Upper gastroscopy in patients with cirrhosis often reveals non-specific lesions, which are usually oriented as portal hypertensive gastropathy (PHG). However, the diagnosis of PHG can be difficult, both from an endoscopic and histological point of view. The study of CD34 expression, which enhances the endothelial cells of the microvasculature, could help the differential diagnosis. The objectives of this study were to evaluate the correlation between endoscopy and histology in the diagnosis of PHG and to assess the utility of CD34 in the diagnosis of PHG.
Material and methodsThe results of immunostaining with CD34 gastric fundus biopsies from 100 cirrhotic patients and 20 controls were compared with the endoscopic images.
ResultsThe correlation between the histology and the endoscopic diagnosis of PHG was very low (kappa=0.15). In addition, the measurement of the diameter of the gastric vessels enhanced by the use of immunohistochemical staining (CD34) did not show good correlation with the endoscopic diagnosis (p=0.26) and did not provide relevant information for the histological diagnosis of PHG either.
DiscussionThe correlation between histology and endoscopy is low for the diagnosis of PHG. The use of immunostaining for CD34 does not seem to improve the diagnostic yield of the histological study.
En la endoscopia digestiva alta de pacientes con cirrosis hepática a menudo se observan lesiones inespecíficas, que se suelen orientar como gastropatía por hipertensión portal (GHP). Sin embargo, el diagnóstico de GHP puede ser difícil, tanto endoscópica como histológicamente. El estudio de expresión de CD34, que realza las células endoteliales de la microvasculatura podría ayudar al diagnóstico diferencial. Los objetivos del estudio fueron evaluar la correlación entre la endoscopia y la histología en el diagnóstico de la GHP y valorar la utilidad del CD34 en el diagnóstico de la misma.
Material y métodosSe analizaron biopsias fúndicas de 100 pacientes cirróticos y 20 controles, y se realizó inmunotinción para CD34. Se compararon con las imágenes endoscópicas.
ResultadosSe observó una correlación muy baja entre la histología con el diagnóstico endoscópico de GHP (kappa=0,15). Además, la medición del diámetro de los vasos gástricos realzados mediante el uso de la tinción inmunohistoquímica (CD34) no mostró buena correlación con el diagnóstico endoscópico (p=0,26) y tampoco parece aportar información relevante para el diagnóstico histológico de GHP.
DiscusiónExiste una baja correlación entre la histología y la endoscopia para el diagnóstico de GHP. El uso de la inmunotinción para CD34 no mejora la rentabilidad diagnóstica del estudio histológico.
