Available evidence assessing the impact of small intestinal bacterial overgrowth (SIBO) following gastrectomy is limited.
ObjectivesTo evaluate the prevalence of SIBO after gastrectomy and its association with malnutrition. To describe the antibiotic treatment required to correct it and if nutritional status improves.
Material and methodsA prospective cohort study was performed at the Agencia Sanitaria Costa del Sol (Costa del Sol Health Agency) from 2012 to 2015. A hydrogen-methane breath test with oral glucose overload was performed. Demographic variables and nutritional parameters were collected at baseline and one month after effective treatment of SIBO. The antibiotic regimens and the number of treatment lines used were assessed.
ResultsSixty gastrectomy patients were analysed, 58.3% of which were male. A sub-analysis of the curve was performed at 45min to minimise possible false positives, and SIBO was identified in 61.6% of cases. SIBO patients tended to have a lower BMI, although this trend was not statistically significant. After treatment with rifaximin, 94.6% of patients were still positive for SIBO, which fell to 85.7% after metronidazole. The rate of total antibiotic treatment failure was 67.6%. No statistically significant changes were found in nutritional parameters after treatment.
ConclusionsSIBO was identified in 61.6% of patients after gastrectomy. No correlation was found with any malnutrition parameter. Rifaximin and metronidazole were found to be largely ineffective in eradicating SIBO. When treatment was effective, the impact on malnutrition was negligible and may have been associated with other factors.
La evidencia disponible que evalúa el impacto de la presencia de sobrecrecimiento bacteriano de intestino delgado (SIBO) después de una gastrectomía es escasa.
ObjetivosEvaluar la frecuencia de SIBO tras gastrectomía y su asociación con malnutrición. Describir las líneas antibióticas necesarias para su corrección y si mejora el estado nutricional.
Material y métodosEstudio de cohortes prospectivo en el ámbito de la Agencia Sanitaria Costa del Sol desde 2012 hasta 2015. Se realizó test del aliento en hidrógeno y en metano espirado con sobrecarga oral de glucosa. Recogida de variables demográficas y valoración nutricional, basal y al mes del tratamiento eficaz del SIBO. Se evaluaron las pautas antibióticas y el número de tratamientos.
ResultadosSe analizaron 60 pacientes gastrectomizados, 58,3%varones. Se realizó un subanálisis de la curva a los 45min para minimizar los posibles falsos positivos con una frecuencia de SIBO del 61,6%. En presencia de SIBO, se observó una tendencia no significativa a presentar un menor IMC. Tras el tratamiento con rifaximina, el SIBO permaneció positivo en el 94,6% y tras metronidazol, en el 85,7%. El multifracaso de la terapia antibiótica fue de 67,6%. No hay cambios estadísticamente significativos en parámetros nutricionales después del tratamiento.
ConclusionesEl SIBO está presente en el 61,6% de los pacientes gastrectomizados, sin que se demuestre asociación con el deterioro nutricional. Rifaximina y metronidazol son escasamente efectivos en la erradicación del SIBO. Cuando este se consigue, el efecto sobre la malnutrición es escaso, pudiendo correlacionarse con otros factores.
Having a gastrectomy causes significant anatomical changes which modify digestion and the normal absorption of food, and this can lead to malnutrition. In some series of gastrectomy patients, up to 74% developed malnutrition, with the ensuing weight loss and below-normal protein levels.1 There are multiple factors involved in the development of malnutrition. They include the lower intake due to early satiety as a result of the restrictive component of the surgery, the reduced absorption of nutrients resulting from resection of the first parts of the small intestine and accelerated transit, and the possibility of developing complications such as dumping syndrome2 and small intestinal bacterial overgrowth (SIBO).
