The drug-injecting population has a high prevalence of hepatitis C virus (HCV) and high risk of transmission. It is a priority to establish an agile diagnostic and treatment plan.
Objectives1) Assess the effectiveness of a new coordinated care plan of referral from the Comprehensive Care Centre for Drug Addicts (CAID) to specialised care and 2) Determine the prevalence of HCV, clinical characteristics, effectiveness and safety of treatment in this population.
Methods1300 serologies requested by the CAID between 1998 and 2018 were retrospectively analysed, the seroprevalence of HCV was calculated and the efficiency of the traditional CAID-specialised care referral system was evaluated. A care plan was designed and coordinated among specialists involved in diagnosis and treatment. Since October 2018, 11 patients have been included in the new plan and the performance of both referral systems was compared.
ResultsWith the traditional system, 48.2% (83/172) of the patients were lost. 14.5% (172/1300) presented positive HCV serology, compared to the general population OR=19; 95% CI 14.3−25. The prevalence of active infection was 80.3% (90/112). The prevalence of active infection was 80.3% (90/112). Of the 11 patients referred by the new plan, 76.9% (8/11) had active infection and 100% (8/8) were treated with Direct Antiviral Agents successfully.
ConclusionsThe new coordinated CAID-specialised care plan presents high effectiveness in comparison with the traditional referral system. The seroprevalence and prevalence of active infection in the CAID population is very high. Treatments with Direct Antiviral Agents are effective and safe.
La población que se inyecta droga presenta una alta prevalencia de VHC y elevado riesgo de transmisión. Es prioritario establecer un plan ágil de diagnóstico y tratamiento.
Objetivos1) Valorar la efectividad de un nuevo plan asistencial coordinado de derivación desde el Centro Atención Integral al Drogodependiente (CAID) a atención especializada y 2) Conocer la prevalencia del VHC, características clínicas, efectividad y seguridad del tratamiento en esta población.
MetodosSe analizaron retrospectivamente 1300 serologías solicitadas por el CAID entre 1998–2018, se calculó la seroprevalencia de VHC y se valoró la eficiencia del circuito clásico de derivación CAID-atención especializada. Se diseñó un plan asistencial coordinado entre especialistas implicados en el diagnóstico y tratamiento. Desde octubre de 2018 se incluyeron 11 pacientes en el nuevo plan y se comparó el rendimiento de ambos circuitos de derivación.
ResultadosCon el circuito clásico, se perdió un 48,2% (83/172) de los pacientes. Un 14,5% (172/1300) presentaron serología VHC positiva, en comparación con población general OR=19; IC 95% 14,3-25. La prevalencia de infección activa fue del 80,3% (90/112). Con el nuevo circuito acudieron el 100% (11/11) (p=0,0003). De los 11 pacientes derivados mediante el nuevo plan, 76,9% (8/11) tenían infección activa y el 100% (8/8) fueron tratados con Agentes Antivirales Directos con éxito.
ConclusionesEl nuevo plan asistencial coordinado CAID-atención especializada presenta alta efectividad en comparación con el circuito clásico de derivación. La seroprevalencia y prevalencia de infección activa en la población del CAID es muy elevada. Los tratamientos con Agentes Antivirales Directos son efectivos y seguros.