Portal hypertensive gastropathy (PHG) is a gastric mucosa lesion present in patients with portal hypertension (PH), the prevalence of which ranges from 7% to 98%,1–5 according to the published case series. In the case of portal hypertensive enteropathy (PHE), there are very few studies in the literature, with a prevalence of 8.4%–68% described in patients with cirrhosis and PHE.6–12
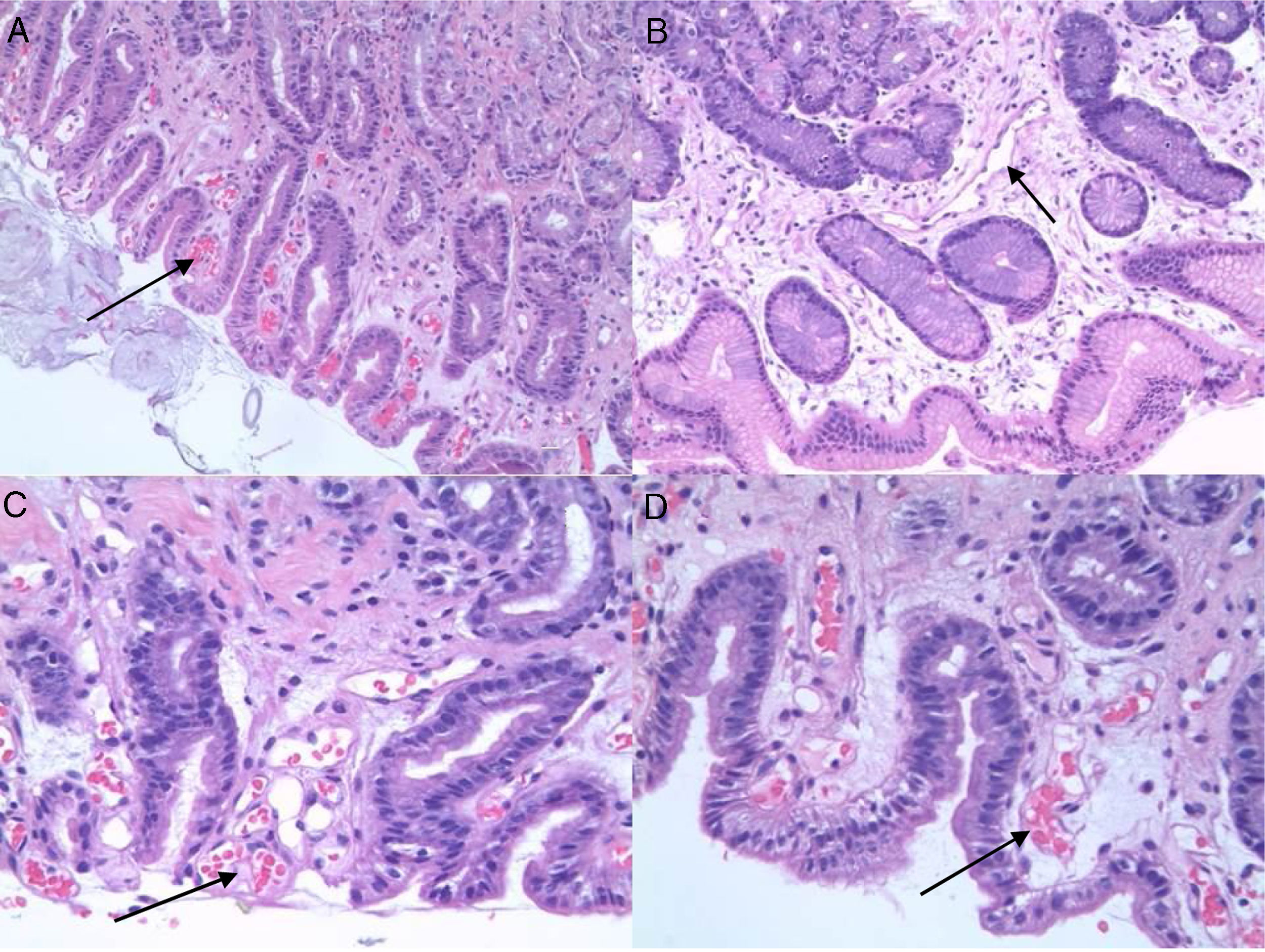
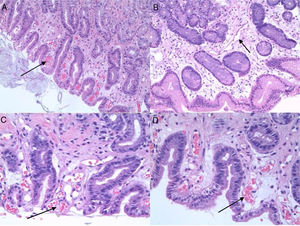
PHG is diagnosed by endoscopy. The most common location is the gastric fundus, although lesions may appear anywhere in the gastrointestinal tract. Nevertheless, it can be difficult to differentiate PHG from other vascular lesions, such as gastric antral vascular ectasia (GAVE) and gastritis due to Helicobacter pylori (H. pylori), etc. Microscopic studies can therefore be useful. Histologically, PHG is characterised by dilation and ectasia of the capillaries and venules of the gastric mucosa and submucosa, as well as oedema and thinning of the arteriole and venule wall in the submucosa, with no evidence of inflammation, underlying erosion or fibrin thrombi13–15 (Fig. 1).
In the context of differential diagnosis, there may be significant difficulties when trying to differentiate PHG from GAVE during both endoscopic and histological studies. Histologically, GAVE is characterised by vascular dilation or ectasia, fibrin thrombi, fibromuscular hyperplasia of the lamina propria, fusiform cell proliferation and fibrohyalinosis, with no underlying inflammatory signs. It has also been suggested that the CD34 vascular marker might prove useful for the histological diagnosis of PHG, although the data are contentious.16,17
The objective of our study was to verify the potential correlation between endoscopic and histological findings suggestive of gastroenteropathy and to assess the utility of measuring the vascular diameter using immunohistochemical staining for CD34 in the histological diagnosis of PHG.
Material and methodsParticipantsAll cirrhotic patients in whom an upper gastrointestinal endoscopy was indicated were selected. The patients were recruited from Outpatient Clinics held at the Hepatology Day Hospital or the Gastrointestinal Department's Inpatient Unit at our institute.
The patients had to fulfil all the inclusion criteria and none of the exclusion criteria.
The inclusion criteria were as follows:
- •
Aged 18 years or older.
- •
Presence of liver cirrhosis diagnosed by anatomical pathology, clinical or analytical criteria, irrespective of aetiology.
- •
Wanting to participate and signing the informed consent form after receiving an explanation of the study objectives and procedures.
