of the physician in charge, either with subcutaneous or intravenous induction, and who have completed at least 16 weeks of follow-up. Intravenous induction has been performed according to the summary of product characteristics, at doses of 6mg/kg. All patients had to have active disease, defined as a Harvey-Bradshaw index greater than 4, at the time of starting ustekinumab.
The clinical response has been assessed at 16, 24, 32 and 48 weeks, using the Harvey-Bradshaw index. Remission has been defined as a Harvey-Bradshaw index less than or equal to 4 points and response as a decrease of at least 3 points in that index without reaching remission. The corticosteroid-free response or remission has been defined according to these same criteria with the complete withdrawal of systemic corticosteroids.
We collect demographic and inflammatory disease data from the medical record, as well as the previous treatment and the reasons for withdrawal of previous biological treatments (primary failure, adverse effects, secondary failure), according to the established definitions.20
The statistical analysis was carried out using the statistical package R. The descriptive analysis was carried out expressing the continuous variables through median and interquartile range and the categorical variables by means of their absolute frequency (N) and percentage. The inferential analysis was performed using an ordinal logistic regression model (No response-Clinical response-Clinical remission).
ResultsBaseline characteristics of the patientsData have been collected from a total of 69 patients treated with ustekinumab. The demographic and clinical characteristics of the sample are summarised in Table 1.
Characteristics of the cohort.
| Characteristic | N=69 |
|---|---|
| Gender: male/female | 37 (54)/32 (46) |
| Age (years) | 42 (32, 57) |
| Evolution of the disease (years) | 9 (6, 14) |
| Smokers | |
| Non-smokers | 46 (66) |
| Ex-smokers | 6 (9) |
| Active smokers | 17 (25) |
| Location | |
| Ileal | 39 (57) |
| Colic | 11 (16) |
| Ileocolic | 18 (26) |
| Upper digestive tract | 1 (1) |
| Perianal disease | 27 (40) |
| Phenotype | |
| Inflammatory | 48 (70) |
| Stenosing | 12 (17) |
| Fistulising | 9 (13) |
| Baseline Harvey-Bradshaw index | 9 (7.10) |
| Baseline CRP | 2.19 (0.55, 7.17) |
| Baseline calprotectin | 324 (141, 609) |
| Extraintestinal manifestations | 31 (45) |
| History of surgical resection: yes | 35 (51) |
| Concomitant medication | |
| Systemic corticosteroids | 20 (29) |
| Immunosuppressants | 15 (22) |
| Previous biologics | |
| 1 | 26 (38) |
| 2 | 25 (36) |
| ≥3 | 18 (26) |
| Previous treatment with vedolizumab | 12 (17) |
| Reason for previous drug discontinuation | |
| Primary failure | 12 (17) |
| Secondary failure | 44 (64) |
| Adverse effect | 13 (19) |
| Previous biological intensification: yes/no | 34 (49)/12 (17) |
| IV induction: yes | 57 (83) |
| Time on treatment with ustekinumab (weeks) | 32 (20, 56) |
| Ustekinumab intensified (every 4 weeks): yes | 10 (14) |
The data are expressed in median (interquartile range) or n (%).
Men and women have a similar distribution, and the cohort is characterised by a long time of evolution of CD (median of nine years), with a complex disease. This is reflected in the high percentage of patients with a history of surgical resection (51%), perianal disease (40%) and extraintestinal manifestations (45%). All patients came from a previous failure to a biologic drug, 25% of them having failed in at least three biologics (anti-TNF and/or vedolizumab).
In 20 cases (29%), the patients continued concomitant corticosteroid treatment and 15 patients (22%) took an immunomodulator when starting ustekinumab.