In 2016, the efficacy of new treatment regimens with direct-acting antivirals (DAAs) led the World Health Organization (WHO) to take on the ambitious goal of eliminating hepatitis C virus (HCV) as a threat to public health by 2030.1 Different countries have implemented specific action plans; in Spain, the Asociación Española para el Estudio del Hígado (AEEH) [Spanish Association for the Study of the Liver] recently published a position statement on the subject.2,3
In 2017, the establishment of microelimination goals4 was suggested as a strategy to face the complex challenge of elimination. These consist of increasing the efficacy of said action by eliminating HCV in specific populations, such as injecting drug users (IDUs) and people who engage in risk behaviours, immigrants from high-prevalence areas and prison inmates.4
IDUs account for 8% of cases of chronic HCV infection.5 They have a high HCV prevalence, estimated at 47-85% in Spain,6–8 and are at high risk of virus transmission, given that they are responsible for 23% of new cases of infections worldwide.9 Thus, treatment is understood to amount to prevention as well. In this population, in addition to damage control measures (supply of needles/syringes and opioid replacement therapy [ORT]), which help to reduce HCV transmission,10–12 a plan for diagnosis and treatment should be established for all patients in a short period of time. This plan should be implemented through a process that includes all parties involved, it should be personalised and its goals should be achievable.13 Some published care models for this population improve access to care and achieve successful treatment results.14,15
We designed a care plan to treat, in a short period of time, patients in our area with HCV cared for at the CAID in Alcorcón.
A significant barrier to achieving these objectives is that it is not possible to directly refer these patients from CAIDs to specialised centres for treatment; they must first be assessed at health centres and referred to specialised centres only after their seropositivity is confirmed. This multi-step process leads to higher rates of missed appointments and loss to follow-up given the idiosyncrasy of this population.
The objectives of the study were: 1) to assess the effectiveness of the new coordinated healthcare plan for direct referral from a CAID to specialised care (SC), and 2) to determine HCV prevalence, clinical characteristics, and treatment effectiveness and safety in this population.
Material and methodsAn ambispective study was conducted. The retrospective part covered recorded data from 1998 to September 2018. Starting from October 2018, prospective follow-up was carried out.