The following were considered exclusion criteria:
- •
Patients with contraindications for undergoing a gastrointestinal endoscopy.
- •
Patient's refusal to undergo an endoscopy.
- •
Patients with active upper gastrointestinal bleeding (UGIB).
- •
Presence of concomitant diseases with a life expectancy of less than one year (BCLC stage 3–4 hepatocellular carcinoma, other active neoplasms, etc.).
Data collection began in May 2009 and ended in April 2013. Control patients were selected from the Anatomical Pathology Department, where we identified normal gastric biopsies from patients with no liver cirrhosis, according to their medical records.
Data collection was done prospectively for patients and retrospectively for controls.
Endoscopic procedureAll the enrolled patients underwent an enteroscopy under sedo-analgesia. Enteroscopy was performed with the paediatric colonoscope (Olympus EVIS EXERA II PCF-Q180AL) in order to explore the entire duodenum and proximal jejunum. All of the enteroscopic interventions were performed by the same endoscopist. During endoscopy, the presence and degree of PHG was assessed in the fundus and antrum, as well as the presence and degree of lesions in the duodenum and jejunum. The degree of PHG was assessed and measured using the Tanoue classification.18
Biopsy collectionDuring the procedure, biopsies were taken for anatomical pathology study using standard biopsy forceps. In total, two jejunum biopsies were taken, as well as two duodenum biopsies, two antrum biopsies and two fundus biopsies. These were preserved in formalin until they were sent to the Anatomical Pathology laboratory.
The endoscopic biopsies were stained with haematoxylin and eosin. The immunohistochemical study for CD34 class II was performed in an automated manner with the Dako Autostainer Link 48 automated immunohistochemistry processor.
Histologically, the presence or absence of biopsy findings suggestive of PHG or GAVE was assessed (capillary congestion, extravasation, oedema, inflammatory changes, fibrin thrombi, fibromuscular hyperplasia of the lamina propria, fusiform cell proliferation, fibrohyalinosis, etc.). In each of the biopsies we assessed the presence or absence of PHG-compatible changes, such as the presence and intensity of ectasia (mild, moderate or severe), vascular congestion, the existence of extravasation and oedema, and the presence or absence of inflammatory changes. In order to differentiate it from GAVE, the existence of fibrin thrombi and fibrohyalinosis was investigated.
The immunohistochemical marker CD34 was used in fundus samples in order to identify the presence of blood vessels. Once identified, three different areas were selected and the smallest diameter of the five largest vessels stained with CD34 was measured.
The presence or absence of H. pylori and other lesions was also assessed. All biopsies were reviewed by the same pathologist. Said pathologist also reviewed each histological study without knowing the endoscopic diagnosis.
Ethical considerations of the studyThe study was submitted and approved by our institute's Ethics Committee. All patients were informed by the principal investigator and signed an informed consent form to participate in the study. In the case of negative controls, anonymous samples were collected.
Statistical methodsSample size: In order to estimate the prevalence of gastrointestinal vascular lesions with a 10% accuracy, assuming a confidence interval of 95% and an expected proportion of 50%, it was necessary to enrol 98 patients in the study. The number of controls was determined empirically.
Descriptive analysis: For qualitative variables, absolute and relative frequencies were calculated. For quantitative variables, the mean, standard deviation (SD) and minimum and maximum values were calculated.
Univariate analysis: For qualitative variables a homogeneity test adapted for discrete distributions was used (chi-squared test, Fisher's exact test or likelihood ratio) based on compliance with the criteria for application. For quantitative variables, we firstly analysed the conditions for applying the different tests (Shapiro–Wilk normality test and Levene's test for equality of variances). The linear or adapted non-parametric model was applied based on compliance with the criteria for application (variance analysis or Mann–Whitney–Wilcoxon test).
For modelling, multivariate linear regression models were adapted for each of the response variables. All variables with a p-value below 0.05 in the baseline homogeneity analysis were considered covariables. Repeated measures were taken into account (three measures per individual). The final model was obtained using backward variable selection, considering a significance level of 0.05.
ResultsParticipantsA total of 100 cirrhotic patients were enrolled, from whom adequate images and biopsies were obtained to conduct the study. An additional 20 patients with normal gastric biopsies were enrolled as controls.