SIBO is the excessive growth and/or a change in the type of bacteria present in the small intestine. The presence of SIBO has been analysed in many different conditions, with a prevalence 9–67% coeliac disease,3 25–88% in Crohn's disease,4 34–92% in chronic pancreatitis,5 and 4–78% in irritable bowel syndrome.6 Less clear, however, is the association with proton-pump inhibitors (PPI), as data are inconsistent.7–10 Recent data suggest that the prevalence of SIBO is up to 43% in surgical series, compared to 13% in controls.11 The hydrogen-methane breath test (HMBT) is positive in 42% of hysterectomy patients and 41% of patients after cholecystectomy. Among gastrectomy patients, several studies estimate a prevalence of 77.5%.11,12 SIBO can be asymptomatic or present with liquid diarrhoea, abdominal pain, abdominal swelling, flatulence, malabsorption and weight loss. It may also be a factor involved in malnutrition, by contributing to the malabsorption of a number of nutrients: fats, fat-soluble vitamins, vitamin B12 and iron.13 SIBO is complex to diagnose. The gold standard is jejunal aspirate culture, but it has considerable limitations, including the cost, the fact that it is an invasive technique, the lack of consensus in quantitative terms, and the fact that the samples are not representative of the distal stretches of the small intestine.14 The HMBT after glucose or lactulose challenge has been put forward as a simple and accessible diagnostic tool for detecting SIBO. The accuracy of the glucose test varies greatly in clinical studies, with a sensitivity of 20% to 93% and a specificity of 30% to 86%, taking as a reference patients diagnosed with SIBO by jejunal aspirate culture.15–17 The glucose-challenge breath tests have greater diagnostic precision than those using lactulose as a substrate, as reflected in the Rome Consensus Conference.18 Some authors have suggested that the rate of false positives may be increased in gastrectomy patients, as the test can lead to conditions such as accelerated transit, making the unabsorbed substrate reach the colon early.19
Unlike the factors relating to the surgery itself or dumping syndrome, SIBO is relatively simple to treat. A recent meta-analysis showed that the administration of antibiotics, considering them overall, induces the remission of SIBO with the breath test returning to normal in 51%.20 The most commonly used antibiotic regimen is rifaximin, with an overall response rate of 70%.21 Correcting SIBO may have an affect on the nutritional status of gastrectomy patients, but the available evidence on the impact of SIBO in these patients is limited. Iivonen et al.22 found a negative correlation between the maximum hydrogen concentration in the HMBT and the main nutritional variables assessed, concluding that there is a relationship between SIBO and the nutritional status of gastrectomy patients. However, although SIBO was common, other authors found no such relationship.12
Consequently, our primary objective was to determine the rate of SIBO in post-gastrectomy patients and to assess its association with malnutrition parameters. Our secondary objective was to determine the antibiotic treatment required to correct SIBO and to assess the impact of treatment on nutritional status.
Patients and methodsPopulationWe conducted a prospective cohort study in which we included patients treated at Agencia Sanitaria Costa del Sol [Costa del Sol Health Agency] from January 2012 to December 2015. Adult patients aged over 18 who had undergone total or subtotal gastrectomy for any reason were included a minimum of three months after the intervention. We excluded patients in advanced stages of cancer (distant metastasis) or with recurrence of cancer, liver cirrhosis or any other cause of maldigestion or malabsorption (chronic pancreatitis, pancreatic resection, inflammatory bowel disease, coeliac disease).
The candidates were asked to participate in the study and included once they had signed the informed consent form.
Study designAll the patients included in the study were initially assessed in the clinic, where a questionnaire was completed with demographic variables (age, gender, smoking and alcohol), the reason for the gastrectomy (benign or cancer and type of cancer), type of surgery (total or subtotal gastrectomy), the type of surgical reconstruction, and clinical assessment parameters by way of a directed presence/absence survey (diarrhoea, abdominal pain, weight loss, suspicion of dumping syndrome, steatorrhoea). The baseline nutritional parameters measured were body mass index (BMI), albumin and prealbumin, cholesterol, absolute lymphocyte count, vitamin D and magnesium. An HMBT was performed according to the protocol described below. The patients were given advice about eating a low-fibre, low-carbohydrate diet in the 48h prior to the test. They came for their appointment having been fasting for at least 12h and without smoking since the previous night; they were also advised not to exercise beforehand and to clean their teeth that morning with 20ml of 0.05% chlorhexidine solution. A baseline sample was taken first of all, which had to be less than 10ppm of hydrogen. Values between 10 and 20 suggested incomplete fasting before the test or intake of slow-digesting food the day before the test. In such cases, specific re-education was provided and an appointment made for another day to repeat the test. The patients were then administered an oral glucose challenge (60g of glucose in 200ml of water), after which, alveolar samples were taken with forced expiration at 15min intervals for 2h. The results were read in the local laboratory with a gas chromatograph (MYCROLIZER®, ISOMED) (Fig. 1), and were sent to the central laboratory for combined reading of hydrogen and methane with QuinTron MYCROLYZER in the reference laboratory ISOMED.
The primary endpoint was the presence of SIBO, considering as positive patients with a peak above 10ppm compared to the baseline hydrogen determination and/or if the methane concentration increased by more than 12ppm with respect to baseline; otherwise, they were considered to be negative.18 In gastrectomy patients, one of the possible causes of false positives in the HMBT is accelerated transit. To minimise the impact of that, and as scintigraphy was not available, we took heed of a recent study23 and performed a sub-analysis of the curve obtained at 45min, such that only the curves which had raised hydrogen and methane peaks in that period were considered as true positives.