First, the classic referral pathway from a CAID to SC, a nine-step process, was assessed. The effectiveness of the pathway was then calculated, with an effective pathway being defined as one with no patient losses throughout the process. Patient losses were analysed at different steps in the pathway: number of patients referred for SC through primary care (PC), number of patients who visited the SC centre out of all referred patients, number of patients who underwent the study requested by SC and number of patients who ultimately received antiviral treatment.
To do this, a retrospective review was conducted of 1300 results of HCV serology tests ordered by the Alcorcón CAID, from the opening of the hospital to the start of the study (1998–2018), to calculate seroprevalence, prevalence of active infection (RNA-HCV positive) and the effectiveness of the classic pathway.
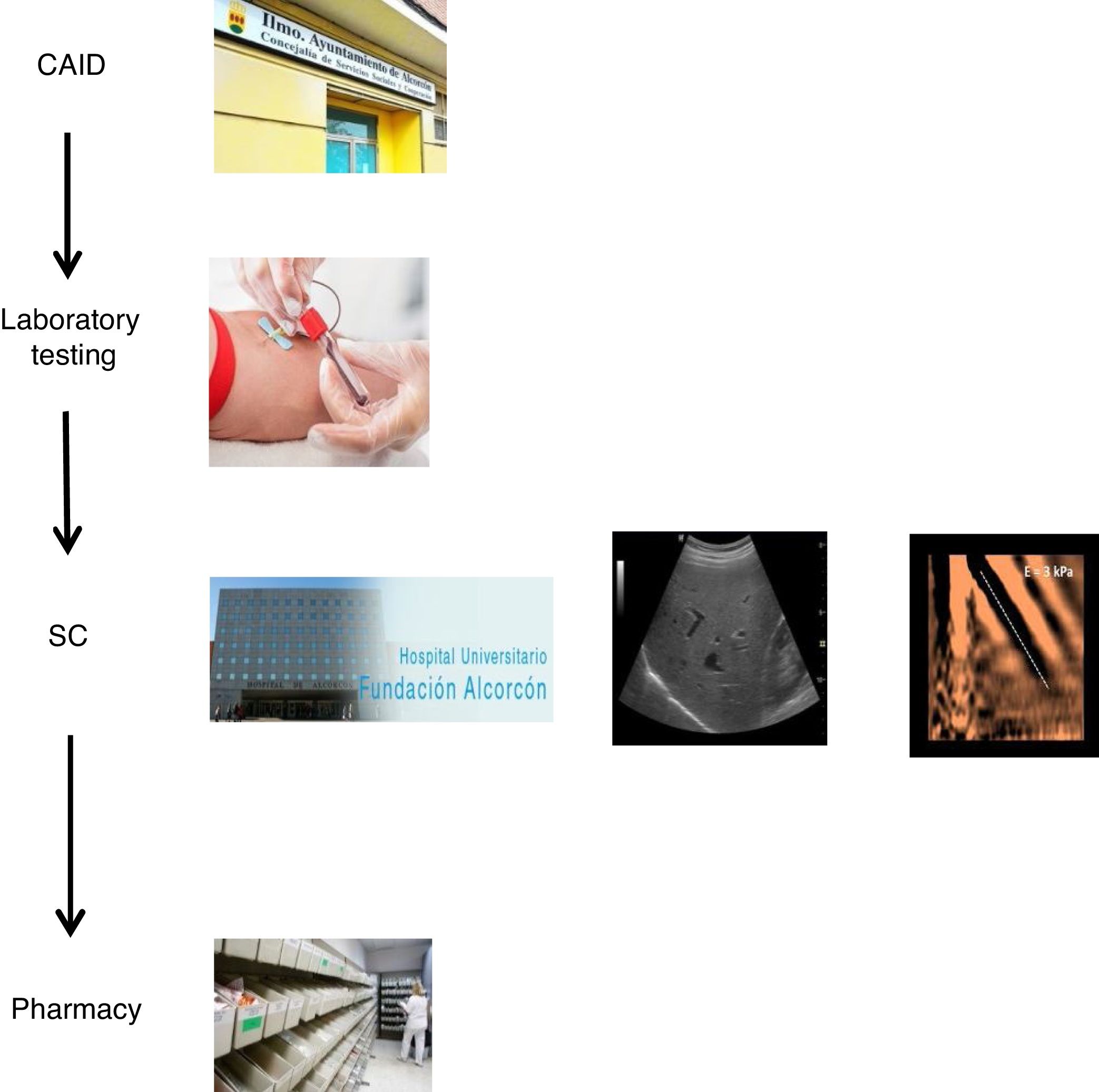
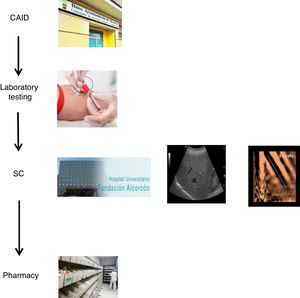
With the goal of improving the effectiveness of the pathway and preventing patient losses along the way, a decision was made to design a coordinated plan. In collaboration with the specialists involved in the care of these patients and the Dirección de Continuidad Asistencial [Directorate of Healthcare Continuity], a care plan was designed that coordinated the different specialists involved in HCV diagnosis and treatment (CAID physicians, laboratory workers/microbiologists, hepatologists and pharmacists). Furthermore, a "one-step" HCV diagnosis technique was used.
The coordinated plan consisted of three steps: 1) a decision was made to perform a single blood draw at the PC referral centre in all patients with known positive HCV serology, who had not been previously assessed/treated, and in those with positive serology not known previously, in order to test viraemia in the same sample and, in the event of a positive result, extend the study with genotyping and determination of viral load. The microbiology department reported cases with active infection by email to hepatologists. 2) Cases with active infection were given an appointment for clinical assessment by Hepatology, an appointment for an abdominal ultrasound/Fibroscan and a prescription for antiviral treatment on the same day. Clinical/laboratory monitoring was scheduled at 12 weeks of follow-up, once treatment was finished. 3) The full treatment regimen would be collected from the hospital pharmacy on the same day as the assessment by Hepatology and taken to the CAID, where the taking of medication would be monitored in-person to ensure proper adherence. In addition, the CAID physicians were responsible for follow-up of patients' side effects and drug interactions (Fig. 1).