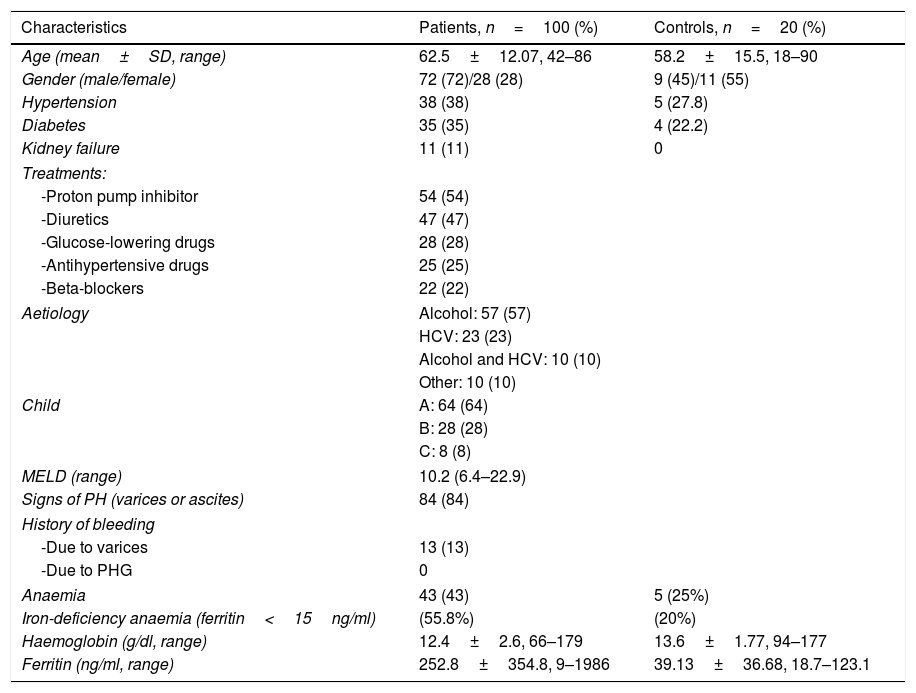
The most important demographic, clinical and analytical data of the patients and controls are shown in Table 1. Given that the endoscopic diagnosis of antral PHG and enteropathy was very low (10% and 4% of patients, respectively), it was not possible to make histological and endoscopic comparisons in these locations. Histological analysis was not performed in one patient because the sample obtained was insufficient for diagnosis. For this reason, the total number of patients analysed is 99.
General characteristics of the patients and controls.
| Characteristics | Patients, n=100 (%) | Controls, n=20 (%) |
|---|---|---|
| Age (mean±SD, range) | 62.5±12.07, 42–86 | 58.2±15.5, 18–90 |
| Gender (male/female) | 72 (72)/28 (28) | 9 (45)/11 (55) |
| Hypertension | 38 (38) | 5 (27.8) |
| Diabetes | 35 (35) | 4 (22.2) |
| Kidney failure | 11 (11) | 0 |
| Treatments: | ||
| -Proton pump inhibitor | 54 (54) | |
| -Diuretics | 47 (47) | |
| -Glucose-lowering drugs | 28 (28) | |
| -Antihypertensive drugs | 25 (25) | |
| -Beta-blockers | 22 (22) | |
| Aetiology | Alcohol: 57 (57) | |
| HCV: 23 (23) | ||
| Alcohol and HCV: 10 (10) | ||
| Other: 10 (10) | ||
| Child | A: 64 (64) | |
| B: 28 (28) | ||
| C: 8 (8) | ||
| MELD (range) | 10.2 (6.4–22.9) | |
| Signs of PH (varices or ascites) | 84 (84) | |
| History of bleeding | ||
| -Due to varices | 13 (13) | |
| -Due to PHG | 0 | |
| Anaemia | 43 (43) | 5 (25%) |
| Iron-deficiency anaemia (ferritin<15ng/ml) | (55.8%) | (20%) |
| Haemoglobin (g/dl, range) | 12.4±2.6, 66–179 | 13.6±1.77, 94–177 |
| Ferritin (ng/ml, range) | 252.8±354.8, 9–1986 | 39.13±36.68, 18.7–123.1 |
HCV: hepatitis C virus; PH: portal hypertension; PHG: portal hypertensive gastropathy; SD: standard deviation.
All of the patients (including controls) tested positive for the CD34 marker, so vascular diameters were successfully measured.
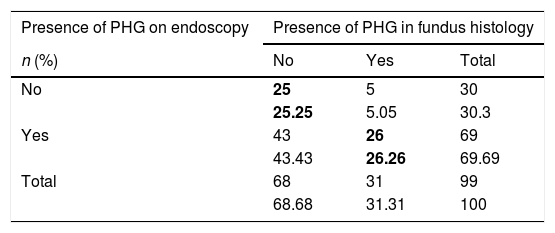
Main results(a) A concordance study was performed to assess the presence or absence of PHG in the fundus histology result and endoscopic image. The correlation between the endoscopic and histological diagnosis of PHG was very low, obtaining a kappa coefficient of 0.15 (Table 2). It should be highlighted that, while 68% of the patients had an endoscopic image compatible with fundal PHG, only 31% had a histological lesion compatible with PHG (29% mild and 2% moderate). Only 5% of patients with an endoscopy reported as normal presented PHG-compatible histological changes.