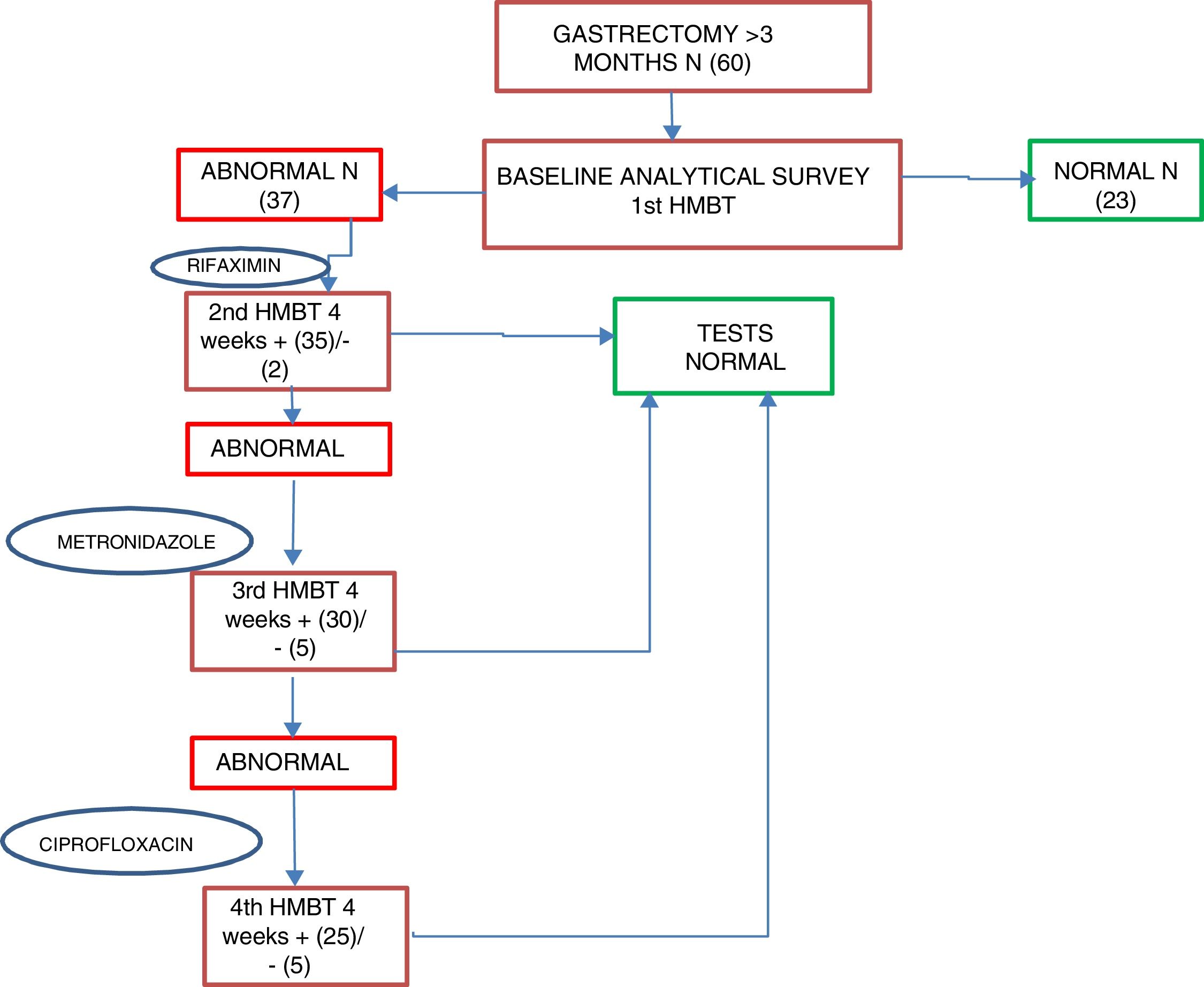
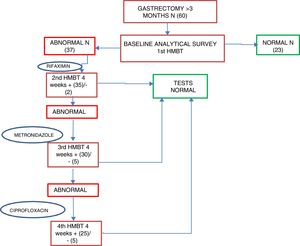
Patients who were positive for SIBO were started on first-line treatment of rifaximin 400mg every 8h for 10 days as standard; second-line treatment was with metronidazole 500mg every 8h for 10 days, and third-line, ciprofloxacin 500mg every 12h for 10 days. An HMBT was performed at four weeks after each antibiotic line to assess the response to treatment and/or the reappearance of SIBO; if after two antibiotic lines the SIBO persisted it was considered as multiple failure. After first confirming that patients had become SIBO negative, after one month, tests were performed to assess their nutritional status, including all the parameters determined in the baseline assessment (Fig. 2).
Statistics and ethical considerationsThis study adhered to the Declaration of Helsinki and the law on the protection of patients’ rights throughout. No clinical data were collected other than those described above. Nevertheless, all data collected in this project were recorded anonymously, strictly adhering to the applicable laws and data protection regulations (Law 41/2002 of 14 November, Law 15/1999 of 15 December). The project was approved in advance in July 2012 by the Ethics and Research Committee of Agencia Sanitaria Costa del Sol.
We performed a descriptive analysis with measures of central tendency and dispersion (mean and standard deviation), along with median and interquartile range (for group comparison) for quantitative variables, and frequency distribution for qualitative variables. The results were compared between the complete curve at 120min and those obtained with the curve at 45min The rate of SIBO was calculated with the test at 45min including the 95% CI. Bivariate analysis was carried out using SIBO positive with the test at 45min as outcome variable, comparing with the Chi-square test for qualitative variables, the Mann–Whitney U test for quantitative baseline malnutrition values and the Wilcoxon test for assessment of the change between nutritional parameters at baseline and one month in patients who became negative. The level of statistical significance was established as p<0.05.
