Evaluation of the impact on the quality of life (QOL) relating to health in patients with chronic urticaria (CSU) treated with omalizumab.
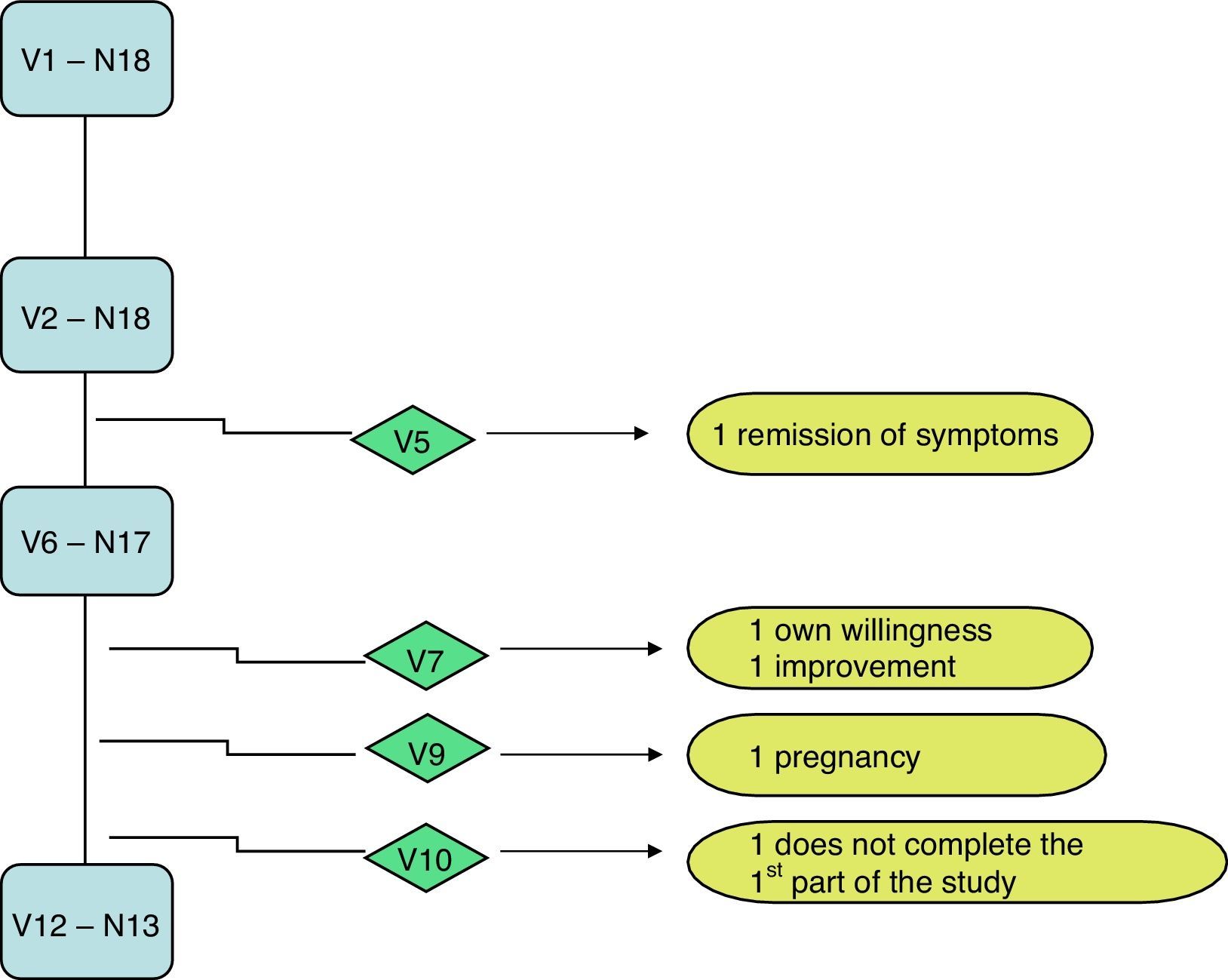
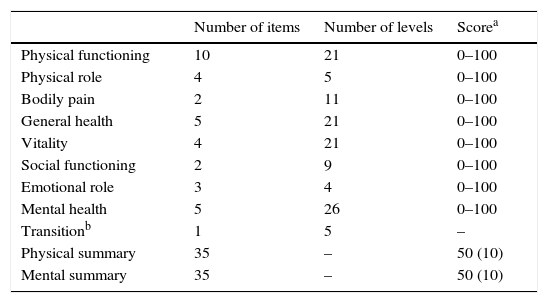
MethodLongitudinal descriptive observational study of quality of life based on18 patients with chronic urticaria ≥12 years treated with omalizumab. Changes in QOL examined at 1 month (T1), at 6 months (T6) and 12 months (T12), by: Visual Analogical Scale (UAS), specific urticaria QOL questionnaire (CU-Q2oL), general health questionnaire (SF-36) and activity of urticaria questionnaire (Score UAS7).
