Carbapenem-resistant Gram-negative bacteria (CRGN) are an urgent public health threat because of the limited treatment options, its rapid spreading and high clinical impact and mortality rates. However, the burden and the use of resources of these infections have not been investigated. The aim of the current study is to understand the use of resources associated to the clinical management of CRGN infections in real clinical practice conditions.
MethodsAn observational retrospective chart review study was performed. Data regarding patient demographics, clinical management and use of resources associated to hospitalization were retrieved from clinical charts of ICU inpatients with a confirmed CRGN infection. Three reference Spanish hospitals were selected according to their patient volume and geographical coverage. Descriptive analyses of the clinical management and the use of resources and its cost were performed and then total costs by type of resource were calculated.
ResultsA total of 130 patients were included in the study. The higher number of patients (n=43; 33%) were between 61 and 70 years old. Ninety-four (72%) patients were male and 115 (88%) suffered from comorbidities. The mean total cost associated to the resources used in patients with CRGN infections hospitalized in ICU was 96,878€ per patient. These total costs included 84,140€ of total hospital stay, 11,021€ of treatments (558€ of antibiotics; 10,463€ of other treatments) and 1717€ costs of diagnostic tests.
ConclusionsCRGN infection causes a high use of hospital resources, being the length of stay either in hospital wards or ICU the driver of the total costs. Diagnostic tests and treatments, including antibiotics, represent the lowest part of the use of resources and costs (13% of total costs).
Las bacterias gramnegativas resistentes a carbapenémicos (CRGN) son una amenaza urgente de salud pública por las limitadas opciones de tratamiento, su rápida dispersión y el alto impacto clínico y tasas de mortalidad. Sin embargo, la carga y el uso de recursos de estas infecciones no han sido investigadas. El objetivo de este estudio es comprender el uso de recursos asociado al manejo clínico de las infecciones por CRGN en condiciones de práctica clínica real.
MétodosSe llevó a cabo un estudio observacional retrospectivo de revisión de historias clínicas. Se recogieron datos demográficos, del manejo clínico y del uso de recursos asociado a la hospitalización de historias clínicas de pacientes hospitalizados en UCI con una infección confirmada por CRGN. Se seleccionaron tres hospitales españoles de referencia por su cobertura geográfica. Se realizaron análisis descriptivos del manejo clínico y el uso de recursos y sus costes en episodios de infecciones por CRGN, y se calcularon los costes totales para cada tipo de recurso.
ResultadosSe incluyeron en el estudio un total de 130 pacientes. La mayoría de los pacientes (n=43;33%) tenían entre 61-70 años. Noventa y cuatro pacientes (72%) eran hombres y 115 (88%) presentaron comorbilidades. El coste medio total asociado a los recursos usados durante el episodio de infección por CRGN por paciente fue de 96.878€. Este coste total incluye 84.140€ de la estancia en el hospital, 11.021€ de los tratamientos (558€ de antibióticos y 10.463€ de otros tratamientos) y 1.717€ del coste de test diagnósticos.
ConclusionesEl episodio de infección por CRGN causa un alto uso de recursos hospitalarios, siendo la duración de la estancia tanto en planta hospitalaria como en UCI el factor con mayor peso de los costes totales. Los test diagnósticos clínicos y los tratamientos, incluyendo los antibióticos, representan la parte más pequeña del uso de recursos y sus costes (13% del coste total).
Multidrug-resistant bacteria (MDR) infections have been prioritized as a global and urgent challenge for public health by the World Health Organization (WHO), the European Commission and the US Department of Health, amongst other institutions.1–3 The resistance of these bacteria to available antibiotics has direct and indirect consequences for patients and society, such as longer patients’ illnesses and hospital stays, loss of protection for patients undergoing invasive procedures, losses of productivity, increasing economic costs or dramatic rates of morbidity and mortality.1 Moreover, infections caused by MDR require strict isolation precautions to limit the spread of these difficult-to-treat bacteria.4 Isolation precautions are associated with clinical adverse effects which may result in poorer hospital outcomes.5 Each year, the incidence of MDR in the United States is estimated in 2.8 million infection with more than 35,000 deaths as a result,3 meanwhile in Europe the estimated number of deaths rise until 33,000.6 Globally, around 700,000 people per annum die due to MDR infections, but this number is likely to be underestimated because of poor surveillance and report.7,8 Moreover, it is expected that if no One Health action is taken against MDR this number would increase until 10 million lives by 2050, replacing cancer as the first cause of death.7
Amongst the different types of pathogenic bacteria, carbapenem-resistant Gram-negative (CRGN) are specially complicated because of their developed antibiotic resistance, their rapid spreading and their aggressive clinical impact. By using different biological mechanisms (e.g., horizontal gene transfer) Gram-negative bacteria become resistant to carbapenems and other antibiotics, limiting the treatment options. As a result, CRGN infections cause a huge number of severe medical emergencies, serious complications to patients, long hospital stays and high mortality rates.8,9 In addition, it has been demonstrated that CRGN bacteria continuously accumulate multidrug-resistance mechanisms, making these bacteria increasingly harmful.9 Different factors are involved in this raising of developed resistance, being one of the main causes the abuse of antibiotic treatment in human population. Spain has been signaled as one of the European countries with a higher community intake rate of antibiotics,10 and CRGN bacteria have been detected as the first source of infections causing a high number of medical emergencies, morbidity and mortality.11 National and regional programs have been established in order to contain this public health issue.12
In addition to the patient suffering, CRGN infections also lead to serious economic consequences and only in the European Union it is estimated that MDR infections suppose €1.5 billion per year in direct and indirect costs.2 Despite the above mentioned clinical impact and the economic consequences, the burden and the use of resources for hospital CRGN infections remain unexplored in real clinical practice. To our knowledge, few studies have attempted to analyze the resources consumption in patients with CRGN infection with public available data.6,13 Recently, in Spain a study estimated that CRGN infections would imply a total cost of €472 million per year to the Spanish National Health System (NHS).13
The aim of the study was to describe the use of hospital resources associated to the clinical management of the CRGN infection in critically ill patients with a laboratory-confirmed infection caused by CRGN bacteria in conditions of real-life clinical practice.