Once the coordinated referral pathway was approved, the second part of the study was conducted. This consisted of a prospective study of patients enrolled in the new pathway from October 2018 to the present.
The study was approved by the Independent Ethics Committee (IEC) of the Hospital Universitario Fundación Alcorcón [Alcorcón Foundation University Hospital]. The patients enrolled in the new referral pathway were informed about the study and signed an informed consent form. For the patients on the classic pathway, assessed retrospectively, the IEC granted an exemption to obtaining informed consent as they represented real clinical practice.
To assess the impact of the coordinated care plan on the referral of patients to SC, the effectiveness of the new coordinated referral pathway was compared to that of the traditional referral plan.
To evaluate the clinical characteristics of HCV patients from the CAID, anthropometric, demographic, risk-behaviour and comorbidity variables were collected. These parameters are normally collected in real clinical practice. The following laboratory variables were gathered: hepatitis B virus (HBV) serology, human immunodeficiency virus (HIV) serology, HCV genotyping and viral load determination, stage of fibrosis, and type and duration of treatment with DAAs.
Data analysis was performed using the statistics software program SPSS 21.0. Categorical variables were expressed in terms of frequency, and continuous (quantitative) variables were expressed in terms of mean and standard deviation or median and ranges.
ResultsA total of 14.5% (172/1300) had positive HCV serology, compared to the general population (OR=19; 95% CI=14.3–25).16
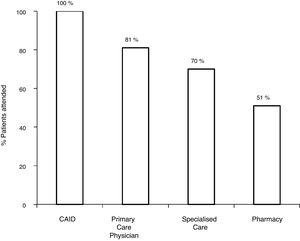