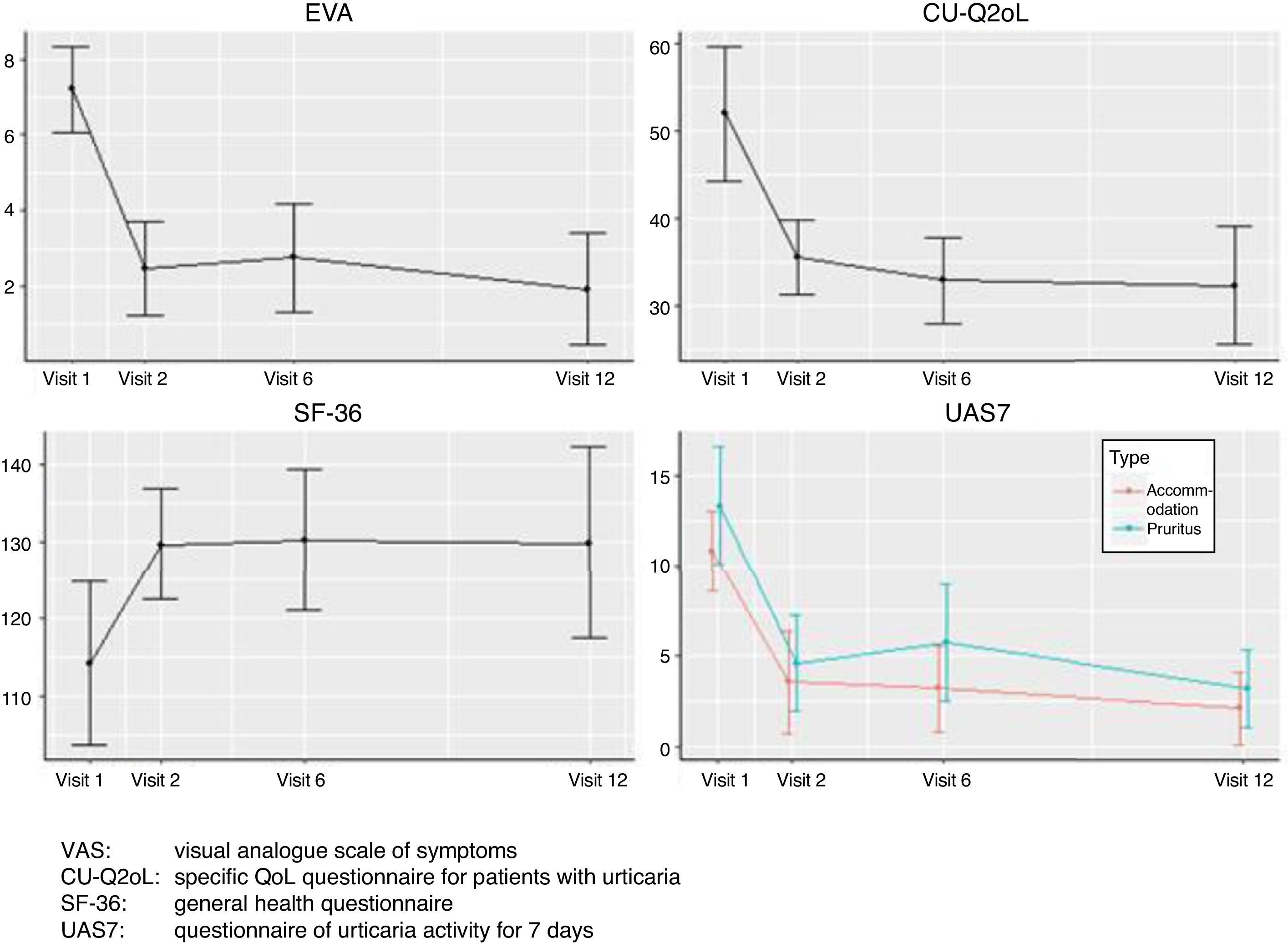
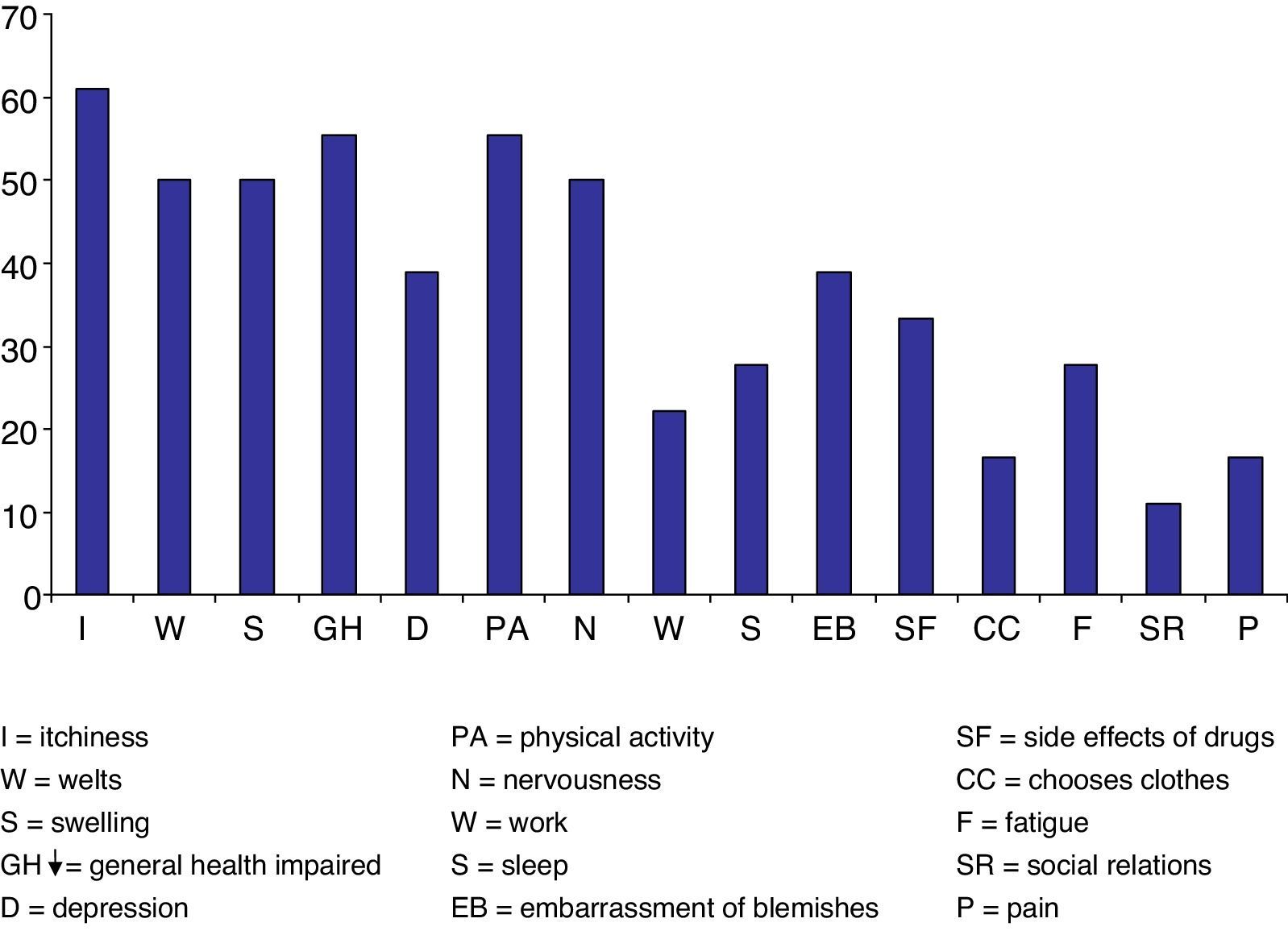
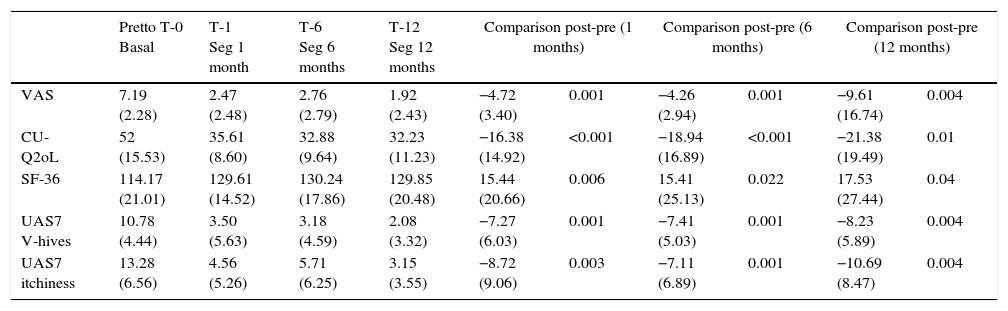
ResultsThe most disabling symptoms for patients were: pruritus (61.1%); decrease in health and physical activity (55.5%), wheals, swelling and nervousness (50%); depression and shame of marks (38.89%); side effects to drugs (33.33%); sleepiness and tiredness (27.78%); work (22.22%); “choosing clothes and pain” 16.67% and social relations (11.11%). Statistically significant differences were observed in T1, T6 and T12. In T1, UAS −4.72 (p=0.001); CU-Q2oL −16.38 (p<0.001); SF-36 15.44 (p=0.006); UAS7 wheals −7.27 (p=0.001), UAS7 pruritus −8.72 (p=0.003). In T6, UAS −4.26 (p=0.001); CU-Q2oL −18.94 (p<0.001); SF-36 15.41 (p<0.001); UAS7 wheals −7.41 (p=0.001), UAS7 pruritus −7.11 (p=0.001). In T12 UAS −9.61 (p=0.004); CU-Q2oL −21.38 (p=0.01); SF-36 17.53 (p=0.04); UAS7 wheals −8.23 (p=0.004), UAS7 pruritus −10.69 (p=0.004).