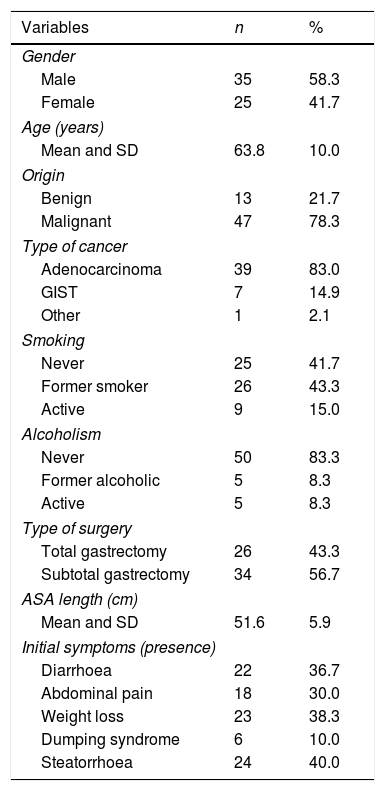
ResultsA total of 76 patients were assessed, with 16 being excluded for presenting exclusion factors: four for pancreatic resection; two for chronic pancreatitis; two for advanced cancer stage; five for atypical gastric resections; one for autoimmune liver cirrhosis; and two were lost after withdrawing their consent during the study. In the end, a total of 60 gastrectomy patients were included, 58.3% male, with a mean age at study inclusion of 63.8 (SD 10 years). Toxic habits are shown in Table 1. Gastrectomy was performed for benign causes in 21.7% (13) and cancer in 78.3% (47). Gastric adenocarcinoma was the most common type of cancer, responsible for 83% of cancer-related gastrectomies (Table 1). Out of the overall group, 56.7% had subtotal gastrectomy and 43.3% total gastrectomy, with Billroth ii reconstruction and a mean loop length in the reconstruction of 51.6cm (SD 5.9). The most common clinical manifestation was steatorrhoea in 40% of the patients; the other signs and symptoms are shown in Table 1.
Description of clinical variables.
| Variables | n | % |
|---|---|---|
| Gender | ||
| Male | 35 | 58.3 |
| Female | 25 | 41.7 |
| Age (years) | ||
| Mean and SD | 63.8 | 10.0 |
| Origin | ||
| Benign | 13 | 21.7 |
| Malignant | 47 | 78.3 |
| Type of cancer | ||
| Adenocarcinoma | 39 | 83.0 |
| GIST | 7 | 14.9 |
| Other | 1 | 2.1 |
| Smoking | ||
| Never | 25 | 41.7 |
| Former smoker | 26 | 43.3 |
| Active | 9 | 15.0 |
| Alcoholism | ||
| Never | 50 | 83.3 |
| Former alcoholic | 5 | 8.3 |
| Active | 5 | 8.3 |
| Type of surgery | ||
| Total gastrectomy | 26 | 43.3 |
| Subtotal gastrectomy | 34 | 56.7 |
| ASA length (cm) | ||
| Mean and SD | 51.6 | 5.9 |
| Initial symptoms (presence) | ||
| Diarrhoea | 22 | 36.7 |
| Abdominal pain | 18 | 30.0 |
| Weight loss | 23 | 38.3 |
| Dumping syndrome | 6 | 10.0 |
| Steatorrhoea | 24 | 40.0 |
ASA: American Society of Anesthesiologists; GIST: gastrointestinal stromal tumours; SD: standard deviation.
When analysing the entire group of gastrectomy patients for SIBO, 70.5% had an HMBT positive for SIBO if the 120min curve was analysed. Analysing the “45min peak” assessment and the complete curve (120min), there was concordance between the two assessments in 55 (91.6%) patients (18 negative and 37 positive). However, in five patients, the assessment at 45min was negative, but the full curve was positive, these being considered as false positives. The rate of SIBO, not including the false positives, was 61.6%. The percentage of absolute agreement between the two tests is 91.7%, with a kappa value of 0.82 (95% CI 0.66–0.97); the kappa value representing an “excellent” strength of agreement.24