MethodsParticipantsThree principal investigators from tertiary university leading Spanish hospitals were selected to participate in this study. These hospitals have a great volume of CRGN infected patients and cover different geographical regions allowing for a more representative sample of the country: Vall d’Hebron Hospital (VdH; Barcelona, beds: 1315, population covered: 430,000), Santiago University Hospital (CHUS; Santiago de Compostela, beds: 1395, population covered: 500,000) and Virgen de la Macarena Hospital (HUVM; Seville, beds: 1279, population covered: 558,000).
Inclusion criteria were defined by presenting a laboratory confirmed CRGN infection episode (including mixed infections of CRGN bacteria) and being hospitalized in the ICU (intensive care unit) between January, 1st 2015 and December, 31st 2019. Exclusion criteria included being under 18 years old at hospitalization time, participation of the subject in randomized clinical trials, active infectious episodes caused by bacteria other than CRGN or CRGN infection coexisting with no CR bacteria.
Sample size was fixed at 130 subjects to achieve the purposes of this descriptive study and to be illustrative of the use of resources in the selected centers.14 A competitive recruitment was performed between centers and all patients whose charts fulfilled the study inclusion and exclusion criteria were consecutively included until the sample size was reached.
The protocol of the study was approved by the Ethical Boards of all participating centers. The investigation followed the ethical principles of the Declaration of Helsinki15 and the requirements of the European Union General Data Protection Regulation (GDPR).16
ProcedureAn observational retrospective chart review design was followed for the purpose of this study. Retrospective data from clinical charts regarding patients’ demographics and the use of resources were collected by researchers via an electronic Case Report Form, including the following interest variables:
- •
Demographic data: gender, age, comorbidities.
- •
Hospitalization related data: main admission diagnosis, dates of hospitalization and ICU admission, date of CRGN infection detection, discharge and patient isolation.
- •
CRGN identification: use of biomarkers (PCR, procalcitonin, interleukins), microbiological (multi-resistance antibiogram, tracheal aspirate, Gram-negative bacilli antibiogram, specific bacteria culture, etc.) and imaging tests, date of CRGN identification.
- •
Clinical management in the ICU: main administered antibiotics, de-escalating process followed, emergence of serious adverse events (SAE: death or life-threatening) and its treatment resources, need for a surgery associated to CRGN infection, required ventilation and dialysis, and the dates of use of ventilation and dialysis.
- •
Hospitalization data out of the ICU: antibiotic treatment out of the ICU, achievement of microbiological eradication and clinical cure and readmission to the hospital within 30 days following an infection recurrence.
After information collection, all data were checked and validated by experimented researchers.
Statistical analysesUse of resourcesDescriptive statistics were performed for demographic variables. The frequencies and percentages of these variables were extracted.
For the data regarding the hospitalization, CRGN identification, clinical management of CRGN infection in the ICU and the hospitalization out of the ICU, the frequencies and percentages were extracted for the categorical variables. We calculated the length of use of different hospital resources and quantitative continuous variables were created: total days in hospital, days in the ICU, days in isolation, days on antibiotic treatment, days on ventilation, days on dialysis, and days from hospital or ICU admission to CRGN identification. For these continuous variables, the frequencies, percentages, mean, standard deviation, minimum and maximum values were extracted.
Different groups were split, based in previously established risk factors17: age, center of recruitment, admission diagnosis, or ward admission (ICU/other ward). Group analyses were performed applying independent-sample t-test to compare the above variables between these groups.
Correlation analyses were also performed between the continuous variables of the length of use of different hospital resources described above. These variables were firstly tested for normality through the Kolmogorov–Smirnov test. Then, non-parametric Spearman's correlations were performed in order to test if the length of the use of these resources was correlated between them.
All these statistical analyses were performed by using SPSS v.23 (SPSS Inc., Chicago, IL).
