The appearance of pressure ulcers (PU) is one of the frequent complications of prone position (PP), due to prolonged pressure and shear forces.
ObjectivesTo compare the incidence of pressure ulcers secondary to prone position and describe their location among four Intensive Care Units (ICU) of public hospitals.
MethodsMulticenter descriptive and retrospective observational study. The population consisted of patients admitted to the ICU between February 2020 and May 2021, diagnosed with Covid-19 who required prone decubitus. The variables studied were sociodemographic, days of admission to the ICU, total hours on PP, PU prevention, location, stage, frequency of postural changes, nutrition and protein intake. Data collection was carried out through the clinical history of the different computerized databases of each hospital. Descriptive analysis and association between variables were performed using SPSS vs.20.0.
ResultsA total of 574 patients were admitted for Covid-19, 43.03% were pronated. 69.6% were men, median age was 66 (IQR 55−74) and BMI 30.7 (RIC 27−34.2). Median ICU stay was 28 days (IQR 17−44.2), median hours on PD per patient 48 h (IQR 24−96). The incidence of PU occurrence was 56.3%, 76.2% of patients presented a PU, the most frequent location was the forehead (74.9%). There were significant differences between hospitals in terms of PU incidence (P = .002), location (P = .000) and median duration of hours per PD episode (P = .001).
ConclusionsThe incidence of pressure ulcers due to the prone position was very high. There is great variability in the incidence of pressure ulcers between hospitals, location and average duration of hours per episode of prone position.
La aparición de úlceras por presión (UPP) es una de las complicaciones frecuentes del decúbito prono (DP), debido a la presión prolongada y las fuerzas de cizallamiento.
ObjetivosComparar la incidencia de úlceras por presión secundarias a la posición del decúbito prono y describir su localización entre cuatro Unidades de Cuidados Intensivos (UCI) de hospitales públicos.
MetodologíaEstudio observacional descriptivo retrospectivo multicéntrico. La población estuvo formada por pacientes ingresados en la UCI entre febrero de 2020 y mayo 2021, diagnosticados de Covid-19 que precisaron decúbito prono. Las variables estudiadas fueron sociodemográficas, días de ingreso en UCI, horas totales en DP, prevención de UPP, localización, estadio, frecuencia de cambios posturales, nutrición y aporte de proteínas. La recogida de datos se realizó a través de la historia clínica de las diferentes bases de datos informatizadas de cada hospital. Se realizó análisis descriptivo y asociación entre las variables, utilizando el programa SPSS vs.20.0.
ResultadosIngresaron 574 pacientes por Covid-19, el 43,03% fueron pronados. 69,6% fueron hombres, la mediana de edad fue 66 (RIC 55–74) y el IMC de 30,7 (RIC 27–34,2). La mediana de estancia en UCI fue de 28 días (RIC 17–44,2), la mediana de horas en DP por paciente 48 h (RIC 24–96). La incidencia de aparición de UPP fue del 56,3%, el 76,2% de pacientes presentaron una UPP, la localización más frecuente fue la frente (74,9%). Existen diferencias significativas entre hospitales en cuanto a incidencia de UPP (P = ,002), localización (P = ,000) y duración media de horas por cada episodio de DP (P = ,001).
ConclusionesLa incidencia de UPP secundarias al DP fue muy elevada. Existe gran variabilidad en cuanto a incidencia de UPP entre hospitales, localización y duración media de horas por cada episodio de DP.
Prone positioning has been shown to be effective in the treatment of patients with COVID-19 who have moderate or severe ARDS. Pressure ulcers (PUs) are the most common adverse event from the technique.
What it contributesThis study is of interest because it reflects the variability in the care of the prone COVID-19 patient in terms of prevention, care, and site of PUs in a critical care setting.
Implications of the studyThe variability of PU care in different hospitals has resulted in different PU incidence rates. Collaboration between centres will help update PU-related procedures based on evidence and health outcomes.