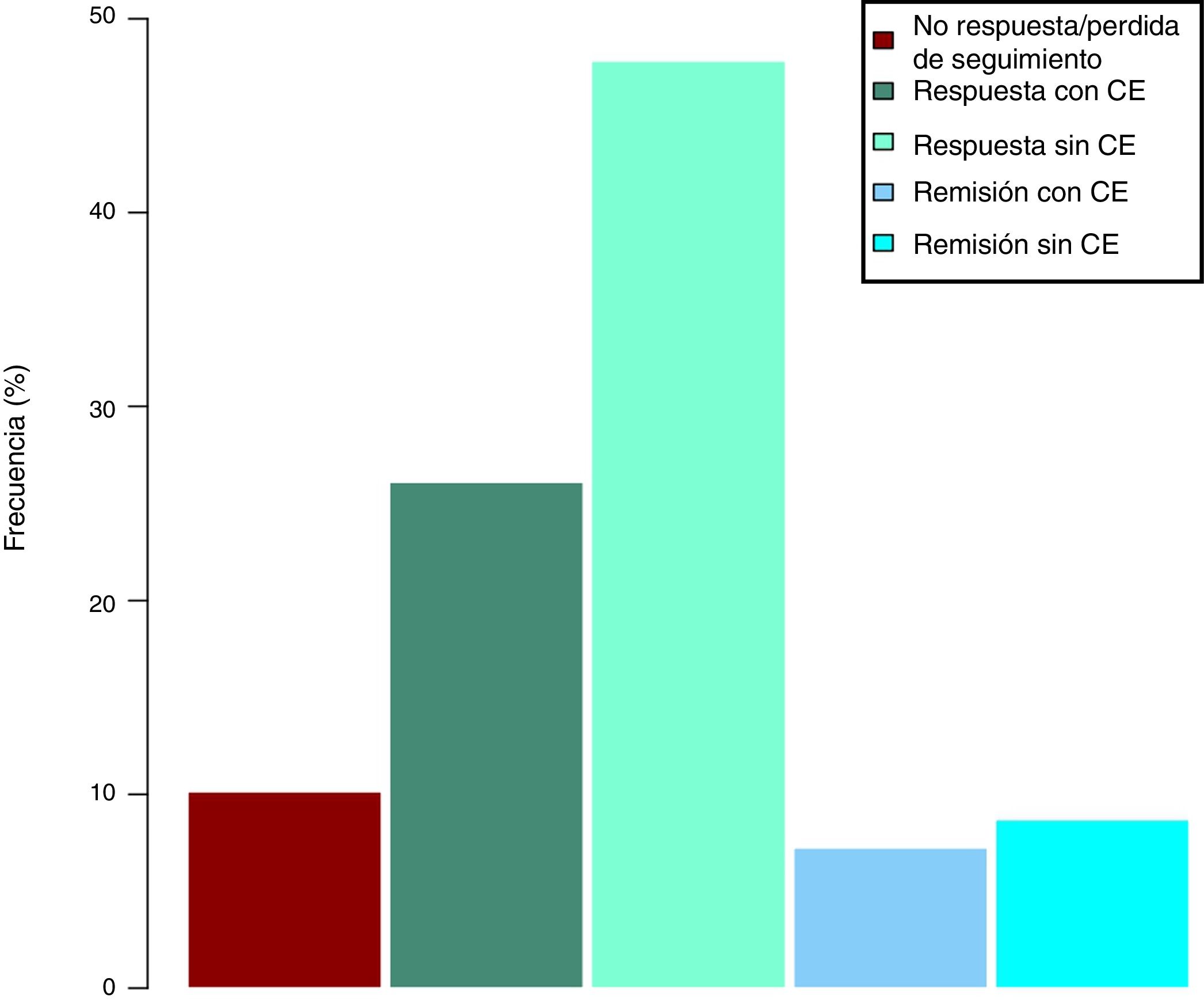
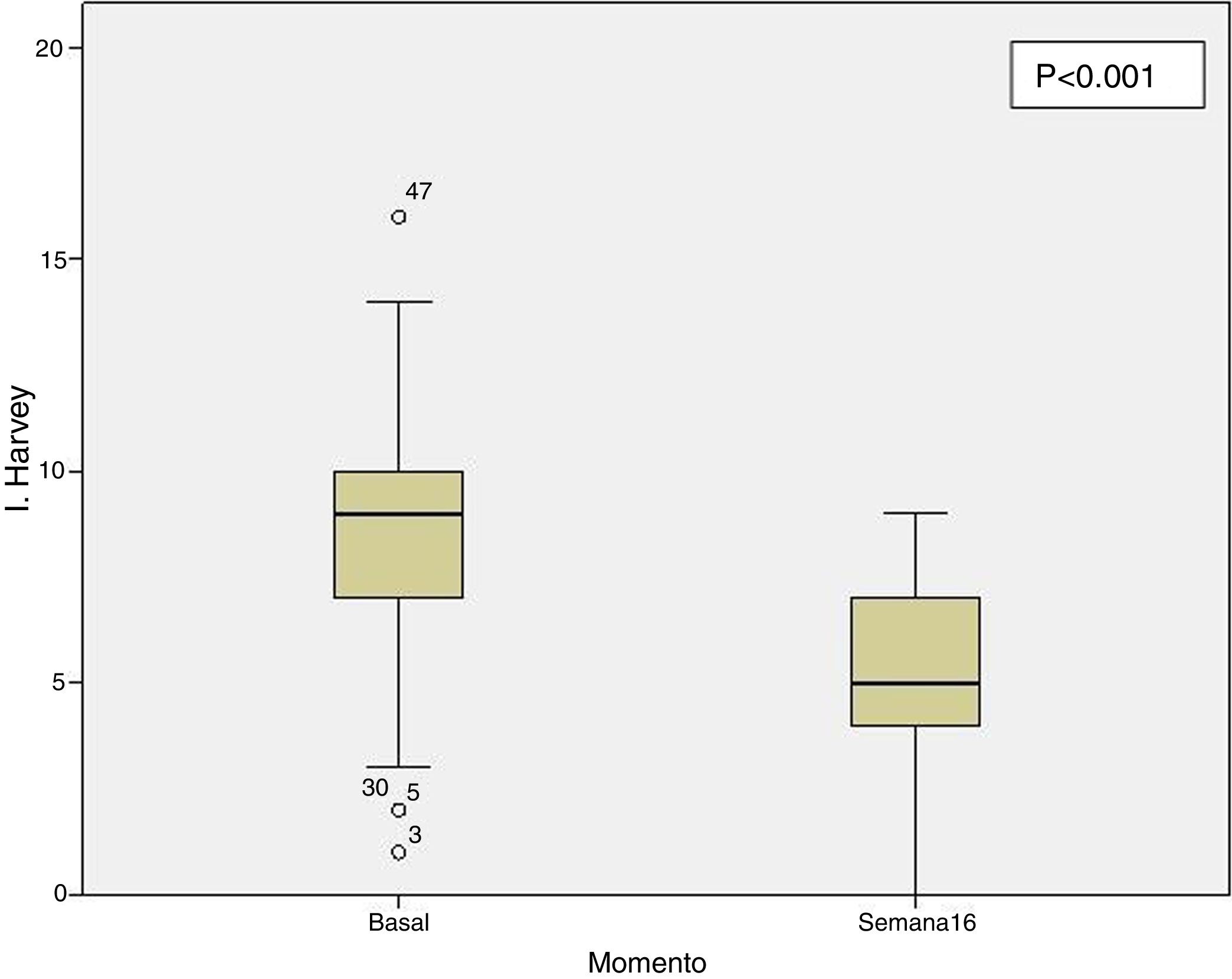
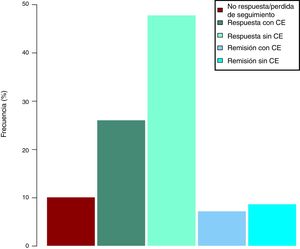
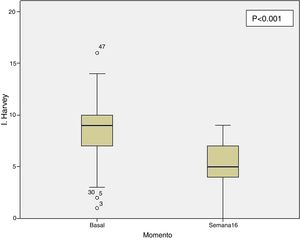
Clinical effectiveness in week 16At the time of starting treatment with ustekinumab, all patients presented clinical activity according to the previously expressed definitions. Of the 69 patients studied, 62 (89.86% [95% CI 0.805–0.949]) presented some type of clinical response, either remission or partial response, at week 16. Depending on whether or not corticosteroids were used, 33 patients (48%) presented a CD-free response, 18 (26%) response with CD, 6 (9%) remission without CD and 5 (7%) remission with CD. These results are summarised in Fig. 1. The Harvey-Bradshaw index has presented a statistically significant decrease (p<0.001) between baseline and week 16, as can be seen in Fig. 2. CRP and calprotectin have not reached statistical significance (p=0.323 and p=0.191, respectively).