Cost of resourcesThe cost of use of resources employed in patients with CRGN infection was also calculated. Data of the costs for the Spanish NHS were extracted from Bot Plus Spanish database (www.botplusweb.portalfarma.com/) and eSalud (www.esalud.oblikue.com) platform.
Mean, maximum and minimum costs were calculated for the resources employed in ICU inpatients with CRGN infection: diagnostic tests (biomarkers, microbiological and radiological tests), stay in ward and ICU, and treatments (including antibiotics, extracorporeal techniques (ventilation, dialysis and surgery) and SAE), as follows:
- •
Mean cost of resource per patient=Unitary cost of resource×Mean units or days of use during the hospitalization×Number of patients using the resource
- •
Maximum cost of resource per patient=Unitary cost of resource×Maximum value of use of the resource×Number of patients using the resource
- •
Minimum cost of resource per patient=Unitary cost of resource×Minimum value of use of the resource×Number of patients using the resource
The calculated costs of the use of resources were aggregated in different sections: costs of diagnostic tests, costs of hospital stay and costs of treatments. The total direct medical costs (mean, maximum and minimum) were finally calculated by the addition of the total costs of each of these sections.
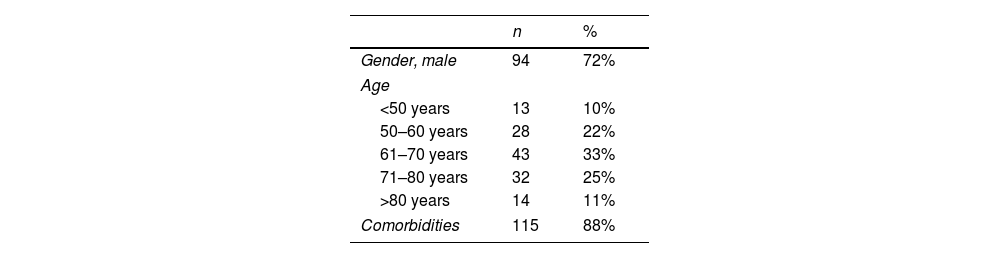
ResultsA total of 134 patients were included in the study: VdH, n=53; CHUS, n=50; HUVM, n=31. All of them accomplished inclusion and exclusion criteria. Four subjects (3, VdH; 1, HUVM) were excluded from the study sample because the information of the chart needed to fulfill the study was incomplete. Demographic characteristics of the study sample (gender, age and comorbidities) are presented in Table 1.
Sociodemographic results and diagnoses at admission of patients with CRGN infection.
| n | % | |
|---|---|---|
| Gender, male | 94 | 72% |
| Age | ||
| <50 years | 13 | 10% |
| 50–60 years | 28 | 22% |
| 61–70 years | 43 | 33% |
| 71–80 years | 32 | 25% |
| >80 years | 14 | 11% |
| Comorbidities | 115 | 88% |
| n | % | |
|---|---|---|
| Main hospital admission diagnostic | ||
| Septic shock | 28 | 21.5% |
| Postoperative | 18 | 13.8% |
| Respiratory infection | 13 | 10.0% |
| Bipulmonar transplant postoperative | 9 | 6.9% |
| Consciousness alteration | 5 | 3.8% |
| Cerebral haemorrhage | 4 | 3.1% |
| Subarachnoid haemorrhage | 3 | 2.3% |
| Ischemic shock | 2 | 1.5% |
| Unipulmonar transplant postoperative | 2 | 1.5% |
| Cardiorespiratory arrest | 2 | 1.5% |
| Hepatic transplant postoperative | 1 | 0.8% |
| Haemorrhagic shock | 1 | 0.8% |
| Othera | 42 | 32.3% |
Patients were admitted in the hospital and ICU with different primary diagnoses, being septic shock and postoperative complications the most common admission diagnoses (Table 1 and Table S1 in the Supplementary Material). Thirty-two percent of patients were directly admitted to the ICU due to their severe condition, and the rest of them were previously admitted to another ward.