The reasons for admission to the intensive care unit (ICU) and patient characteristics may be multiple: sepsis, neurocritical illness, severe trauma, shock, renal failure, cardiac disease, postoperative surgery…. Critical patient care is complex given the pathophysiological disturbances and the severity of the clinical situation requiring their admission, whether due to severe illness or the need for continuous nursing care.1,2 Patients were admitted to ICU during the COVID-19 pandemic primarily for respiratory reasons, specifically coronavirus pneumonia, and acute respiratory distress syndrome (ARDS) was one of the most frequent associated complications. ARDS is an acute, diffuse lung disease that causes hypoxaemia, decreased pulmonary compliance, and increased dead space.3 Prone positioning is advised in the treatment of ARDS patients with arterial oxygen pressure/inspired oxygen fraction (PaO2/FiO2) < 150 mmHg.4 Prone positioning is a postural therapy to optimise respiratory function by increasing oxygenation levels, lung compliance, secretion management, increasing redistribution of perfusion, and improving ventilation in previously collapsed areas.5 The main benefit of this therapy is that it improves the respiratory outcome of patients with severe COVID-19.6
The Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMYCIUC) recommends prone positioning for at least 16 h for the first 24 h in patients with ARDS diagnosed with COVID-19, repeating cycles until the patient improves.7 However, prone positioning is not a complication-free procedure. The most frequent complications reported in the literature include catheter loss, obstruction or loss of the endotracheal tube, haemodynamic instability, brachial plexus injuries, and/or pressure ulcers (PU).8 A PU is a localised injury to the skin and/or underlying tissue, usually over a bony prominence, as a result of pressure, or pressure in combination with shear. They can sometimes also occur on soft tissues subjected to external pressure by different materials or clinical devices. Intrinsic factors such as immobility, respiratory/circulatory impairment, low blood pressure, anaemia, age, malnutrition, and dehydration, and extrinsic factors such as humidity, hospital stay, support surface, medication, and catheterisation all predispose to the development of PUs.9
PUs are frequent complications of prone positioning (PP), with an incidence of 25.7%, and are described as possible factors related to time in this position and nutritional intake.8 During the first wave of the pandemic, an increase in pressure injuries was detected, especially in patients requiring PP,10 with an incidence of 38%–57%.8,11 Malnutrition and hydration deficits are factors related to the incidence and severity of PUs, especially low protein intake.8,9
Regular inspection of the skin is a recommended interventions for PU care, systematically monitoring bony prominences, especially in people admitted to the ICU with risk factors for developing PUs. To treat category 1 PUs, the Health Service of the Balearic Islands’ guidelines for the prevention and treatment of PUs recommend applying hyperoxygenated fatty acids (HOFA) to the areas at risk, without massage, because they produce optimal skin hydration, promote capillary circulation and improve local conditions of skin exposed to prolonged ischaemia.9 Other authors and expert groups recommend hydrocolloid and/or silicone dressings on areas of pressure or on early detection of signs such as oedema and reddening of the skin.8,12,13
From the recommendations of the ZERO Projects during the SARS-CoV-2 pandemic, the SEMYCIUC proposes focussing on the training of new professionals, implementing protocols for prone positioning, and a standardised nursing care plan in intensive care units.10
The COVID-19 pandemic marked a turning point in critical patient care. The rapid spread of the virus led to the saturation of most Spanish ICUs, which precipitated adaptation and restructuring of the care environment. It also had a major impact on healthcare, which was hampered by human factors, such as incorporating professionals with no experience in the care of critically ill patients, the risk of contagion resulting in the modification of work dynamics, causing some hospitals to change their shifts. Other factors that affected this healthcare crisis were the poor availability of resources and the high burden of care.14–16 The development of PUs is closely linked to nursing care, and the question is whether the recommendations made by the different scientific societies on PP and PU prevention have been sufficient to prevent the process. The aims of this study are to compare the incidence of PUs secondary to PP in 4 intensive care units in public hospitals, to describe the characteristics and sites of PUs secondary to PP and to analyse the possible causes of their onset.
MethodologyDesignWe conducted a multicentre retrospective observational study.
Scope of the study and subjectsThe study was conducted in 4 public hospitals of different levels of care in Mallorca:
- -
Hospital 1(H1): polyvalent ICU with 6 beds, with capacity to care for 6 critical patients diagnosed with COVID-19 and 2 beds in resuscitation for non-COVID-19 patients.
- -
Hospital 2 (H2): Multi-purpose ICU with 6 beds. Due to the pandemic, 8 beds were made available for COVID-19 patients and 5 beds for non-COVID-19 patients.
- -
Hospital 3 (H3): Multi-purpose ICU with an initial capacity of 18 beds. With the contingency plan, 31 beds were made available for COVID-19 patients and 6 for non-COVID-19 patients.
- -
Hospital 4 (H4): Multi-purpose ICU with a total of 38 beds. Forty-four beds were made available for COVID-19 patients and 26 beds for non-COVID-19 critical patients.
All of the hospitals reconverted spaces to meet the high demand for COVID-19 admissions to ICU, almost doubling the baseline capacity of each hospital (from 64 beds, a total of 114 beds were made available among the 4 hospitals), and adapting to the needs of each wave of the pandemic.
The sample was selected by purposive convenience sampling. The inclusion criteria were patients admitted to the hospitals under study, diagnosed with COVID-19 from 1 March 2020 to 1 May 2021, over 18 years of age, who at any time during their admission to the ICU had required PP. The exclusion criteria were patients diagnosed with COVID-19 admitted to the ICU, admitted for less than 24 h and/or dying during the first 24 h. No sample calculation was performed, because all patients who met the inclusion criteria were included in the study.
Data collection technique and instrumentsData collection was undertaken by all the researchers, consulting the different computerised databases of each hospital. An initial meeting was held among the researchers to establish unique codes for recording the variables, with the aim of making them understandable to all, given that there are 2 different computerised programmes. Those using the same programme adapted it to their clinical practice. A shared-use Excel spreadsheet was designed to compile the variables, consensus was reached on the discrepant points between centres, and then a pilot test was used to corroborate that the spreadsheet would allow the recording of all the variables under study and be easy to fill in. The researchers themselves transcribed the data into the Excel spreadsheet for subsequent analysis and data cleaning.
The study variables were:
- -
Demographic variables: age (in years), sex (male or female), body mass index (BMI), date of admission, date of discharge from the ICU service, and days of admission.