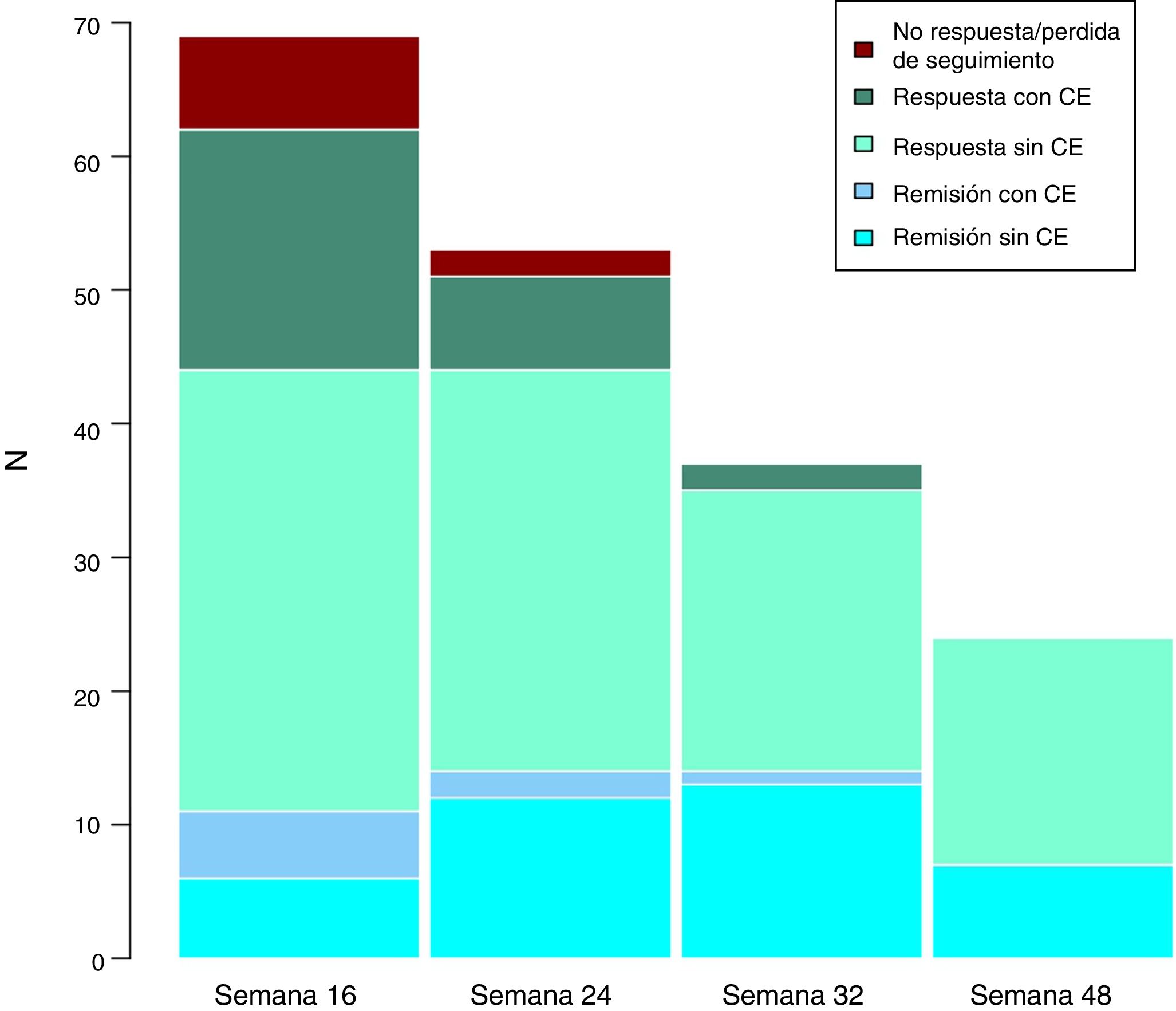
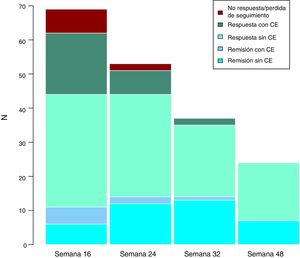
Long-term clinical effectivenessThe median follow-up of patients is 32 (interquartile range 20.56) weeks. Patients were evaluated at weeks 24, 32 and 48. Throughout the follow-up, no patient presented secondary failure to ustekinumab. These results are shown in Fig. 3.
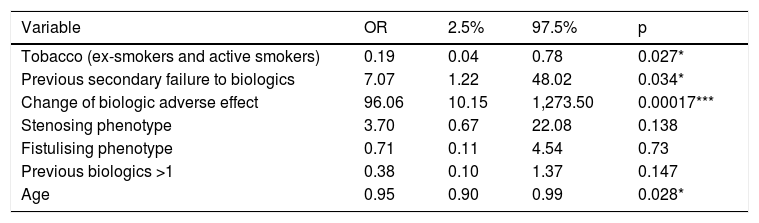
Predictive factors of induction responseOld age and smoking have been linked to the lack of response in week 16. On the contrary, starting ustekinumab due to adverse effect or secondary failure to the previous biological treatment has been associated with a better response. However, the concomitant use of corticosteroids or immunomodulators, the pattern of the disease, the number of previous biologics, previous use of vedolizumab, the route of induction or the time of evolution of the disease have not obtained statistical significance. The adjustment of the ordinal regression model can be seen in Table 2.