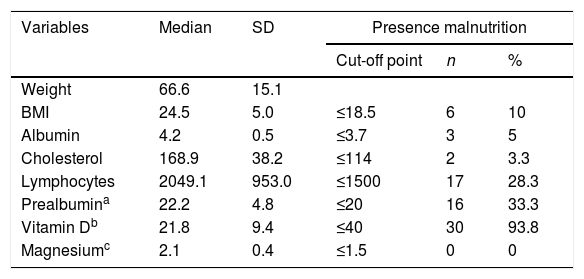
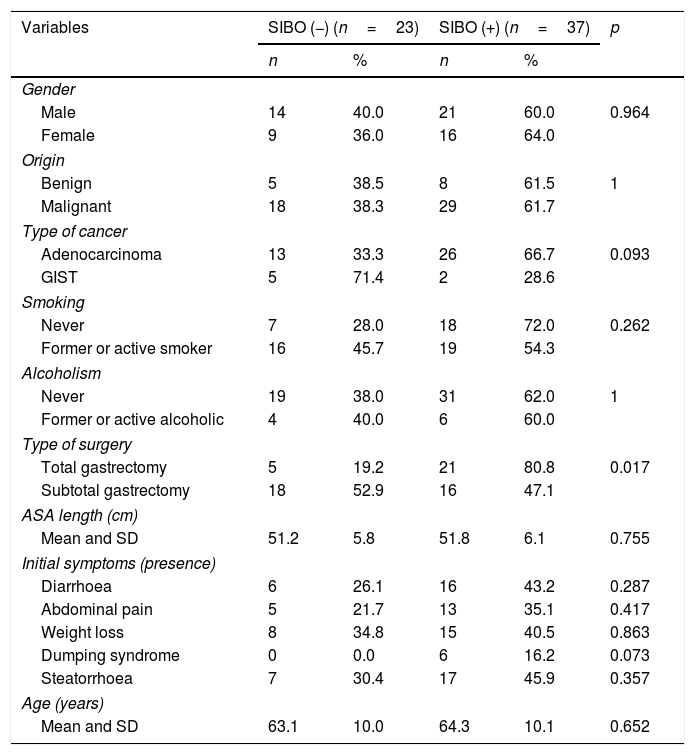
Measurement of the baseline malnutrition parameters in this series of patients revealed a low lymphocyte count in 28.3%, low levels of prealbumin in 33.3% and low vitamin D in 93.8%, while 10% had a BMI≤18.5kg/m2 and 3.3% had low cholesterol levels. None of the patients had low magnesium levels (Table 2). The patients’ clinical characteristics according to presence of SIBO are shown in Table 3. In the bivariate analysis of these data, a statistically significant difference was only found in the presence of SIBO in the group of patients who had undergone total gastrectomy.
Description of malnutrition parameters.
| Variables | Median | SD | Presence malnutrition | ||
|---|---|---|---|---|---|
| Cut-off point | n | % | |||
| Weight | 66.6 | 15.1 | |||
| BMI | 24.5 | 5.0 | ≤18.5 | 6 | 10 |
| Albumin | 4.2 | 0.5 | ≤3.7 | 3 | 5 |
| Cholesterol | 168.9 | 38.2 | ≤114 | 2 | 3.3 |
| Lymphocytes | 2049.1 | 953.0 | ≤1500 | 17 | 28.3 |
| Prealbumina | 22.2 | 4.8 | ≤20 | 16 | 33.3 |
| Vitamin Db | 21.8 | 9.4 | ≤40 | 30 | 93.8 |
| Magnesiumc | 2.1 | 0.4 | ≤1.5 | 0 | 0 |
BMI: body mass index; SD: standard deviation.
Bivariate analysis between clinical variables and presence of “45-min peak”.
| Variables | SIBO (−) (n=23) | SIBO (+) (n=37) | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | |||||
| Male | 14 | 40.0 | 21 | 60.0 | 0.964 |
| Female | 9 | 36.0 | 16 | 64.0 | |
| Origin | |||||
| Benign | 5 | 38.5 | 8 | 61.5 | 1 |
| Malignant | 18 | 38.3 | 29 | 61.7 | |
| Type of cancer | |||||
| Adenocarcinoma | 13 | 33.3 | 26 | 66.7 | 0.093 |
| GIST | 5 | 71.4 | 2 | 28.6 | |
| Smoking | |||||
| Never | 7 | 28.0 | 18 | 72.0 | 0.262 |
| Former or active smoker | 16 | 45.7 | 19 | 54.3 | |
| Alcoholism | |||||
| Never | 19 | 38.0 | 31 | 62.0 | 1 |
| Former or active alcoholic | 4 | 40.0 | 6 | 60.0 | |
| Type of surgery | |||||
| Total gastrectomy | 5 | 19.2 | 21 | 80.8 | 0.017 |
| Subtotal gastrectomy | 18 | 52.9 | 16 | 47.1 | |
| ASA length (cm) | |||||
| Mean and SD | 51.2 | 5.8 | 51.8 | 6.1 | 0.755 |
| Initial symptoms (presence) | |||||
| Diarrhoea | 6 | 26.1 | 16 | 43.2 | 0.287 |
| Abdominal pain | 5 | 21.7 | 13 | 35.1 | 0.417 |
| Weight loss | 8 | 34.8 | 15 | 40.5 | 0.863 |
| Dumping syndrome | 0 | 0.0 | 6 | 16.2 | 0.073 |
| Steatorrhoea | 7 | 30.4 | 17 | 45.9 | 0.357 |
| Age (years) | |||||
| Mean and SD | 63.1 | 10.0 | 64.3 | 10.1 | 0.652 |
ASA: American Society of Anesthesiologists; GIST: gastrointestinal stromal tumours; SD: standard deviation; SIBO: small intestinal bacterial overgrowth.