With the old pathway, of the 172 patients with positive serology, 81.3% (140/172) were referred to SC, and 70.3% (121/172) attended the specialist visit. Of the 121 patients assessed by a specialist, 7.5% (9/121) did not undergo the study ordered by the specialist. Among the 112 with positive serology who completed the study, 80.3% (90/112) had positive viraemia, and 19.7% (22/112) had negative viraemia. Out of the 90 patients with active infection, 75.5% (67/90) were treated and achieved a sustained virological response (SVR): 27 with interferon (IFN) and 40 with DAAs (Fig. 2).
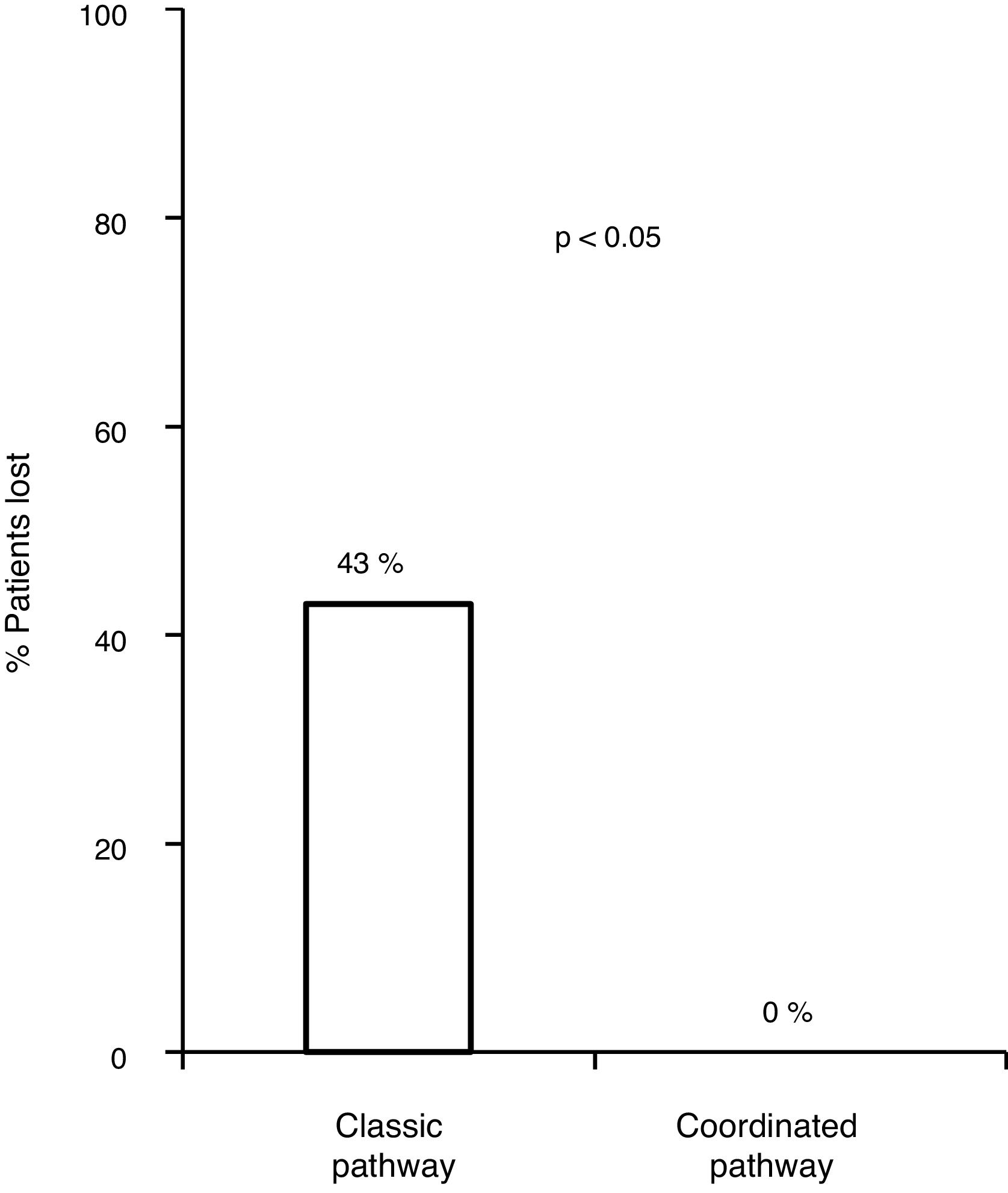
With the traditional referral system, 48.2% (83/172) of all patients with positive serology were lost along the different steps of the pathway. Of these, 18.6% (32/172) were not referred, 11% (19/172) did not attend the first specialist visit, 5.2% (9/172) did not undergo the study ordered by the specialist or did not get the results and 13.3% (23/172) were not treated.
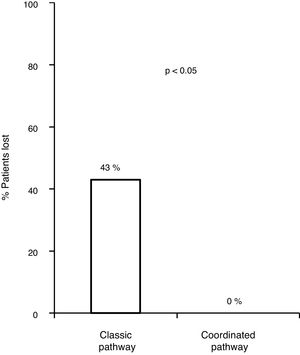
Of the 83 lost patients, 11 were referred by the CAID physicians using the new coordinated healthcare plan, and 8 (76%) had active infection. The 8 patients (100%) were diagnosed and treated according to the plan designed without any losses along the pathway, whereas there were 83/172 (43.8%) losses along the classic pathway. Hence, the referral rate was higher for the coordinated pathway compared to the classic pathway (p=0.0008). All 8 patients (100%) received treatment with DAAs and achieved an SVR, with adherence rates close to 100% and no serious adverse events reported (Fig. 3).
When the medical records of the 72 lost patients not being followed up by the CAID were reviewed, 14 patients (20%) had died and 49 (70%) were in other areas of the Autonomous Community of Madrid or another autonomous community in Spain, and just 9 (10%) remained in our area. Once their PC physicians had been informed, the 9 patients who remained in the area were contacted. Only two responded; one of them had positive viraemia.