Concordance between the presence or absence of PHG in the fundus histology and endoscopic image.
| Presence of PHG on endoscopy | Presence of PHG in fundus histology | ||
|---|---|---|---|
| n (%) | No | Yes | Total |
| No | 25 | 5 | 30 |
| 25.25 | 5.05 | 30.3 | |
| Yes | 43 | 26 | 69 |
| 43.43 | 26.26 | 69.69 | |
| Total | 68 | 31 | 99 |
| 68.68 | 31.31 | 100 | |
PHG: portal hypertensive gastropathy.
The values in bold indicate the boxes in which concordance between the presence or absence of PHG was observed.
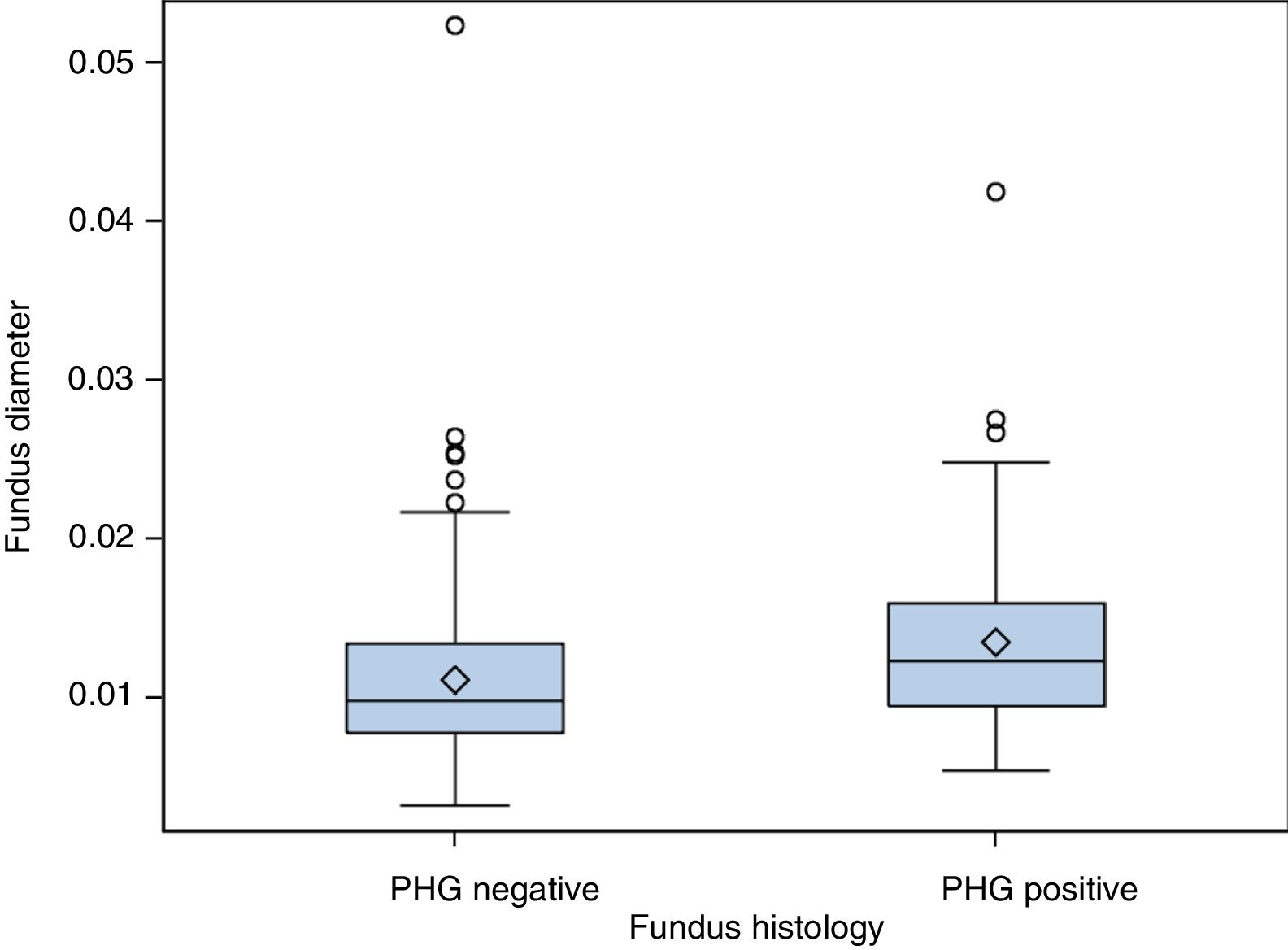
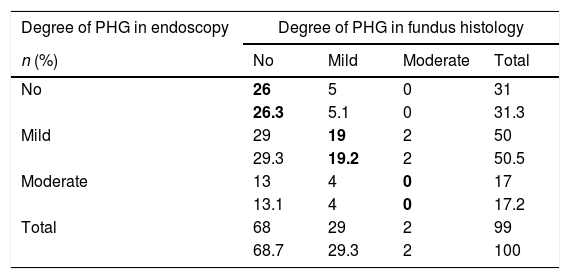
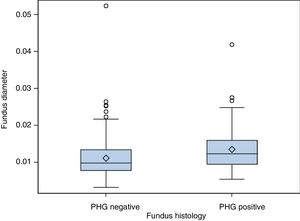
(b) The degree of concordance between the severity of fundal PHG observed endoscopically and histologically was assessed (mild, moderate or severe), obtaining a kappa coefficient of 0.12 (Table 3). No statistically significant differences were observed between the mean diameter of the five largest vessels, in three different areas of the fundus, between the patients and controls (p=0.26). The patients presented a mean of 0.0118mm±0.0054 (0.0032–0.0524) and the controls 0.0124mm±0.0055 (0.0057–0.0425). It was assessed whether or not there were statistically significant differences in the fundal vessel diameter of patients with and without PHG (assessed by means of a conventional histological study). The patients with PHG presented a significantly higher mean