- -
PP-related variables:
- •
Number of PP episodes per patient.
- •
Duration in hours of each PP episode.
- •
Number of total hours of PP per patient.
- •
- -
PU-related variables:
- •
Site of PU: nose, lip, pinna, chin, forehead, cheeks, chest, iliac crest, genitalia, knees, back of the foot and toes.
- •
PU stage:
- o
Stage 1: non-blanching erythema on intact skin.
- o
Stage 2: partial loss of skin thickness (phlyctena).
- o
Stage 3: total loss of skin thickness.
- o
Stage 4: total loss of tissue thickness (visible muscle/bone).
- o
- •
Prevention: HOFA, almond oil, hydrocolloid dressings, and heel pads (for knees and toes).
- •
Type of mattress: viscoelastic or air mattress.
- •
Frequency of head and arm postural changes: every 4 h, every 6 h, or not performed.
- •
- -
Nutrition-related variables:
- •
Time from admission to start of nutrition in hours: at 24 h, 48 h, 72 h, 96 h, or not started.
- •
Type of nutrition: enteral, parenteral, or mixed.
- •
Type of enteral nutrition: diabetic, hyperproteic, or normocaloric.
- •
Extra protein intake: with extra protein intake (yes), without extra protein intake (no).
- •
Authorisation for the study was requested from the Research Ethics Committee of the Balearic Islands (CEI-IB) and was approved, n° IB4461/21 PI. The project was also submitted for evaluation to the research committees of the different hospitals. In accordance with the provisions of Royal Decree 957/2020, of 3 November, which regulates observational studies with medicinal products, informed consent was not requested, because the data were obtained by reviewing clinical records; no intervention was performed, nor was there any change in treatment, and therefore there was no risk to patients. Data confidentiality was guaranteed in accordance with Organic Law 3/2018 of December 5 on Protection of Personal Data and Guarantee of Digital Rights.
Data analysisA descriptive analysis of all variables was performed to define their characteristics, with frequencies and percentages for qualitative variables and quantitative variables with median and interquartile range. The H-Kruskal Wallis and χ2 tests were used to evaluate the differences between the 4 hospitals. Variables were then compared between patients with and without PUs using the Mann-Whitney U test, χ2 test, or Fisher's exact test. Correlations between 2 numerical variables were assessed using the Rho Spearman correlation coefficient. In the case of significant differences in the bivariate analyses, in relation to the presence of PUs, multivariate logistic regression methods were used to eliminate potential confounding factors, adjusting for age, sex, BMI, and total number of hours on PP. A P-value of <.05 was considered a significant difference. IBM-SPSS v.2 was used to analyse the data2.
ResultsDuring the data collection phase, 574 patients diagnosed with COVID-19 were admitted (H1 n: 60; H2 n: 60; H3 n: 177; H4 n: 277). A total of 43.03% (n: 247) required PP at some time; the incidence of PP per hospital was: H1 51.7%, H2 50%, H3 52.5%, and H4 33.6%.
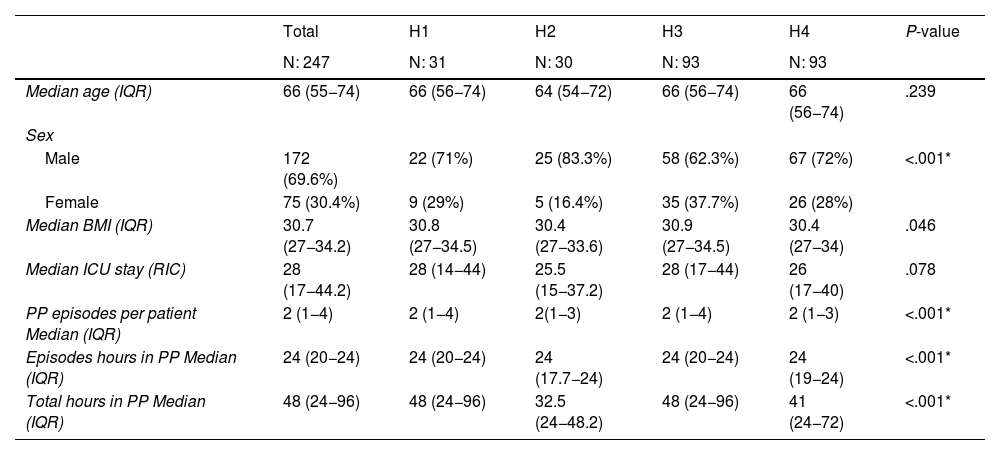
Of the patients, 69.6% were male, with a median age of 66 (IQR: 55−74) years, median BMI of 30.7 (IQR: 27−34.2), and median hospital stay of 28 (IQR: 17−44.2) days. The median duration of each PP was 24 h (IQR: 20−24), with a median (IQR: 1−4) 2 PP episodes recorded per patient during admission, and a median total hours in PP of 48 (IQR: 24−96). Statistically significant differences were observed between the variables sex, BMI, PP episodes per patient, median hours per PP episode, and total PP hours during admission per patient (P < .005) (Table 1).