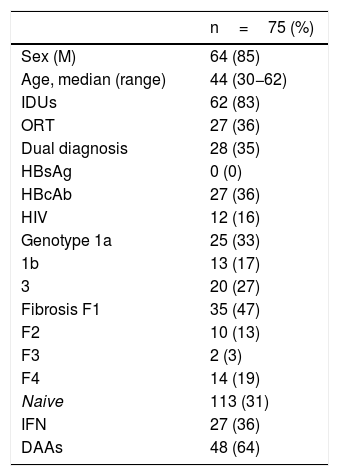
Altogether, including both referral pathways, 75 patients were treated and achieved an SVR; among them, 27 (36%) were treated with IFN and 48 (64%) were treated with DAAs. Of these 75 patients, 64 were male (85%), with a mean age of 44 (range 30−62). Regarding comorbidities, 62 (82%) acknowledged having used parenteral drugs and 28 (35%) had a dual diagnosis, 8 (10%) had schizophrenia, 4 (5%) had depression, 3 (4%) had personality disorders, 3 (4%) had adjustment disorders, 9 (12%) had anxiety and 1 (1.3%) had another unspecified disorder.
At the time of starting antiviral treatment, 12 (16%) acknowledged active drug use, 27 (36%) were receiving ORT, 66 (88%) were active smokers and 47 (62%) acknowledged alcohol use.
As was to be expected in the infected population, the predominant genotypes were 1a and 3 (60%), while 21% had advanced-stage fibrosis (F3-F4), 36% were anti-HBc positive and 16% were HIV positive.
The clinical characteristics of the patients treated are shown in Table 1.
Characteristics of the CAID patients treated.
| n=75 (%) | |
|---|---|
| Sex (M) | 64 (85) |
| Age, median (range) | 44 (30−62) |
| IDUs | 62 (83) |
| ORT | 27 (36) |
| Dual diagnosis | 28 (35) |
| HBsAg | 0 (0) |
| HBcAb | 27 (36) |
| HIV | 12 (16) |
| Genotype 1a | 25 (33) |
| 1b | 13 (17) |
| 3 | 20 (27) |
| Fibrosis F1 | 35 (47) |
| F2 | 10 (13) |
| F3 | 2 (3) |
| F4 | 14 (19) |
| Naive | 113 (31) |
| IFN | 27 (36) |
| DAAs | 48 (64) |
The concept of microelimination of HCV in specific populations such as IDUs represents one strategy for tackling the WHO's complex challenge of HCV elimination.1,2 IDUs are a stigmatised population, sometimes engaged in illegal activity, who may be reluctant to seek or accept medical help. The health system itself imposes some barriers on this population, such as care at out-of-the-way sites and inflexible schedules and appointments. It is important to establish plans that are implemented through a process involving multiple interested parties, with interventions adapted to population needs. Some published care models for this population have been shown to be effective in improving access to care and to achieve successful outcomes.14,15
We designed a care plan to treat, in a brief period of time, patients with HCV in our area cared for at the Alcorcón CAID. This plan coordinated the care provided by the different specialists involved in HCV diagnosis and treatment (CAID physicians, laboratory workers/microbiologists, hepatologists and pharmacists) and used a "one-step" HCV diagnosis technique.
This study shows that the new coordinated CAID/SC healthcare plan is highly effective with regard to referral rates compared to the classic referral pathway. The seroprevalence and prevalence of active infection in the CAID population are very high. This was a younger population with a major predominance of men, some engaging in active drug use, some on ORT, some with a dual diagnosis and some on concomitant treatment with psychoactive drugs. Treatments with DAAs in this population are effective and safe.