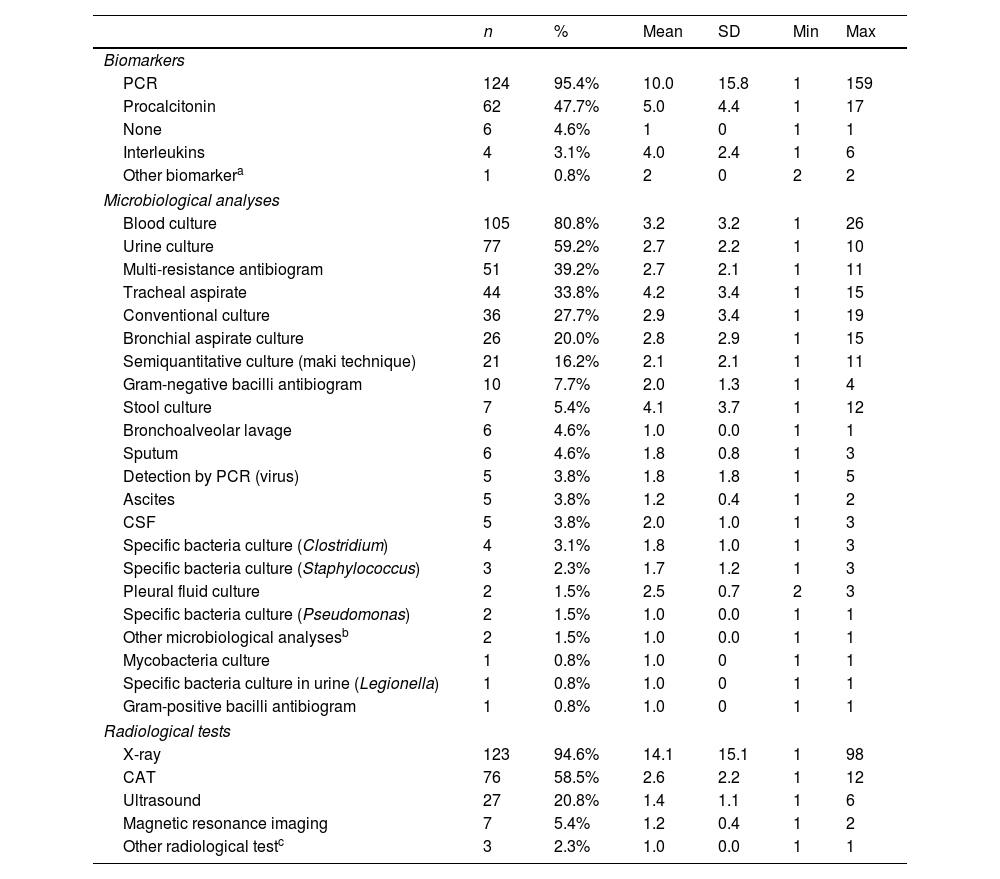
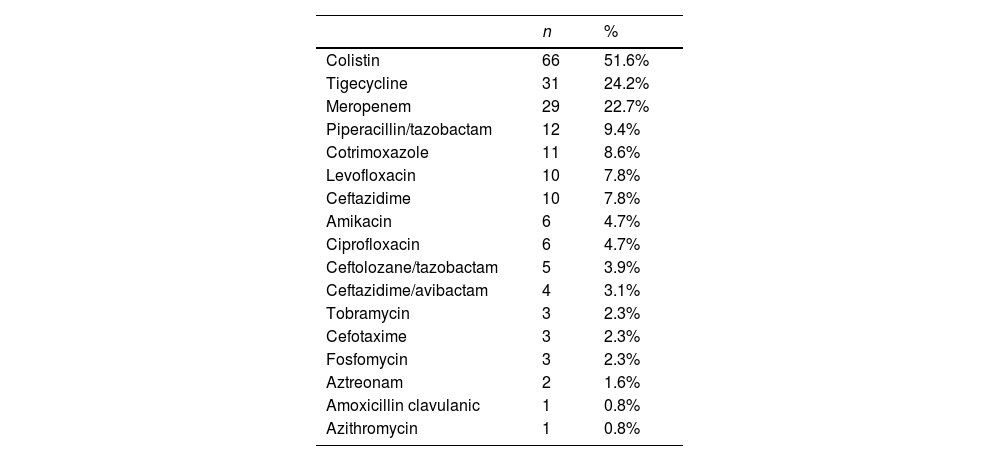
The use of biomarkers, microbiological analyses and radiological tests are shown in Table 2. Main antibiotics used for infection management at the ICU are fully presented in Table 3, being colistin, tigecycline and meropenem the most frequently administered. A single-antibiotic therapy was employed in 54% of patients, and the rest of patients received a combined therapy of 2-4 different antibiotics (Table S2 in the Supplementary Material). The delay of the antibiotic treatment was also calculated with the dates of CRGN identification and the beginning of the antibiotic treatment, and ranges between −10 days (identification before antibiotic) and 19 days (antibiotic before treatment, empirical diagnosis). An antibiotic de-escalating process was performed in only 15% of patients. SAEs related to antibiotic treatment were observed in only 4 subjects of the sample (being kidney failure, cardiorespiratory arrest, atrial fibrillation and death due to respiratory insufficiency).