vessel diameter (p<0.001), with mean values of 0.0101mm±0.0035 (0.0032–0.0194), while the patients without PHG had mean values of 0.0125mm±0.0059 (0.0046–0.0524) (Fig. 2). No differences were observed when we compared the vessel diameters of patients with mild PHG to those with moderate or severe PHG (p=0.44). The patients with mild PHG had a mean of 0.0123mm±0.0058 (0.0046–0.0524) and those with moderate or severe PHG had a mean of 0.0129mm±0.0061 (0.0056–0.0418).
Concordance between the severity observed in the endoscopic image and fundus histology.
| Degree of PHG in endoscopy | Degree of PHG in fundus histology | |||
|---|---|---|---|---|
| n (%) | No | Mild | Moderate | Total |
| No | 26 | 5 | 0 | 31 |
| 26.3 | 5.1 | 0 | 31.3 | |
| Mild | 29 | 19 | 2 | 50 |
| 29.3 | 19.2 | 2 | 50.5 | |
| Moderate | 13 | 4 | 0 | 17 |
| 13.1 | 4 | 0 | 17.2 | |
| Total | 68 | 29 | 2 | 99 |
| 68.7 | 29.3 | 2 | 100 | |
PHG: portal hypertensive gastropathy.
The values in bold indicate the boxes in which there was concordance between the severity observed by means of endoscopy and histology.
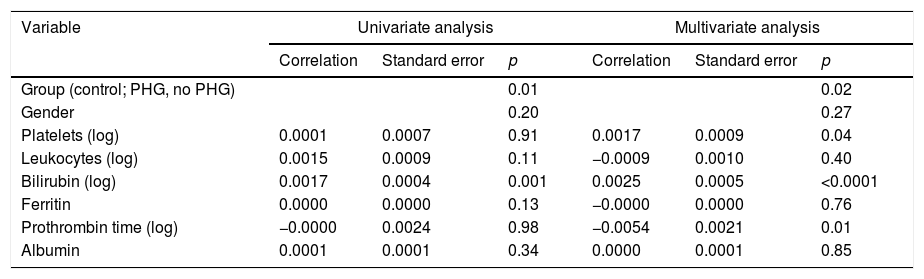
(c) It was assessed whether the vessel diameter was related to any of the patients’ characteristics, particularly the presence of anaemia. To do so, a univariate analysis was performed which included the group (control, PHG, no PHG), age, gender and analytical parameters (Table 4).