Sociodemographic and clinical characteristics of the sample.
| Total | H1 | H2 | H3 | H4 | P-value | |
|---|---|---|---|---|---|---|
| N: 247 | N: 31 | N: 30 | N: 93 | N: 93 | ||
| Median age (IQR) | 66 (55−74) | 66 (56−74) | 64 (54−72) | 66 (56−74) | 66 (56−74) | .239 |
| Sex | ||||||
| Male | 172 (69.6%) | 22 (71%) | 25 (83.3%) | 58 (62.3%) | 67 (72%) | <.001* |
| Female | 75 (30.4%) | 9 (29%) | 5 (16.4%) | 35 (37.7%) | 26 (28%) | |
| Median BMI (IQR) | 30.7 (27−34.2) | 30.8 (27−34.5) | 30.4 (27−33.6) | 30.9 (27−34.5) | 30.4 (27−34) | .046 |
| Median ICU stay (RIC) | 28 (17−44.2) | 28 (14−44) | 25.5 (15−37.2) | 28 (17−44) | 26 (17−40) | .078 |
| PP episodes per patient Median (IQR) | 2 (1−4) | 2 (1−4) | 2(1−3) | 2 (1−4) | 2 (1−3) | <.001* |
| Episodes hours in PP Median (IQR) | 24 (20−24) | 24 (20−24) | 24 (17.7−24) | 24 (20−24) | 24 (19−24) | <.001* |
| Total hours in PP Median (IQR) | 48 (24−96) | 48 (24−96) | 32.5 (24−48.2) | 48 (24−96) | 41 (24−72) | <.001* |
Values expressed as median and interquartile range (IQR).
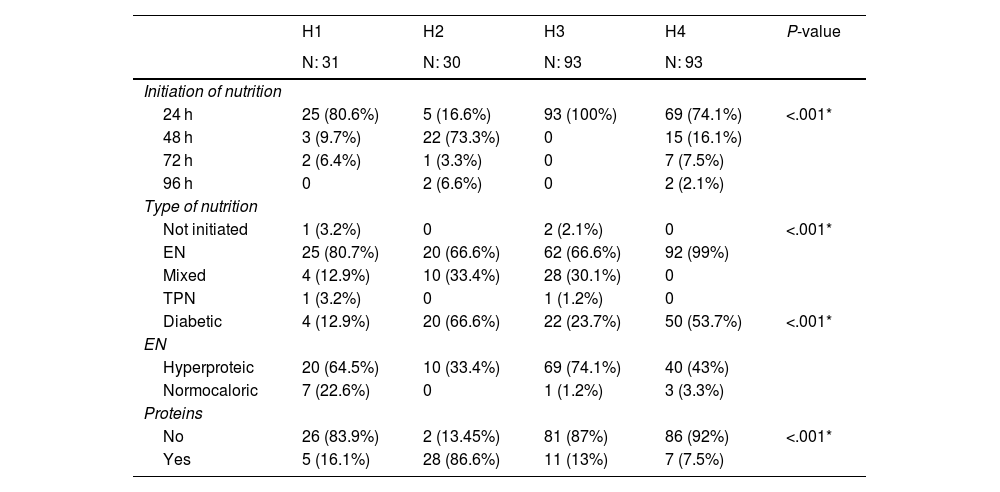
The variables of nutrition initiation, type of nutrition, and protein supplementation were statistically significant (P < .001). Nutrition was started at 24 h in 3 hospitals and at 48 h in only one hospital. In terms of type of feeding, 81% of patients were on enteral nutrition and 17% were supplemented with parenteral nutrition. Protein supplementation was given in all the hospitals, in H2 it was given to 86.6% of the patients. Table 2 describes the variables related to nutrition.
Characteristics related to nutrition administered to the critically ill patients in the study.
| H1 | H2 | H3 | H4 | P-value | |
|---|---|---|---|---|---|
| N: 31 | N: 30 | N: 93 | N: 93 | ||
| Initiation of nutrition | |||||
| 24 h | 25 (80.6%) | 5 (16.6%) | 93 (100%) | 69 (74.1%) | <.001* |
| 48 h | 3 (9.7%) | 22 (73.3%) | 0 | 15 (16.1%) | |
| 72 h | 2 (6.4%) | 1 (3.3%) | 0 | 7 (7.5%) | |
| 96 h | 0 | 2 (6.6%) | 0 | 2 (2.1%) | |
| Type of nutrition | |||||
| Not initiated | 1 (3.2%) | 0 | 2 (2.1%) | 0 | <.001* |
| EN | 25 (80.7%) | 20 (66.6%) | 62 (66.6%) | 92 (99%) | |
| Mixed | 4 (12.9%) | 10 (33.4%) | 28 (30.1%) | 0 | |
| TPN | 1 (3.2%) | 0 | 1 (1.2%) | 0 | |
| Diabetic | 4 (12.9%) | 20 (66.6%) | 22 (23.7%) | 50 (53.7%) | <.001* |
| EN | |||||
| Hyperproteic | 20 (64.5%) | 10 (33.4%) | 69 (74.1%) | 40 (43%) | |
| Normocaloric | 7 (22.6%) | 0 | 1 (1.2%) | 3 (3.3%) | |
| Proteins | |||||
| No | 26 (83.9%) | 2 (13.45%) | 81 (87%) | 86 (92%) | <.001* |
| Yes | 5 (16.1%) | 28 (86.6%) | 11 (13%) | 7 (7.5%) | |
Values expressed as absolute (n), relative (%) frequencies.