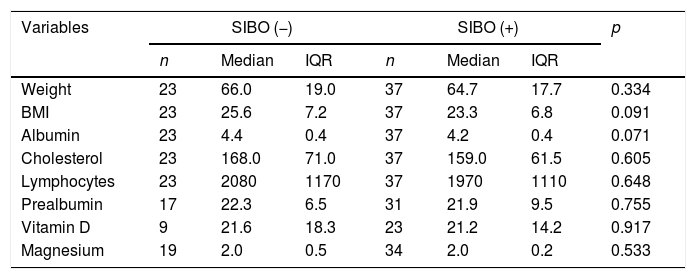
Relating the presence of SIBO and baseline nutritional status, a tendency to have low vitamin D levels and lower BMI was found in those positive for SIBO compared to those negative for SIBO, although without reaching statistical significance (Table 4). There were also no differences for cholesterol levels, albumin, absolute lymphocyte count or magnesium levels, probably due to the small sample size. In those who were positive for SIBO, 68.8% had prealbumin levels≤20mg/dl, 73.3% vitamin D levels≤40mg/dl and 66.7% a BMI≤18.5kg/m2.
Bivariate analysis between malnutrition parameters and SIBO.
| Variables | SIBO (−) | SIBO (+) | p | ||||
|---|---|---|---|---|---|---|---|
| n | Median | IQR | n | Median | IQR | ||
| Weight | 23 | 66.0 | 19.0 | 37 | 64.7 | 17.7 | 0.334 |
| BMI | 23 | 25.6 | 7.2 | 37 | 23.3 | 6.8 | 0.091 |
| Albumin | 23 | 4.4 | 0.4 | 37 | 4.2 | 0.4 | 0.071 |
| Cholesterol | 23 | 168.0 | 71.0 | 37 | 159.0 | 61.5 | 0.605 |
| Lymphocytes | 23 | 2080 | 1170 | 37 | 1970 | 1110 | 0.648 |
| Prealbumin | 17 | 22.3 | 6.5 | 31 | 21.9 | 9.5 | 0.755 |
| Vitamin D | 9 | 21.6 | 18.3 | 23 | 21.2 | 14.2 | 0.917 |
| Magnesium | 19 | 2.0 | 0.5 | 34 | 2.0 | 0.2 | 0.533 |
BMI: body mass index; IQR: interquartile range; SIBO: small intestinal bacterial overgrowth.
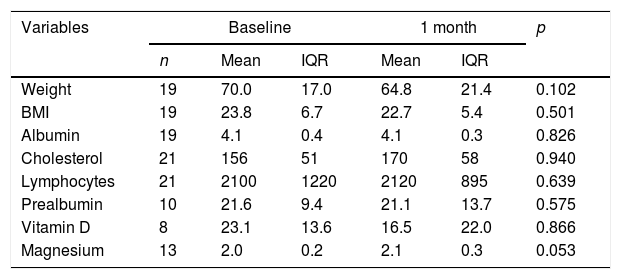
Of all the patients positive for SIBO, only two (2/37) were found to be negative after the first line of treatment with rifaximin, with 94.6% once again being positive. After the second line of treatment with metronidazole, 14.3% (5/35) were found to be negative, and after the third line with ciprofloxacin, 16.6% (5/25) were negative. Multiple failure of antibiotic therapy was therefore high in these patients, occurring in 67.6% of cases. Analysis of the nutritional and anthropometric parameters in the group of patients with multiple antibiotic failure showed no statistically significant changes in any of them with respect to the baseline figures. Evaluation of changes in the nutritional parameters between baseline and one month revealed a median reduction of 5.2kg and a tendency for the magnesium value to increase by 0.1 points. However, neither of these differences were statistically significant (Table 5). In the group whose HMBT became negative, there was a tendency towards a higher prealbumin and absolute lymphocyte count, but complete post-treatment data were only available in 21 and 10 patients, respectively.
Paired analysis between baseline malnutrition parameters and after one month with negative SIBO.
| Variables | Baseline | 1 month | p | |||
|---|---|---|---|---|---|---|
| n | Mean | IQR | Mean | IQR | ||
| Weight | 19 | 70.0 | 17.0 | 64.8 | 21.4 | 0.102 |
| BMI | 19 | 23.8 | 6.7 | 22.7 | 5.4 | 0.501 |
| Albumin | 19 | 4.1 | 0.4 | 4.1 | 0.3 | 0.826 |
| Cholesterol | 21 | 156 | 51 | 170 | 58 | 0.940 |
| Lymphocytes | 21 | 2100 | 1220 | 2120 | 895 | 0.639 |
| Prealbumin | 10 | 21.6 | 9.4 | 21.1 | 13.7 | 0.575 |
| Vitamin D | 8 | 23.1 | 13.6 | 16.5 | 22.0 | 0.866 |
| Magnesium | 13 | 2.0 | 0.2 | 2.1 | 0.3 | 0.053 |
BMI: body mass index; IQR: interquartile range.
Malnutrition is more common among gastrectomy patients because of changes in the digestion and absorption of food. Probable causative factors include the surgery itself, early satiety due to the restrictive component, accelerated transit, and the development of complications such as SIBO and dumping syndrome.1,2
One factor which is modifiable with adequate diagnosis and treatment is SIBO. Our aim was therefore to determine the rate of SIBO in gastrectomy patients and its correlation with malnutrition data. Our secondary objective was to determine the antibiotic treatment required to correct SIBO and to assess the impact of treatment on nutritional status.