Univariate and multivariate analysis results. Relationship between blood vessel diameter and different parameters studied.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Correlation | Standard error | p | Correlation | Standard error | p | |
| Group (control; PHG, no PHG) | 0.01 | 0.02 | ||||
| Gender | 0.20 | 0.27 | ||||
| Platelets (log) | 0.0001 | 0.0007 | 0.91 | 0.0017 | 0.0009 | 0.04 |
| Leukocytes (log) | 0.0015 | 0.0009 | 0.11 | −0.0009 | 0.0010 | 0.40 |
| Bilirubin (log) | 0.0017 | 0.0004 | 0.001 | 0.0025 | 0.0005 | <0.0001 |
| Ferritin | 0.0000 | 0.0000 | 0.13 | −0.0000 | 0.0000 | 0.76 |
| Prothrombin time (log) | −0.0000 | 0.0024 | 0.98 | −0.0054 | 0.0021 | 0.01 |
| Albumin | 0.0001 | 0.0001 | 0.34 | 0.0000 | 0.0001 | 0.85 |
PHG: portal hypertensive gastropathy.
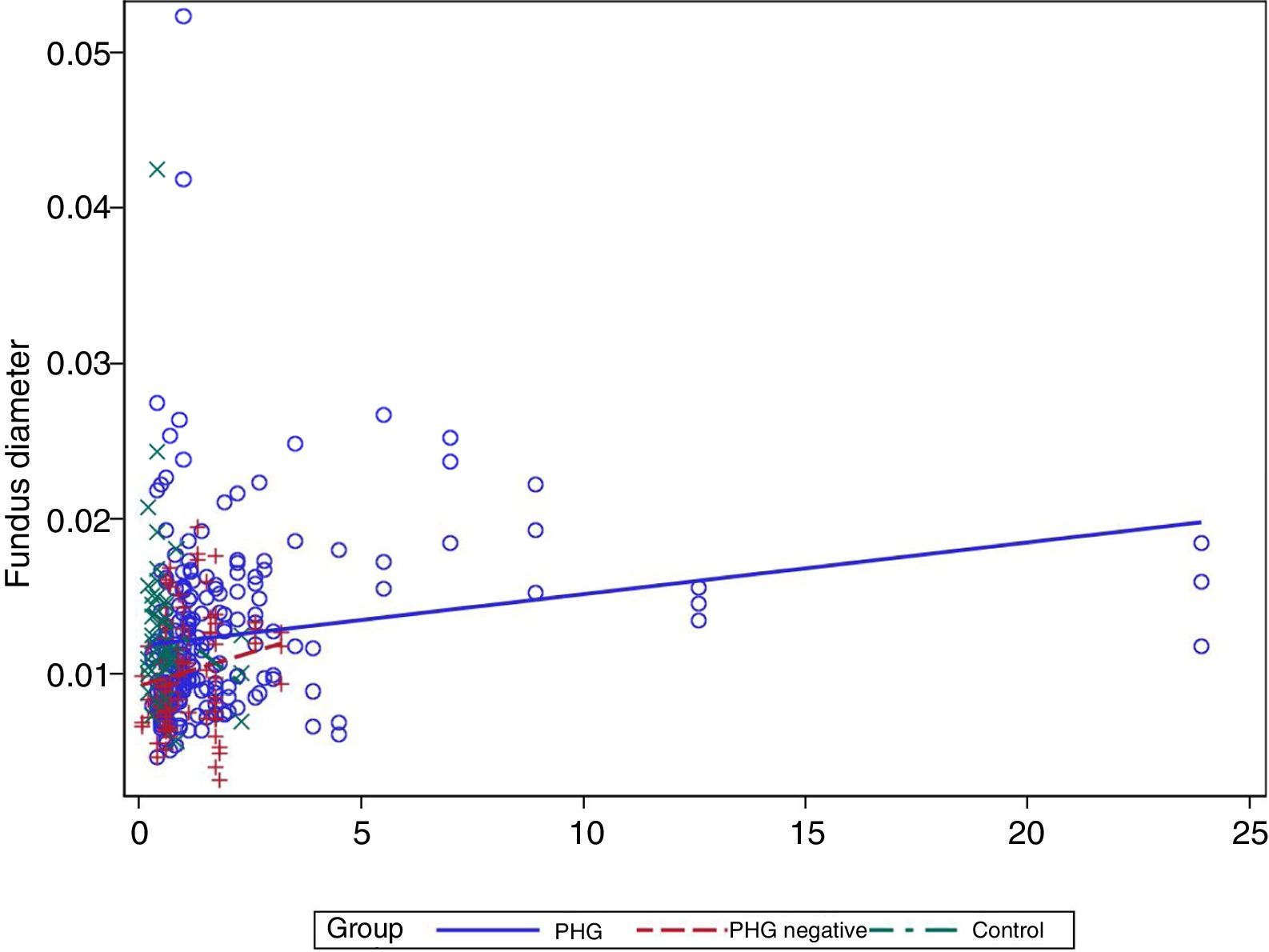
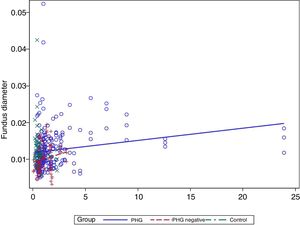
In the multivariate analysis the group, platelets, bilirubin (Fig. 3) and prothrombin time were related to the vessel diameter (Table 4).