In relation to PU prevention, 98% of the admitted patients had an anti-bedsore air mattress. There was variable use of HOFA and hydrocolloid dressings. In H1, 22.5% used only hydrocolloid dressings. In H2 96% used only HOFA. In H3 75.2% used HOFA and silicone dressings. In H4 91.3% used HOFA and heel pads.
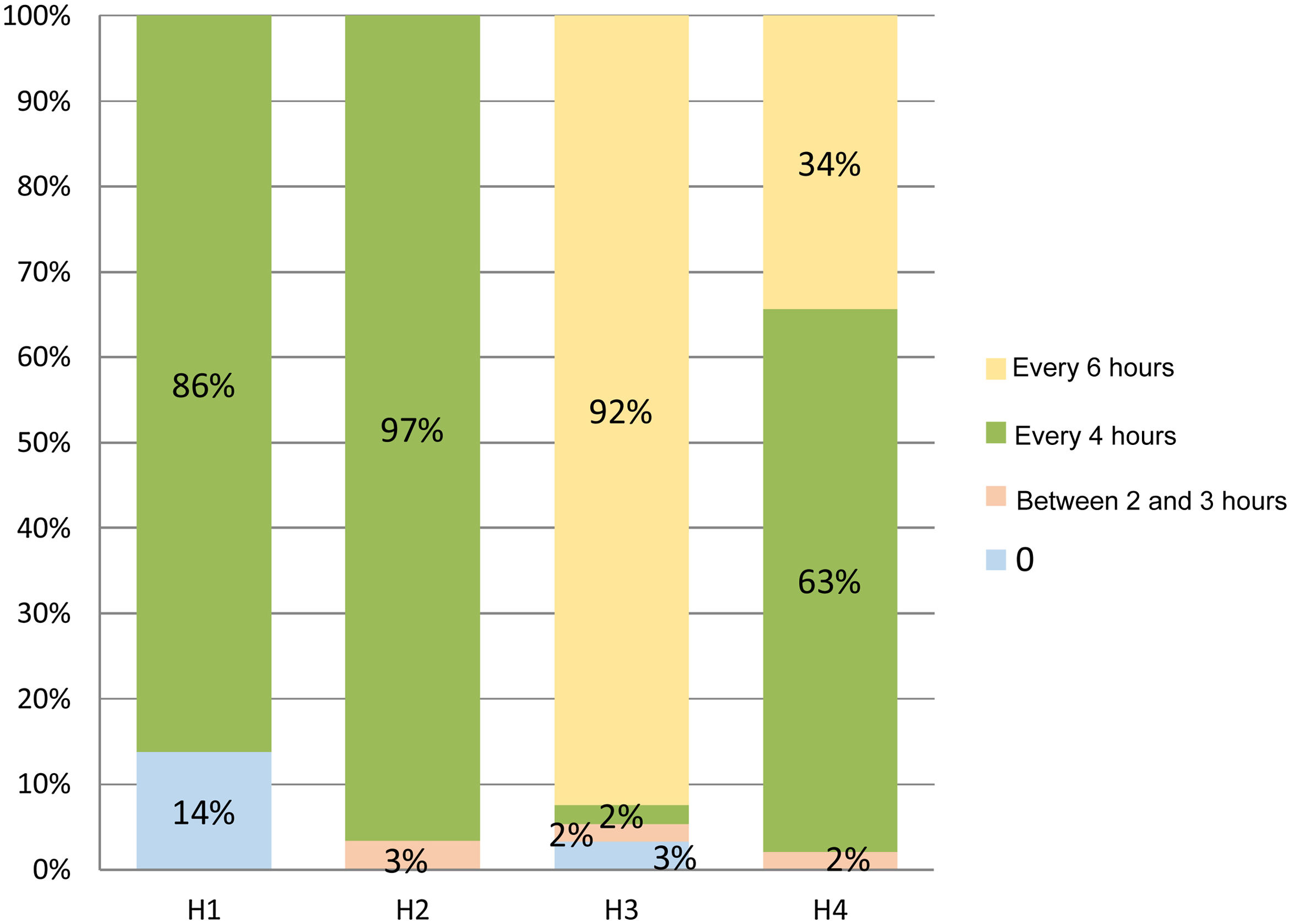
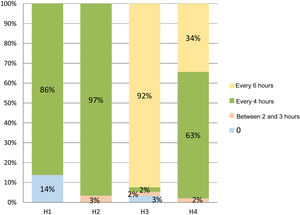
There were statistically significant differences in the frequency of postural changes between the different hospitals (P < .001), they were performed every 4 h in most of the ICUs (Fig. 1).
The overall incidence of PU secondary to PP was 56.3%, varied considerably between hospitals (H1: 32.2%, H2: 43.3%, H3: 96.7%, and H4:26.9%), and was statistically significant (P < .001).