Clinical diagnostic tests performed in CRGN infected patients.
| n | % | Mean | SD | Min | Max | |
|---|---|---|---|---|---|---|
| Biomarkers | ||||||
| PCR | 124 | 95.4% | 10.0 | 15.8 | 1 | 159 |
| Procalcitonin | 62 | 47.7% | 5.0 | 4.4 | 1 | 17 |
| None | 6 | 4.6% | 1 | 0 | 1 | 1 |
| Interleukins | 4 | 3.1% | 4.0 | 2.4 | 1 | 6 |
| Other biomarkera | 1 | 0.8% | 2 | 0 | 2 | 2 |
| Microbiological analyses | ||||||
| Blood culture | 105 | 80.8% | 3.2 | 3.2 | 1 | 26 |
| Urine culture | 77 | 59.2% | 2.7 | 2.2 | 1 | 10 |
| Multi-resistance antibiogram | 51 | 39.2% | 2.7 | 2.1 | 1 | 11 |
| Tracheal aspirate | 44 | 33.8% | 4.2 | 3.4 | 1 | 15 |
| Conventional culture | 36 | 27.7% | 2.9 | 3.4 | 1 | 19 |
| Bronchial aspirate culture | 26 | 20.0% | 2.8 | 2.9 | 1 | 15 |
| Semiquantitative culture (maki technique) | 21 | 16.2% | 2.1 | 2.1 | 1 | 11 |
| Gram-negative bacilli antibiogram | 10 | 7.7% | 2.0 | 1.3 | 1 | 4 |
| Stool culture | 7 | 5.4% | 4.1 | 3.7 | 1 | 12 |
| Bronchoalveolar lavage | 6 | 4.6% | 1.0 | 0.0 | 1 | 1 |
| Sputum | 6 | 4.6% | 1.8 | 0.8 | 1 | 3 |
| Detection by PCR (virus) | 5 | 3.8% | 1.8 | 1.8 | 1 | 5 |
| Ascites | 5 | 3.8% | 1.2 | 0.4 | 1 | 2 |
| CSF | 5 | 3.8% | 2.0 | 1.0 | 1 | 3 |
| Specific bacteria culture (Clostridium) | 4 | 3.1% | 1.8 | 1.0 | 1 | 3 |
| Specific bacteria culture (Staphylococcus) | 3 | 2.3% | 1.7 | 1.2 | 1 | 3 |
| Pleural fluid culture | 2 | 1.5% | 2.5 | 0.7 | 2 | 3 |
| Specific bacteria culture (Pseudomonas) | 2 | 1.5% | 1.0 | 0.0 | 1 | 1 |
| Other microbiological analysesb | 2 | 1.5% | 1.0 | 0.0 | 1 | 1 |
| Mycobacteria culture | 1 | 0.8% | 1.0 | 0 | 1 | 1 |
| Specific bacteria culture in urine (Legionella) | 1 | 0.8% | 1.0 | 0 | 1 | 1 |
| Gram-positive bacilli antibiogram | 1 | 0.8% | 1.0 | 0 | 1 | 1 |
| Radiological tests | ||||||
| X-ray | 123 | 94.6% | 14.1 | 15.1 | 1 | 98 |
| CAT | 76 | 58.5% | 2.6 | 2.2 | 1 | 12 |
| Ultrasound | 27 | 20.8% | 1.4 | 1.1 | 1 | 6 |