Predictive factors of induction response.
| Variable | OR | 2.5% | 97.5% | p |
|---|---|---|---|---|
| Tobacco (ex-smokers and active smokers) | 0.19 | 0.04 | 0.78 | 0.027* |
| Previous secondary failure to biologics | 7.07 | 1.22 | 48.02 | 0.034* |
| Change of biologic adverse effect | 96.06 | 10.15 | 1,273.50 | 0.00017*** |
| Stenosing phenotype | 3.70 | 0.67 | 22.08 | 0.138 |
| Fistulising phenotype | 0.71 | 0.11 | 4.54 | 0.73 |
| Previous biologics >1 | 0.38 | 0.10 | 1.37 | 0.147 |
| Age | 0.95 | 0.90 | 0.99 | 0.028* |
p: individual nullity test values in ordinal logistic regression model.
No adverse effects that caused treatment to be discontinued have been reported in our cohort. Minor adverse effects have been reported, consisting of two cases of pruritus, one case of arthralgia and another case of drowsiness. One patient required surgery after starting ustekinumab and another presented a worsening of his perianal condition.
DiscussionThere are few studies about the effectiveness of ustekinumab in real clinical practice, most of them with subcutaneous induction. In our study, although not all patients started intravenous induction treatment, the majority did (83%). Ustekinumab has been shown to be effective in patients with long-term CD and refractory to multiple treatments (62% had received two or more previous biologics) and all patients had failed at least one previously biological treatment. Despite this, 89.86% (95% CI [0.80-0.94]) of the patients presented a clinical response at week 16.
This response was considerably higher than that observed in the UNITI-1 and UNITI-2 authorisation trials of the drug, where the response observed was evaluated at week 8, and was 20.9 and 40.2%, respectively, as had already been seen in previous work.
Regarding the study by Iborra et al.,21 58% of patients reached clinical response at week 14, figures similar to those of Ma et al.,13 where the response was also 58%, although the induction in this cohort was subcutaneous in 89% of the cases.
Studies in real clinical practice that evaluate the response beyond the induction period (evaluated at 12, 14 or 16 weeks depending on the study) are even more limited. Our results, despite the limited sample size, indicate a very good medium-long term effectiveness. There has been no loss of secondary response, and we observed a tendency to remission and to be able to do without corticosteroid treatment.
As for the predictive response factors, although they have been widely studied in the case of anti-TNF drugs, they are less known in the new molecules. There are two recent papers that provide information about this in patients treated with ustekinumab. On the one hand, a review by Barré et al.,22 and on the other, the aforementioned work of Iborra et al.21 In these studies, the following were proposed as factors associated with the response: treatment with immunomodulators; the severity of the disease; the number of previous biological treatments; the severity of the activity assessed by endoscopy, aggressive patterns (stenosing and fistulising) and the location of the disease.
In our work, we have evaluated several factors that can determine the response to treatment, performing an analysis not only to predict it, but to predict the greater likelihood of remission versus response and response versus no response. We have found statistical significance in the reason for indication, in the patient's age and smoking habit, while we have not found it in other factors indicated in previous studies, such as the number of previous anti-TNFs or the phenotype of the disease.
Regarding the reason for the indication, both the indication due to a previous adverse effect to a biological treatment and a secondary loss of response have obtained statistical significance, which is consistent with the literature. This leaves the indication for primary failure of previous biological treatment as the indication with the highest probability of induction failure with ustekinumab.
On the other hand, we found a negative correlation in smoking, for active smokers and ex-smokers with respect to non-smoking patients, and for age. Both factors showed statistical significance, predicting a worse response to the drug.
As for the previously described factors for which we did not find significance in our model, this may be due to several reasons. On the one hand, our sample size, smaller than in other series, limits our statistical power. Regarding the number of previous biological treatments, especially anti-TNF drugs, described in the work of Iborra et al., significance was found, especially since the patients naïve to biologics presented a significantly higher response compared to those who had received treatment with biologics. In our cohort, all patients had previously received treatment with biologics, which prevented us from seeing these differences.
LimitationsOur study is retrospective, with the limitations that this entails. CRP and calprotectin are not available for all patients. The sample size of the follow-up, especially in week 48, is limited.
ConclusionsOur data show the effectiveness and safety of ustekinumab in real clinical practice, even in highly complex patients who have been refractory to numerous previous therapies. Our results show that the effectiveness in induction with ustekinumab is greater in patients with indication of change of biologic due to adverse effect or loss of secondary response, while it is lower in smokers or ex-smokers and in older patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Casas Deza D, García López S, Lafuente Blasco M, Vicente Lidón R, Nerín de la Puerta J, Peña Gonzalez E, et al. Eficacia y seguridad de ustekinumab en la práctica clínica real. Estudio multicéntrico retrospectivo. Cohorte ARAINF. Gastroenterol Hepatol. 2020;43:126–132.