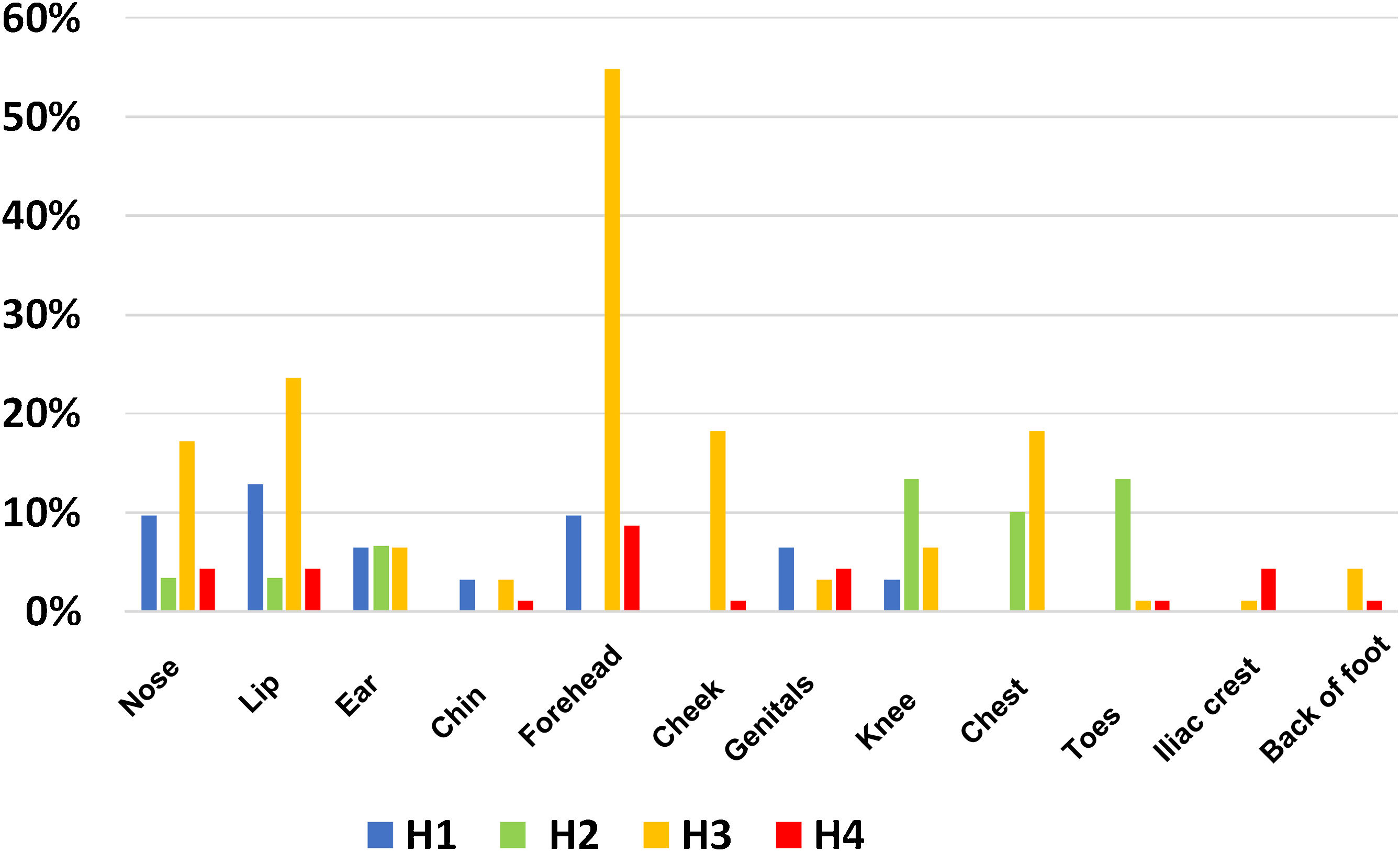
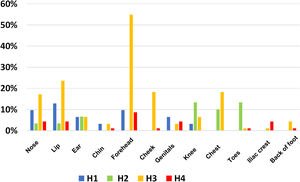
The site of PUs also varied between the different ICUs. Fig. 2, site, and frequency of PUs in the different hospitals, shows a higher incidence of lip and nasal PUs (H1: 10%), knee and back of the feet (H2: 13%), and forehead (H3: 55% and in H4: 9%).
In terms of the stage of the PUs, 33.8% were first-degree and 66.2% were second-degree. Regarding the number of PUs per patient, 76.8% had one PU and 12.3% had two PUs. When analysing this variable between hospitals H2 and H4, there was a maximum of 2 PUs per patient and in H3 some patients had up to 6 PUs.