| Magnetic resonance imaging | 7 | 5.4% | 1.2 | 0.4 | 1 | 2 |
| Other radiological testc | 3 | 2.3% | 1.0 | 0.0 | 1 | 1 |
CAT=computerized axial tomography; CSF=cerebrospinal fluid; PCR=polymerase chain reaction; SD=standard deviation; n=number of patients who underwent the clinical diagnostic test; %=percentage of patients who underwent the test; mean=mean of tests per patient; SD=standard deviation of the mean; min=minimum value of tests performed in the patients with the test; max=maximum value of tests performed in the patients with the test.
Main antibiotic treatment administered to patients with CRGN infection.
| n | % | |
|---|---|---|
| Colistin | 66 | 51.6% |
| Tigecycline | 31 | 24.2% |
| Meropenem | 29 | 22.7% |
| Piperacillin/tazobactam | 12 | 9.4% |
| Cotrimoxazole | 11 | 8.6% |
| Levofloxacin | 10 | 7.8% |
| Ceftazidime | 10 | 7.8% |
| Amikacin | 6 | 4.7% |
| Ciprofloxacin | 6 | 4.7% |
| Ceftolozane/tazobactam | 5 | 3.9% |
| Ceftazidime/avibactam | 4 | 3.1% |
| Tobramycin | 3 | 2.3% |
| Cefotaxime | 3 | 2.3% |
| Fosfomycin | 3 | 2.3% |
| Aztreonam | 2 | 1.6% |
| Amoxicillin clavulanic | 1 | 0.8% |
| Azithromycin | 1 | 0.8% |
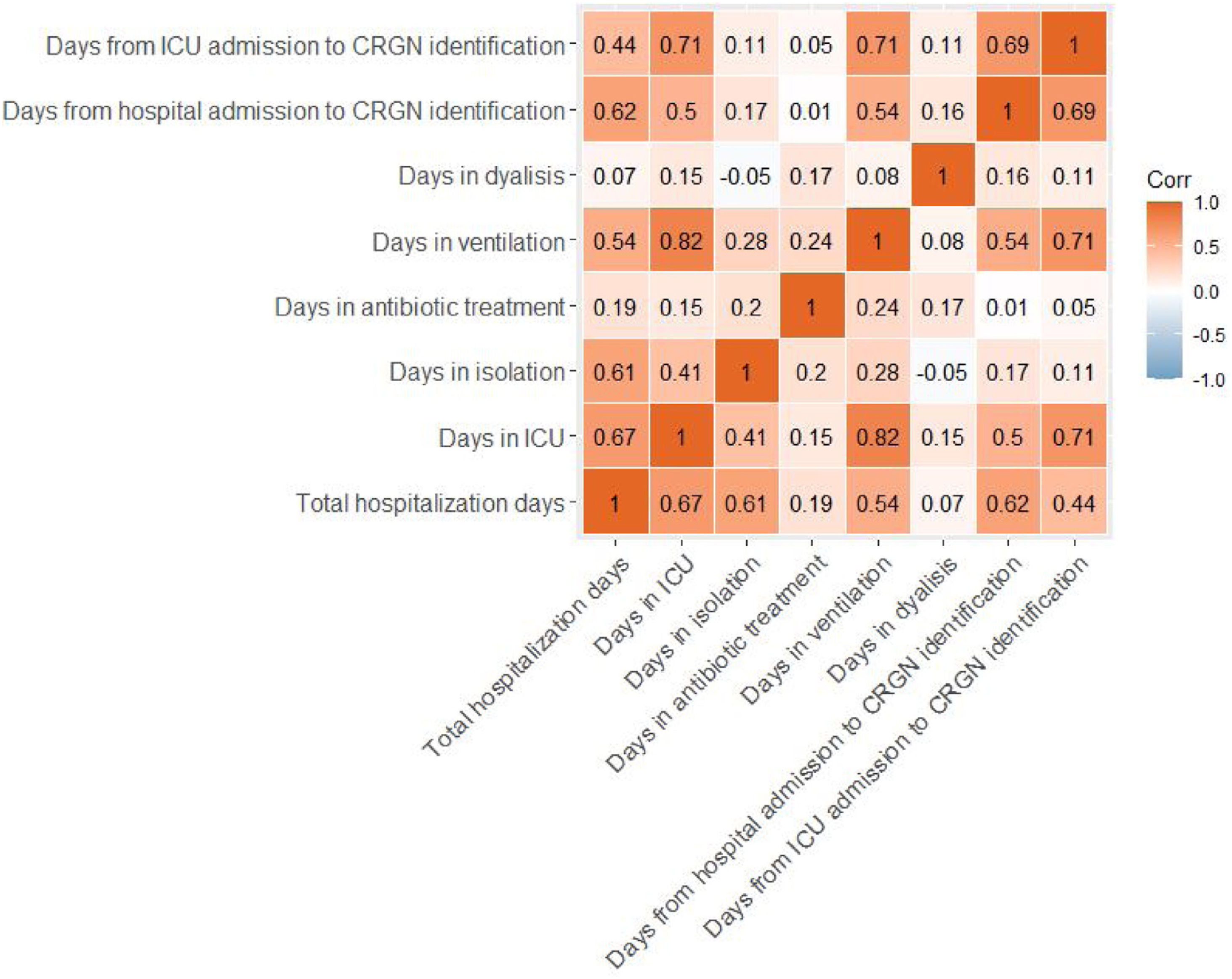
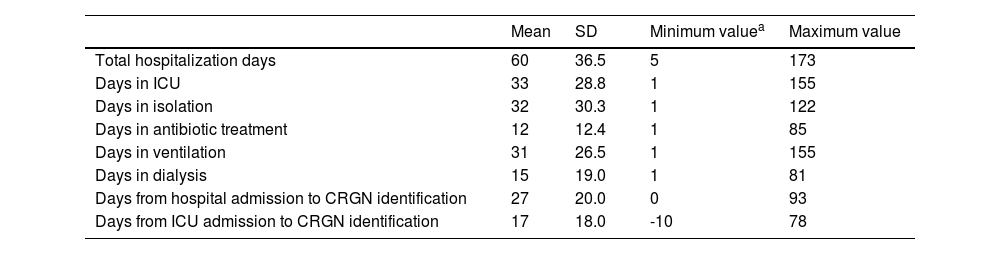
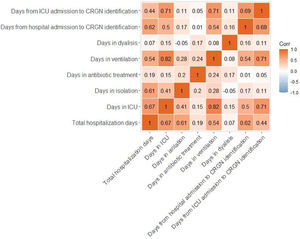
Eighty-two percent of patients required isolation during their stay in the hospital. Surgical procedures were followed in 11% of patients and 20% received dialysis. Moreover, 85% of subjects received ventilation for 17 days on average before the CRGN identification. Ninety-one percent of this ventilation was invasive. Total hospital stay, length of stay (LOS) in the ICU, days in isolation, antibiotic treatment, ventilation, dialysis and days from hospital or ICU admission to CRGN identification are presented in Table 4. With exception of the length of dialysis, the other quantitative continuous variables showed strong positive associations between them (see Fig. 1).