Our study demonstrated the ineffectiveness of the classic CAID/SC referral pathway, a lengthy pathway consisting of 9 steps altogether, and highlighted patient losses at different steps in the pathway, finding that nearly half of patients ultimately did not reach the end goal of receiving antiviral treatment. Analysis of the pathway revealed that nearly two-thirds of losses occurred at SC access and that the remaining one-third of lost patients did not receive antiviral treatment, either because they had not completed the study ordered by SC or because of contraindications, since this was a retrospective study in which IFN treatment was assessed in most patients. Thus, there is a demonstrated need to establish agile, speedy pathways that facilitate access to SC and keep patients within the system, thus preventing losses, using one-step diagnosis, a single location for multiple services and treatment outsourcing.
The new referral pathway was simplified to three steps. Without a doubt, one of the strengths of the new pathway is that it uses a one-step diagnosis technique. Another is the fact that clinical assessment, disease staging and treatment prescription are done in a single visit. Finally, antiviral treatment was externalised to the CAID, with patients taking their treatment there in person and their usual physicians doing follow-up, thus taking advantage of follow-up appointments with those physicians, with no need for duplicate visits.
The efficacy of the new CAID/SC coordinated healthcare plan was confirmed, since all the patients completed the study and the treatment in a short period of time and it was superior to the classic referral pathway.
Regarding the prevalence of HCV in IDUs, data from our study were consistent with previously published series that reported high rates of seroprevalence and prevalence of active infection in this population. Our study might have reflected somewhat lower seroprevalence figures than other series. This was likely tied to the fact that our study enrolled all CAID patients, who were not necessarily all IDUs.
Concerning the clinical characteristics of patients with HCV from the CAID in our area, compared to the rest of the population with HCV, these patients represented a younger population, with a male predominance, a lesser degree of fibrosis and a dual diagnosis, while some were active drug users, some were on methadone replacement therapy and some were being treated with psychoactive drugs. Given this population's characteristics, it is very important to simplify healthcare pathways that facilitate access to the system. These people are stigmatised, show limited inclination to seek medical help, have very unstable social, work and family environments, and are very uninformed about their disease with regard to diagnosis, prognosis and current safe and effective treatments.
Despite these characteristics, patients showed good adherence, good tolerance and no serious side effects. Treatment with DAAs is safe and effective in a population on ORT and engaging in active drug use,17 and treatment regimens are also simple and brief, enabling more professionals to prescribe them (CAID physicians, PC physicians and pharmacists).
Apart from the related benefits (HCV curation), there are other benefits for IDUs, such as reduction of drug use risk behaviours18 and prevention of infection transmission by those who continue to engage in risk behaviours. It is very important to take advantage of instances in which these patients come into contact with the system to inform them of the risk of reinfection (0-5% people per year) if they continue to engage in risk behaviours,19,20 to educate them about means of damage control such as sterile equipment and to broach other health topics such as the risk of transmission of other infections (HBV/HIV).
Our study's limitations include the limited number of patients enrolled in the new pathway. Its strengths include the design of the new referral pathway, which incorporates improvements at different points in the pathway, such as one-step diagnosis, a single location for multiple services and externalising treatment. Thanks to the study design and results, these health models being implemented in a research context and translated to regular clinical practice, represent a good example of "implementation science".
This study found that the new coordinated CAID/SC healthcare plan shows high effectiveness compared to the classic referral pathway, that the seroprevalence and prevalence of active infection in the CAID population are very high and that current treatments with DAAs are effective and safe. Concerning the characteristics of the population, these patients represented a younger population, with a lesser degree of fibrosis and a dual diagnosis, some of whom were active drug users, were on methadone replacement therapy or were being treated with psychoactive drugs.
FundingNo funding was received for this work.
Conflicts of interestThe authors declare that they have no conflicts of interest.
To the authors for data collection and critical reading of the article.
Please cite this article as: Gutiérrez García ML, Gómez Perosanz R, Acedo Sanz JM, Delgado-Iribarren García-Campero A, Claudio Domínguez I, Domenech Gómez-Imaz A, et al. Plan asistencial coordinado para la eliminacion del virus de la hepatitis C en el centro de ayuda integral al drogodependiente (CAID). Gastroenterol Hepatol. 2021;44:214–220.