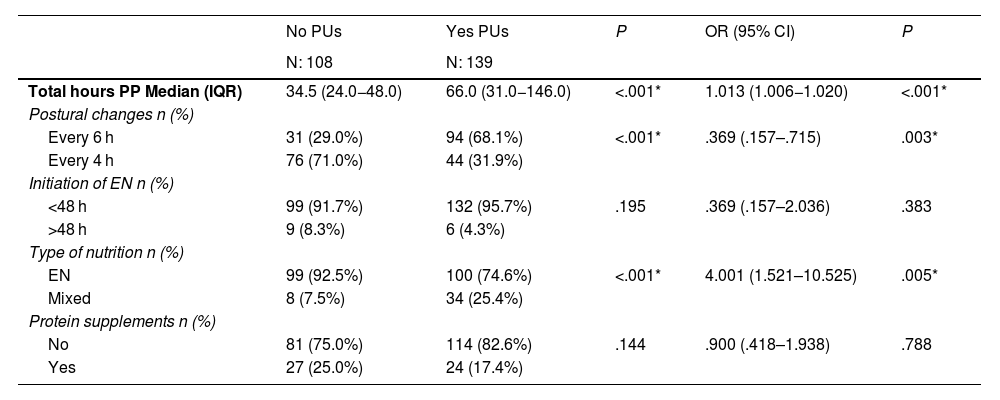
There are statistically significant differences between patients who had PUs and those who did not. Table 3 shows the association between ulcer prevalence and admission characteristics. Subsequent multiple regression analysis showed that the variable total hours in PP had significant impact (OR: 1.013; 95% CI: 1.006−1.020), therefore, the longer the time in PP the likelihood of PUs increased (P < .001). Postural changes also had a significant effect (P = .003) (OR: .369; 95% CI: .157–.715). Performing changes at a frequency of less than 4 h is a protective factor in the development of PUs. Early initiation of nutrition, although not statistically significant, was a protective factor (OR: .369; 95% CI: .157–2.036), P = .383, as was extra protein intake (OR: .900; 95% CI: .418–1.938), P = .788. Patients on mixed nutrition have a higher likelihood of PU compared to exclusive enteral nutrition (OR: 4.001; 95% CI: 1.521–10.525), P = .005.
Bivariate analysis in relation to the onset of PUs and multivariate logistic regression adjusting for age, sex, BMI, and total number of hours in PP.
| No PUs | Yes PUs | P | OR (95% CI) | P | |
|---|---|---|---|---|---|
| N: 108 | N: 139 | ||||
| Total hours PP Median (IQR) | 34.5 (24.0−48.0) | 66.0 (31.0−146.0) | <.001* | 1.013 (1.006−1.020) | <.001* |
| Postural changes n (%) | |||||
| Every 6 h | 31 (29.0%) | 94 (68.1%) | <.001* | .369 (.157–.715) | .003* |
| Every 4 h | 76 (71.0%) | 44 (31.9%) | |||
| Initiation of EN n (%) | |||||
| <48 h | 99 (91.7%) | 132 (95.7%) | .195 | .369 (.157–2.036) | .383 |
| >48 h | 9 (8.3%) | 6 (4.3%) | |||
| Type of nutrition n (%) | |||||
| EN | 99 (92.5%) | 100 (74.6%) | <.001* | 4.001 (1.521–10.525) | .005* |
| Mixed | 8 (7.5%) | 34 (25.4%) | |||
| Protein supplements n (%) | |||||
| No | 81 (75.0%) | 114 (82.6%) | .144 | .900 (.418–1.938) | .788 |
| Yes | 27 (25.0%) | 24 (17.4%) | |||
Quantitative variables expressed as median and interquartile range (IQR), categorical variables as absolute and relative frequencies: n (%). Multivariate binary logistic regression analysis expressed as odds ratio (OR) and confidence interval (95% CI%).
On analysis of the data, it was observed that the number of admissions of men was higher than that of women and the median age was 64 years, which is somewhat higher than the figures found in the literature of 57, 61, and 62 years.17–19
Recent studies report that obese patient affected by COVID-19 have a higher rate of severe complications and worse outcome than non-obese patients.20 The WHO international classification of obesity considers people with a BMI equal to or greater than kg/m2 of either sex to be obese.21 BMI is associated with the onset of PUs; however, we did not find a statistical relationship between onset of PUs and BMI. Obese patients have higher rates of PUs compared to normal weight patients. The formation of adipose tissue reduces the vascularisation of the skin surface, favouring tissue ischaemia and the development of PUs.13 Most of the patients admitted during the study period were obese, with a BMI over 30, and therefore the risk of developing PUs is high. We agree with other studies that patients admitted due to COVID-19 had a BMI > 25.17,20
The current literature also describes the risk of developing PUs in relation to nutritional status.22,23 The recommendations of scientific societies for the management of critically ill patients diagnosed with COVID-19 include early initiation of nutrition within the first 24 h–48 h of admission10,24,25; in all the hospitals nutrition was initiated within the recommended time period. In intubated, sedated, and relaxed patients the enteral route is considered the treatment of first choice, and in this study early enteral nutrition was initiated in all the ICUs. One of the concerns regarding the safety of enteral nutrition (EN) administration in PP is the risk of patients presenting with large residual gastric volumes, vomiting, or bronchial aspiration, and therefore to improve tolerance, the 25° anti-Trendelenburg position and the administration of prokinetic drugs are recommended.24,25
Another recommendation for patients intolerant to EN, or when the digestive route fails to cover more than 60% of energy expenditure, is to initiate parenteral nutrition, maintaining trophic enteral nutrition whenever possible.10,25,26 Mixed nutrition was used in three of the hospitals, and it was observed that these patients were more likely to develop PUs than patients with EN; this finding was statistically significant. Nutritional assessment and early management of the nutritional care of patients with COVID-19 should be integrated into the overall therapeutic strategy.27 There are recommendations to avoid malnutrition during ICU admission, such as the administration of hyperproteic nutrition and protein intake. Although it is a protective factor in the prevention of PUs, we found no statistical relationship between protein intake and the onset of PUs.