Length of use of different hospital resources by the patients with CRGN infection episode.
| Mean | SD | Minimum valuea | Maximum value | |
|---|---|---|---|---|
| Total hospitalization days | 60 | 36.5 | 5 | 173 |
| Days in ICU | 33 | 28.8 | 1 | 155 |
| Days in isolation | 32 | 30.3 | 1 | 122 |
| Days in antibiotic treatment | 12 | 12.4 | 1 | 85 |
| Days in ventilation | 31 | 26.5 | 1 | 155 |
| Days in dialysis | 15 | 19.0 | 1 | 81 |
| Days from hospital admission to CRGN identification | 27 | 20.0 | 0 | 93 |
| Days from ICU admission to CRGN identification | 17 | 18.0 | -10 | 78 |
After the ICU discharge, 40% of patients continued with antibiotic treatment. The clinical cure and the microbiological eradication were obtained in 61% and 46% of the patients, respectively. Only 12% of patients were re-admitted to the hospital 30 days after discharge.
No differences were found between groups split by gender or age. Differences found between sites, admission diagnostic and ward admission groups were observed mainly in the LOS in hospital and ICU. A detailed description of these results can be found in the Supplementary Material, Tables S3–S5.
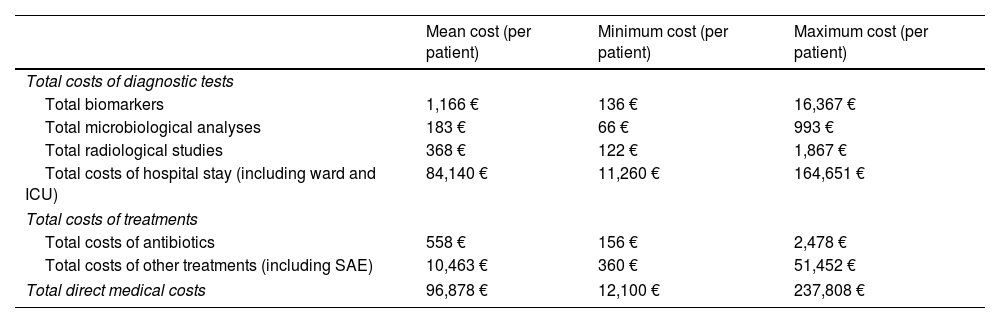
Cost of resourcesThe mean, maximum and minimum costs of the used resources in patients with CRGN infection are presented in Table 5. The mean total cost in patients with CRGN infection hospitalized in ICU, per patient, was 96,978€. The higher costs in the CRGN infection episode were those related to the LOS in hospital and ICU with 84,140€, since the cost of antibiotic treatment was 558€ that represented less than the 1% of the total costs of the CRGN infection episode and 5% of the total cost of the other treatments involved in infection management during the complete hospital stay.
Costs of the resources used in patients with an episode of CRGN infection.
| Mean cost (per patient) | Minimum cost (per patient) | Maximum cost (per patient) | |
|---|---|---|---|
| Total costs of diagnostic tests | |||
| Total biomarkers | 1,166 € | 136 € | 16,367 € |
| Total microbiological analyses | 183 € | 66 € | 993 € |
| Total radiological studies | 368 € | 122 € | 1,867 € |
| Total costs of hospital stay (including ward and ICU) | 84,140 € | 11,260 € | 164,651 € |
| Total costs of treatments | |||
| Total costs of antibiotics | 558 € | 156 € | 2,478 € |
| Total costs of other treatments (including SAE) | 10,463 € | 360 € | 51,452 € |
| Total direct medical costs | 96,878 € | 12,100 € | 237,808 € |
This study demonstrated that episodes of CRGN infections in conditions of real clinical practice imply a high utilization of hospital resources in hospitalized patients in ICU. The management of these infections requires an intensive use of hospital resources, such as strict isolation measures, treatments, or diagnostic tests. To our knowledge, this is the first work studying the use of resources in the process of CRGN infection management in patients that are admitted to the ICU with real clinical practice data in Spain.
We found that the majority of patients infected by a CRGN presented previously risk factors, as advanced age or comorbidities.18–20 The identification of the CRGN bacteria was confirmed on average 17 days after the admission to the ICU, in accordance with previous research reporting similar ranges for CRGN identification21 and supporting the evidence regarding the risk of CRGN infection acquisition in hospitalized patients.22,23 Mechanical ventilation has been also identified as a risk factor for hospital bacterial infection and has been associated with an increased mortality in CRGN infection patients.18,24,25
In our sample, a very high percentage of patients received invasive ventilation before the CRGN pathogen identification, and we also found a positive correlation between diagnosis of CRGN infection with LOS and number of ventilation days. Invasive ventilation has been previously pointed out as a risk factor for CRGN infection,19 but our data do not allow to confirm that in the patients of our study a causal relation exists. In our study, the LOS in hospital and ICU were long and correlated positively with the duration of antibiotic treatment (r=0.19, p=0.03; r=0.15, p=0.09, respectively), ventilation (r=0.54, p=0.01; r=0.82, p=0.01, respectively) and isolation (r=0.61, p=0.01; r=0.41, p=0.01, respectively). Previously, the LOS in hospital has been directly associated with higher antibiotic resistance and with positive cultures for P. aeruginosa, one of the CRGN pathogens causing the highest mortality rates.13,26 The most used antibiotics for the treatment of the infectious episodes in our sample were colistin, tigecycline and meropenem, in line with CRGN treatments followed in previous publications despite the previous evidence about their high toxicity.25,27 Clinical cure and the microbiological eradication were not achieved in a high percentage of our patients, signaling the need for developing and access of new effective drugs that allows a fast intervention in order to reduce the time of hospital stay and its associated risks.28 Differences between the split groups were not significant and should be deeply studied in the future with more specific data.