GAVE was not observed in any patient.
DiscussionIn our study, gastric biopsy histology in patients with cirrhosis had a very low correlation with both the presence of PHG in endoscopy and the endoscopic degree thereof. This result is in line with previous studies.19–21 One possible explanation for this low correlation could be that biopsies are superficial and do not allow PHG-compatible changes to be seen in the submucosa. It has also been suggested that the lesions may be focal and, as a result, a sampling error is possible. Finally, this lack of correlation may simply reflect the fact that diagnosing PHG endoscopically is not particularly reliable.
No differences were observed in vessel size between the patients and controls, as in a study performed by Misra, where the diameter of the mucosal capillary wall was measured in 73 cirrhotic patients and 64 healthy volunteers.22 There were differences, however, between cirrhotic patients who presented PHG in the histological study and those who did not. Moreover, no differences were observed in vessel size according to the severity of PHG assessed using a histological image. Only two other studies have assessed capillary diameter in PHG. Quintero et al.19 assessed gastric antrum biopsies in patients with UGIB due to PHG, observing that the patients presented larger vessel diameters than the controls. In the second study, performed by Khomeriki et al.,23 gastric body and antrum biopsies were assessed. In this case, the patients with PHG presented a smaller vessel diameter than the patients without PHG, which surprised the authors. Conversely, it is logical that patients with a PHG-compatible histology will also have a larger vessel diameter since this is a diagnostic criterion for PHG.
Although the use of CD34 staining allowed vessel diameters to be assessed in more detail, this fact does not seem to provide significantly greater diagnostic reliability with regard to conventional staining techniques.
Finally, a modest positive correlation is observed between vessel diameter and bilirubin levels. No previous studies have analysed this finding, but it may well be coherent, since the presence of PHG is correlated with the degree of liver failure.
The limitations found in this study are, on the one hand, that no macrobiopsies were performed. The studies published on macrobiopsy are very old, with a limited number of patients, and have not been subsequently validated. Therefore, although they may show the technique to be safe, it does not form part of routine clinical practice and, in our case, due to the high number of biopsies performed, it was not considered ethical to perform a macrobiopsy given that the patient group in question had a greater baseline risk of bleeding due to their cirrhosis. Another limitation was that changes suggestive of PHG present in the submucosa might not have been detected due to the biopsies being superficial. Moreover, only two fundus biopsies were taken, so there is a certain risk that localised lesions may have been overlooked.
Another limitation is the low number of patients with liver disease and moderate or severe gastropathy. In the study by Quintero19 the patients with PHG were enrolled after presenting with UGIB due to PHG, so a greater prevalence of advanced liver disease and moderate or severe PHG lesions was to be expected. In our case, the prevalence of PHG in the sample of stable patients who underwent endoscopy was lower than expected. This suggests that PHG is now less common and probably arises in very advanced stages of liver disease progression.
Lastly, an additional limitation is that there is no gold standard diagnostic test, since in endoscopic imaging it may be confused with other pathologies and its severity under- or overestimated, and we have already seen how histology is also non-specific. Given these limitations, in our opinion, the diagnosis of gastropathy should be reserved for patients with compatible symptoms (anaemia) who present signs of moderate or severe gastropathy during endoscopy. It would be interesting to determine histological findings in this specific patient group.
In conclusion, there is a low correlation between histology and endoscopy for the diagnosis of PHG. Only bilirubin levels present a very modest positive correlation with vessel diameter. The use of CD34 staining does not appear to improve the diagnostic utility of histology.
Authors/contributorsThe authors Maria Rosa Bella and Meritxell Casas contributed equally to the preparation of this manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bella MR, Casas M, Vergara M, Brullet E, Junquera F, Martínez-Bauer E, et al. Utilidad de la histología para el diagnóstico de la gastroenteropatía por hipertensión portal. Concordancia entre la imagen endoscópica y las biopsias gastrointestinales. Papel del marcador CD34. Gastroenterol Hepatol. 2019;42:150–156.