The measures used in the 4 hospitals for the prevention of PUs were air mattresses, HOFA, and dressings. Dynamic positioning devices (alternating air mattresses, lateral positioning, air-fluidised and bariatric beds) are recommended in addition to repositioning, to relieve pressure points on the face and body. The use of hydrocolloid dressings, transparent film, and silicone may be beneficial in reducing facial skin lesions.11 In our study no dressings were used to protect the face, but pillows and foams were used. Despite the preventive measures used, there was a high incidence of PU development in the facial area. The author Binda reveals that hydrocolloid dressings to protect facial pressure areas were not sufficient to prevent onset of PUs. These results are not far from recent studies, which used the same preventive actions17,18,28 and the recorded rates are similar to ours. Perrillat’s study explains this higher incidence of the onset of PUs on the face as due to microvascular injury and thrombosis related to facial decubitus, severe hypoxaemia of the patient, and decreased peripheral perfusion.29
There is variability between the different hospitals on the frequency of postural changes. It was observed that performing them every 4 h was a protective factor, since patients who underwent them every 6 h developed more PUs. It should be noted that, at the beginning of the pandemic, lack of knowledge, fear of contagion, and the lack of individual protection resources meant that contact with patients was reduced and the frequency of entering and exiting the cubicle was reduced. The Binda study describes a frequency of repositioning every 2 h, and this is perhaps why they recorded a lower number of PUs compared to our results.17
Prone positioning is associated with an increased risk of PUs. Mora states that it is the most common adverse event from the procedure.30 The most frequent sites related to PP were the forehead and lip, similar to the results of other authors who state that the facial area was the area with the highest risk,17,18,31 followed by the knee and the back of the feet. When comparing the anatomical areas of PUs secondary to PP between the different hospitals, it is striking that in the larger ICUs there were a greater number of affected areas, between 10–12 compared to 6–7 in the smaller ICUs. In her study, Jové describes 8 sites, 4 of which were facial; we agree with this author on the stage of the PUs, the highest percentage being second-degree.8
The overall incidence of PUs in PP patients in our study was higher than in other similar articles, 56.3% versus 30.2%17, 47%18, and 50%,31 with great variability between hospitals. The high incidence of PUs may be due to longer periods in the prone position and the median number of hours per patient in PP, which was 24 h in all hospitals, although there was variability in duration between centres. The literature reports periods of 8 h per day, 16 h, and even up to 48 h.30–32 Other studies report intervals from 12 h to 18 h.17,18,31 American and European scientific societies recommend PP episodes of 12 h–16 h.27,31 During the pandemic, prolonged prone positioning episodes for longer than 16 h were recommended.10 When assessing the risk-benefit of PP, respiratory benefits were prioritised over late complications, such as pressure injuries with time spent in PP.9,17,18,33 The author Concha states that due to the work overload of health professionals during the pandemic, new professionals’ inexperience in the prone positioning technique, and its complexity, they opted for prolonged periods of PP, and in 85% the duration of PP episodes was longer than 24 h.32
The limitations of the study are those inherent to retrospective designs in which care may be under-recorded. The use of different recording programmes in the ICUs that participated in the study may have led to variability in coding and different assessment criteria, which led us to discard some variables, such as the severity scales of the critical patient. The development of PUs in the critical patient is multi-causal, and factors such as treatment, poor peripheral perfusion, and the administration of vasoactive drugs also affect onset. In this study, only variables related to nursing care and PU prevention were considered; the lack of a single procedure may be a limitation when comparing the incidence between hospitals. It was not possible to analyse the efficacy of the different treatments to prevent PUs, because the same patient could receive different care to the risk areas. In future studies it would be interesting to compare the efficacy of HOFA and the different dressings.
ConclusionsThe incidence of PUs in patients with COVID-19 in PP was high. Care related to PU prevention in PP patients (use of HOFA, frequency of postural changes, duration of PP episodes, initiation of nutrition, and protein-energy administration) was uneven. There is variability between hospitals in ulcer sites. The most frequent were facial PUs, especially on the forehead and lips, followed by the knees and the back of the feet, most were second-degree PUs. The likelihood of PU occurrence in PP is influenced by the number of hours on PP and the frequency of postural changes; the more hours in PP and the lower the frequency of postural changes, the higher the risk of PU. The type of nutrition and protein supplementation did not have a significant impact in this study.
The data from this study demonstrate the importance of unifying criteria for the prevention of PUs in PP patients admitted to the ICU. The variability of practice and the results obtained from the 4 units highlight the need to develop standardised protocols based on the recommendations and best evidence to reduce variability and improve the quality of care for critical patients.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Aina Millán Pons, Institut d'Investigació Sanitària de les Illes Balears-IdISBa, for the statistical support.