The high use of hospital resources leads to a high economic impact. In Spain, it has been estimated that CRGN total economic costs suppose €472 million per year to the Spanish NHS.13 Based in real-life data, we calculated that the total mean economic impact of CRGN infections in ICU patients is about 96,878€. Importantly, we observed that the cost of hospital stay, with 84,140€ per CRGN infected patient, was the resource with the biggest weight in the total medical costs of these patients. We also observed that the cost of antibiotics was almost insignificant (only 558€) in comparison with all the other resources used, representing only 1% of them. These results point to the fact that even if the costs of the CRGN infection episodes are elevated, the weight of antibiotics in the whole episode is not significant. Therefore, the potential impact of employing more expensive but effective drugs that may potentially reduce the use of other resources (e.g., the length of hospital and ICU stay) should be investigated and should be eventually considered in clinical practice.
There are some limitations in our study that should be acknowledged. Some data as the type of bacteria or the site of infection have not been collected, since the objective of the study was to get a general picture of the use of hospital resources dedicated to treat and manage the CRGN infection episodes, but not to specifically compare between the different pathogens or the systems affected by the infection. However, the study of the associated use of resources of each pathogen would be useful for the development of more targeted interventions in the future.6 Eighteen percent of patients were not isolated. Usually, all patients with CRGN infections are isolated due to the pathogens spreading.29 In our sample, exitus of the patient or missing data can explain this result and this result should be cautiously interpreted. Data regarding the mortality were not collected. Given the high mortality and morbidity associated to CRGN infection, including data related to these variables would be interesting in future studies to calculate the mortality, the years of life lost or the years of productive life lost due to CRGN infections.6,7,9 Since possible nephrotoxic effect of antibiotics (e.g., colistin) are covered in this study by dialysis and the number of days of dialysis, other adverse events (AEs) were not gathered. Only SAEs were collected because their high impact in the total cost of CRGN infections and because AEs are defined as any untoward effect after the administration of a product that is not necessarily related with the treatment.30 It would be therefore difficult to distinguish if AEs were caused by antibiotics and the estimation of the use of resources could be contaminated by AEs not related with CRGN infection.
Another limitation of the present study is the difficulty to establish a period of active infection. The length of stay in hospital was then calculated using the total days of stay at hospital. Different reasons made difficult to define cut-off points of active infection: the sample presents heterogenous diagnoses (sepsis or infection at the admission or other diagnoses), we did not collect the information regarding comorbidities or patient's infection as a secondary diagnose at admission, the days of antibiotic after ICU was neither gathered, so a follow-up of the treatment was not possible to be performed. A prospective methodology would be more appropriate to control these variables and to define an accurate active infection period, and the results of the present study should be cautiously interpreted as merely descriptive. The sample size of the study is representative of the centers included but it cannot be extrapolated to all Spanish population, especially to low-level hospitals. Nevertheless, the differences in the resources used for the management of CRGN infections are large and we would not expect to observe many changes in this distribution with bigger samples.
More studies focused on the use of resources of CRGN infection episodes in this and other populations (e.g., pediatric, immunosuppressed patients, other type of Spanish hospitals like low-level hospitals or from other geographical areas) should be conducted to gain a deeper knowledge of the clinical impact of these spreading pathogens and its impact to the NHS. Also, international multicentric studies would be useful to create awareness about this global problem. If antibiotics that could improve or cure the CRGN episodes were available, it would be expected that the use of resources and the hospital and ICU stays associated costs, which are the most important cost, would decrease. In conclusion, in our sample CRGN infections lead to a high use of hospital resources, being the length of stay in ICU and other wards the driver of the total costs. The lowest part of the resource consumption in these hospitals (13%) was represented by diagnostic tests and treatments, including antibiotics. Further studies would be necessary to replicate this study in other Spanish hospitals, to get a more representative picture of the burden of CRGN infection and it's cost to the National Health System.
FundingThis study has been financed by Shionogi Spain.
Conflict of interestsRF has received fees for advisory boards and conferences from Shionogi, MSD, Pfizer, Gilead, GSK and Menarini. JG-M has participated in educational activities for Shionogi and Pfizer. PR reports grants from Shionogi, grants and personal fees from Pfizer, and personal fees from Shionogi, MSD, and Menarini. LB-C has received fees for conferences from Shionogi. IdC and XB received funding for the design and analysis of the data of the study from Shionogi Inc.