ConclusionsThere was a very good response in T1, which was maintained in T6 and T12. Patients with chronic urticaria treated with omalizumab presented good results with a reduction of CUE activity and improvement of their QOL.
Evaluación del impacto en la calidad de vida (CdV) relacionada con la salud en los pacientes con urticaria crónica espontánea en tratamiento con omalizumab.
MétodoEstudio observacional descriptivo longitudinal de calidad de vida partiendo de 18 pacientes con urticaria crónica espontánea ≥ 12 años tratados con omalizumab. Se analizaron los cambios en la CdV al mes (T1), a los 6 (T6) y a los 12 meses (T12), mediante: escala analógica visual (EVA), cuestionario de CdV específico de urticaria (CU-Q2oL), cuestionario de salud general (SF-36) y cuestionario de actividad de urticaria (Score UAS7).
ResultadosLos síntomas más condicionantes para los pacientes fueron: prurito (61,1%); disminución de la salud y actividad física (55,5%); ronchas, hinchazón y nerviosismo (50%); depresión y vergüenza de marcas (38,89%); efectos secundarios a fármacos (33,33%); sueño y cansancio (27,78%); trabajo (22,22%); «elección de ropa y dolor» 16,67% y relaciones sociales (11,11%). Se observaron diferencias estadísticamente significativas en T1, T6 y T12. En T1, EVA -4,72 (p=0,001); CU-Q2oL -16,38 (p<0,001); SF-36 15,44 (p=0,006); UAS7 ronchas -7,27 (p=0,001), UAS7 prurito -8,72 (p=0,003). En T6, EVA -4,26 (p=0,001); CU-Q2oL -18,94 (p<0,001); SF-36 15,41 (p<0,001); UAS7 ronchas -7,41 (p=0,001), UAS7 prurito -7,11 (p=0,001). En T12 EVA -9,61 (p=0,004); CU-Q2oL -21,38 (p=0,01); SF-36 17,53 (p=0,04); UAS7 ronchas -8,23 (p=0,004), UAS7 prurito -10,69 (p=0,004).
ConclusionesExistió muy buena respuesta en T1, que se mantuvo en T6 y T12. Los pacientes con urticaria crónica espontánea tratados con omalizumab presentaron buenos resultados con reducción de la actividad de UCE y mejora de su CdV.