In the analysis of the 120min curve, the rate of SIBO in our series was 70.5%. This is in line with previous studies, which report a rate of 77.6%.3 After gastrectomy, the primary defensive barrier diminishes, predisposing patients to the development of SIBO.4–6 The factors involved in deterioration of the barrier include a decrease in or absence of acid secretion, altered intestinal motility with a reduction in antegrade waves which facilitates intraluminal growth of the bacteria, and a decrease in antibacterial secretions. Direct culture of small intestinal aspirate (>105CFU/ml) was used as the gold standard for the diagnosis of SIBO. However, the invasive nature of the procedure, the risk of contamination with flora from segments higher up, the location of the sample (false negatives), the lack of consensus on the definition of positive culture and the lack of reproducibility all now mean that it is less than ideal.7,8 In this context, the HMBT, a simple, inexpensive and minimally invasive test, is currently the most used, although there is no validated breath test for the diagnosis of SIBO.7 The glucose breath test has a sensitivity of 62.5% and a specificity of 81.8%, and is considered positive if exhaled hydrogen increases by more than 10–20ppm above baseline.8 When interpreting the curve in gastrectomy patients, we have to take into account the risk of false positives due to accelerated transit and/or loss of mucosal surface for absorption; in this case the glucose escapes absorption in the small intestine and is fermented in the colon.4,7 To overcome this problem, and as scintigraphy was not available, in our study, we reappraised the criteria and considered positive SIBO as that occurring in the first 45min of the curve.23 This meant that of the 42 patients positive for SIBO, 37 fulfilled the new criteria, with the remaining five being considered as possible false positives due to fermentation of glucose in the colon. Consequently, with the change in method, the rate of SIBO in our series was established at 61.6%. The percentage of absolute agreement between the two tests is 91.7%, with a kappa value of 0.82 (95% CI 0.66–0.97); the kappa value representing an “excellent” strength of agreement.24
There have been suggestions that SIBO is one of the factors involved in malnutrition in gastrectomy patients. However, the association has not been well established and there is no standardised method for measuring malnutrition in these patients. From our results, we found that among patients with positive HMBT (SIBO present) there was a trend towards lower levels of prealbumin (68.8% had prealbumin≤20mg/dl) and vitamin D (73.3% had levels≤40mg/dl), and a low BMI (66.7% had a BMI≤18.5kg/m2), although the differences were not statistically significant. The data in the literature are contradictory. Some authors report that 74% of gastrectomy patients have a BMI<18.5kg/m2, up to 50% have anaemia (although this is usually mild) and up to 58% have hypoproteinaemia and albuminaemia (<3.5mg/dl).1 In another study, in gastrectomy patients with Roux-en-Y reconstruction, an inverse relationship was found between the highest concentrations of exhaled hydrogen on the breath test curve for SIBO and iron, albumin and weight loss levels.6 However, Paik et al.12 found no relationship between the presence of SIBO and lower levels of calcium, albumin, iron and haemoglobin.
The malnutrition associated with SIBO involves both malabsorption and maldigestion as pathophysiological determinants.4 The malabsorption of carbohydrates is caused by the fact that these disaccharides are metabolised in the intestinal lumen by the metabolising bacteria of fructose, sorbitol and lactose, with the result that part of them is not absorbed.9 Added to that is the secretion of inflammatory cytokines, which can damage the enterocytes’ brush border, with the consequent decrease in disaccharidase activity and less absorption of carbohydrates.10–12 As a result of the deconjugation of bile acids by the bacteria, there is an alteration in the formation of micelles, which leads to malabsorption of fats and, consequently, steatorrhoea and deficiency in fat-soluble vitamins.13,14 Vitamin D deficiency is the most common and usually presents with the development of osteomalacia or even osteoporosis.15 The absorption of proteins can also be altered, as the bacteria split the nitrogen in the diet into urea, in which form it is no longer of use for protein anabolism.16 Our results did not reach statistical significance, probably in relation to the size of the sample and the fact that our analysis of this condition was only carried out in the short-term; we perhaps should have allowed more time for the SIBO to exercise its harmful effects on the patients’ nutritional status. Another element that needs to be assessed is whether or not the standard of care for these patients in our hospital setting includes a full nutritional assessment with standardised recommendations on diet, with nutritional support if necessary prior to surgery in order to optimise conditions pre-intervention, and systematic assessments post-intervention. Paik et al.12 identify that a significant amount of input by nursing staff, providing both symptomatic and nutritional support, could explain the adequate nutritional status of the patients in their study.
To improve the nutritional status of gastrectomy patients with SIBO, providing them with specific treatment has been proposed. The most commonly used and studied antibiotic for the treatment of SIBO in any indication is rifaximin, which achieves post-treatment normal breath test results in 21.7–70% of cases.20,21 Rifaximin is a good option as first-line treatment as this antibiotic has a low level of systemic absorption and so acts primarily in the intestinal lumen, and it has a very low side effect rate. The second most used antibiotic is metronidazole, which achieves normal breath test results post-treatment in 51.2% of cases.20 Other antibiotics which are less studied, but which could also be useful, are ciprofloxacin, doxycycline, norfloxacin and amoxicillin-clavulanic acid, but there have been very few studies on their effectiveness. In the last few years, the use of probiotics has also been proposed, but supporting evidence is still limited.25,26 There is no consensus in the literature on the best treatment regimen for SIBO after a gastrectomy, and with the reference of the effectiveness of rifaximin in other conditions, we used it in our series as first-line. However, the rate of elimination of SIBO was only 5.4%. As second-line we used metronidazole, which eliminated SIBO in 14.3% of the cases, and, in third-line, we achieved a 16.7% response to ciprofloxacin. Our data show that at 67.6%, the rate of repeated failure of antibiotic treatments in these patients is high. It is therefore evident that, despite being the treatments of choice for SIBO in general, rifaximin and metronidazole do not obtain good responses in gastrectomy patients; hence the need to prescribe antibiotic therapy with caution and to test its effectiveness with the HMBT. There are a number of different explanations for the results of the series presented. Gastrectomy is a permanent structural alteration that creates a favourable environment for SIBO, so not acting perpetuates the situation. After several lines of unsuccessful antibiotic treatment, it therefore seems likely that patients would benefit from cyclical antibiotic treatment, provided that the impact and the theoretical benefits to be obtained are always assessed. For a non-absorbable antibiotic such as rifaximin to be effective, two conditions must be met: it has to reach the location where the SIBO is produced and achieve an adequate concentration in that location. Therefore, after Billroth ii gastrectomy with Roux-en-Y reconstruction, with the creation of blind loops, these two conditions may not be met by non-absorbable antibiotics, thus explaining the low therapeutic efficacy.
Although SIBO has been postulated as a risk factor for malnutrition in gastrectomy patients, it is not the only factor that may affect these patients’ nutritional status. Other factors to consider are the type of gastrectomy and reconstruction, the patient's age and the possible development of exocrine pancreatic insufficiency due to inactivation of lipase leading to fat maldigestion, a hypothesis our group is currently working on.
One of the limitations of our study is the sample size; although similar to that of other studies reported in the literature, it diminished over the course of the follow-up process. This was the result of a number of factors, one of which was recurrence and/or progression of the cancer, which led to losses because of the death of the patient or deterioration in their general condition. The high percentage of patients with repeated failure of antibiotic treatment for SIBO also had an effect. Another limitation may be the short follow-up for the assessment of nutritional parameters, as these may take longer to become modified. We should point out that in the design of the study, we did not plan to perform follow-up analysis in patients in whom antibiotic treatment failed. This design may explain the bias if the baseline situation is only compared with that obtained after the successful treatment of SIBO several weeks or months later.
Among the strengths of the study is the fact that it addresses a problem little studied up to now. Particularly important are its prospective design, with systematic assessment by HMBT with glucose challenge of the presence of SIBO, the correlation with nutritional data and the re-assessment of the curve at 45min to minimise the impact of accelerated intestinal transit, and thus avoid false positives. There are limited data in the literature on the treatment of SIBO in gastrectomy patients and they do not discuss the evolving nature of this condition and its influence on nutritional status.
In conclusion, the results of this study show that SIBO is common in gastrectomy patients, with 61.6% of our patients testing positive. However, in our series, we were unable to make a statistically significant correlation with any malnutrition parameters. Rifaximin and metronidazole do not obtain good results in the control of SIBO after gastrectomy. Antibiotic failure occurs in 83% of cases, and the response needs to be re-assessed after each antibiotic treatment. Once the SIBO has been resolved, it has little effect on malnutrition and it may be correlated with other factors.
Authors’ contributionsM.C. García Gavilán: data acquisition; analysis and interpretation of data; writing of the manuscript; critical review of the manuscript providing relevant intellectual content.
J. Alcaide García: study concept and design; data acquisition; analysis and interpretation of the data.
J.M. Méndez Sánchez: data acquisition.
R. Rivera Irigoin: study concept and design; data acquisition; analysis and interpretation of the data.
F. Fernández Cano: data acquisition; analysis and interpretation of the data.
T. Pereda Salguero: data acquisition; analysis and interpretation of the data.
F. Rivas Ruiz: analysis and interpretation of data; writing of the manuscript; critical review of the manuscript providing relevant intellectual content.
A. Pérez Aisa: study concept and design; data acquisition; analysis and interpretation of data; writing of the manuscript; critical review of the manuscript providing relevant intellectual content; statistical analysis, obtaining of funding; administrative, technical and material support; supervision of the study.
All the authors approved the final version of the manuscript.
Conflicts of interestNone declared.
Please cite this article as: Pérez Aisa A, García Gavilán MC, Alcaide García J, Méndez Sánchez IM, Rivera Irigoin R, Fernández Cano F, et al. El sobrecrecimiento bacteriano de intestino delgado es una entidad frecuente tras gastrectomía, pero con escasa relevancia en el estado nutricional. Gastroenterol Hepatol. 2019;42:1–10.